Abstract
Two distinct subpopulations of endogenous cardiac stem cells (CSCs) are identified in the adult heart; myogenic CSCs (mCSCs), which are characterized by the presence of c-kit receptor, reside in the myocyte niche surrounded by matured cardiomyocytes, whereas vasculogenic CSCs (vCSCs), which express c-kit as well as KDR, are stored in the vascular niche found in the vessel wall. They both possess the fundamental properties of stem cells: self -renewing, clonogenic, and multipotent. While mCSCs preferentially differentiate into myocyte lineage in vitro, vCSCs tend to commit to vascular endothelial or smooth muscle lineages upon stimulation. Intramyocardial injection of human mCSCs to the region bordering infarct of immunosuppressed animals induces cardiac regeneration involving intensive muscle and vessel formation. On the other hand, the administration of human vCSCs near the critical stenosis created in the dog coronary artery results in the formation of large conductive vessels, i.e. “biological coronary bypass", coupled with the improved tissue perfusion. A clinical trial utilizing autologous c-kit-positive CSCs on patients with chronic ischemic heart failure was launched recently. Upon coronary artery bypass graft (CABG) surgery, a small portion of the right atrial appendage was obtained and used for isolation and expansion of CSCs. Intracoronary CSC implantation was performed 4 months after the surgery, and the symptom and the cardiac function were followed. Although the study is still ongoing, no adverse event due to cell infusion has been reported in any of the cell-treated patients, and the initial outcome is very promising. Disease-based customized cell therapy employing various combinations of autologous mCSCs and vCSCs would become available in the near future.
Keywords: Cardiac stem cells, c-kit, myocardial regeneration, heart failure
Introduction
The mammalian heart had been considered a post-mitotic terminally differentiated organ, in which the cardiomyocytes present at birth would persist throughout the life of the organism. However, recent identification of tissue-specific stem cells in the adult heart, i.e. cardiac stem cells (CSCs), of mammals including humans has challenged this traditional biological view. By definition, stem cells must satisfy three fundamental properties: self-renewing, clonogenic, and multipotent.
Now the heart has been appeared as a dynamic organ, in which newly formed cells derived from stem cell compartment continuously substitute old ones. Additionally, the latest research revealed that the heart contains two distinct sub-populations of endogenous stem cells: myogenic CSCs (mCSCs), which are mainly responsible for regenerating cardiomyocytes [1], and vasculogenic CSCs (vCSCs), which are more dedicated to the turnover of coronary vessels [2].
Distribution of mCSCs and vCSCs
In general, tissue-specific stem/progenitor cells are stored in structures called niche, which is located deep in the organ protecting primitive cells from harmful external stimuli [3]. Stem/ progenitor cells in niches are physically connected to specific supporting cells.
In the human heart, mCSCs express stem cell antigen c-kit, which is a receptor for a stem cell factor. mCSCs are clustered in niches located in the myocardial interstitium (Figure 1A), where mCSCs form connections with supporting cells, i.e. cardiomyocytes and fibroblasts, through connexins and cadherins (Figure 1B, C). Although mCSCs are present throughout myocardium and contribute to myocyte renewal, they are enriched at the atria and apex with low hemodynamic stress [1].
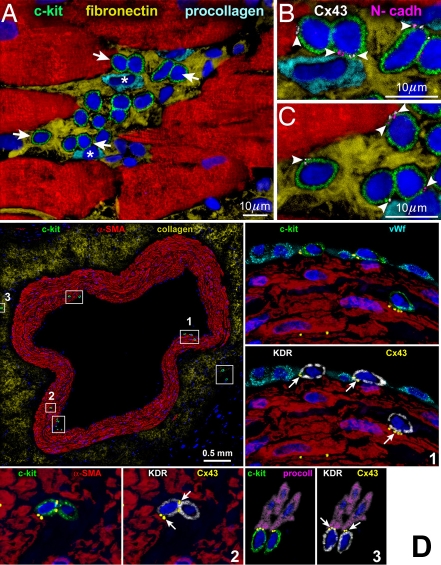
Figure 1.
Niches in the normal human heart. (A-C) Cluster of c-kitPOS cells (green). Arrows in A define the areas in B and C. Gap (connexin 43: Cx43, white; arrowheads) and adherens (N-cadherin: N-cadh, magenta; arrowheads) junctions are shown at higher magnification. Cx43 and N-cadh are present between c-kitPOS cells and myocytes (α-SA, red) and fibroblasts (procollagen, light blue) [1]. (D) Cross-section of human epicardial coronary artery composed of several layers of smooth muscle cells (α-smooth-muscle-actin: α-SMA, red). The c-kit-positive cells (green) are included in six rectangles. Three of the six rectangles are shown at higher magnification in the adjacent panels. The c-kit-positive cells express KDR (white). Cx43 (yellow dots; arrows) is seen between c-kit-KDR-positive cells and endothelial cells (von Willebrand factor: vWf, bright blue), smooth muscle cells (α-SMA, red), and adventitial fibroblasts (procoll, magenta) [2].
Human vCSCs express c-kit as well as KDR, a receptor for a vascular endothelial growth factor. They are nested in vascular niches distributed along the coronary circulation and primarily responsible for the turnover of vascular smooth muscle cells (SMCs) and endothelial cells (ECs). SMCs, ECs, and fibroblasts function as supporting cells in vascular niches found in endothelial, medial, and adventitial layers of epicardial coronary arteries (Figure 1D) as well as in arterioles and capillaries [2].
Clonogenicity of mCSCs and vCSCs
Quantitatively, there is one c-kit-positive cells in every ∼30,000 cardiac cells. By digesting human myocardium with solution containing collagenase, a small cell fraction is obtained. About 1% of these small cells express c-kit [1], and this population is subdivided to KDR-negative and positive cells corresponding to mCSCs and vCSCs, respectively. The lack of CD45 and tryptase excludes the contribution of mast cells to this pool in the preparation. Moreover, in the contrast with c-kit-positive bone marrow cells that express various hematopoietic markers, CSC populations do not express these antigens [2].
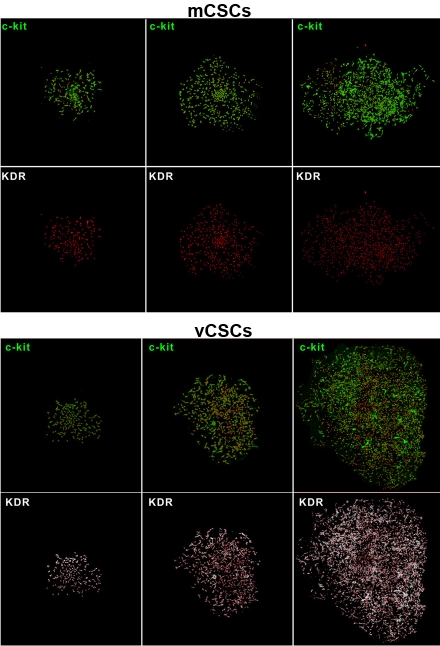
When single mCSCs or vCSCs are cultured, multicellular colonies are formed. As shown in Figure 2, clonogenic mCSCs and vCSCs continue to express c-kit alone and c-kit together with KDR, respectively. Importantly, single human mCSC can generate up to 1015 cells [1]; in other words, its proliferative potential is limited and different from those of cancer cells.
Figure 2.
Clones derived from single sorted human mCSC and vCSCs [2].
Division and differentiation of mCSCs and vCSCs
Both stem cells divide in two distinct ways. Whereas symmetric division generates two un-differentiated cells or two committed progenies, asymmetric division results in a formation of one stem cell and one differentiated cell. The latter form of division allows the organ to produce parenchymal cells while preserving the size of stem cell pool and thereby to maintain the tissue homeostasis.
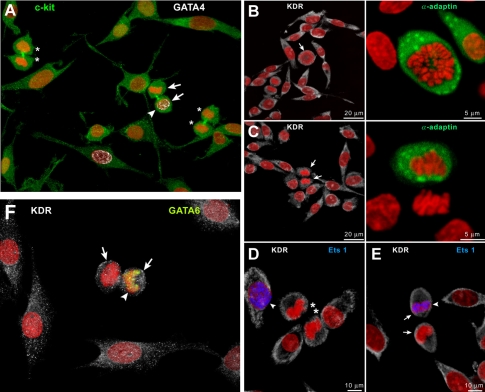
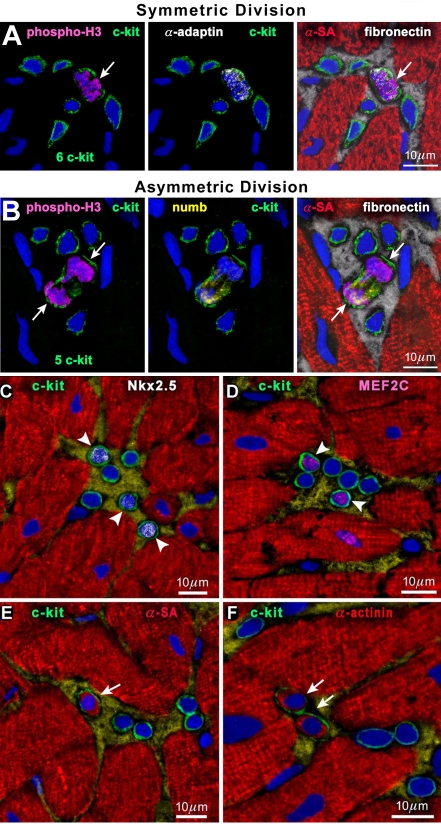
Proliferating mCSC may form either two mCSCs or one mCSC and one committed cell (Figure 3A) through symmetric or asymmetric divisions, respectively. Similarly, cultured vCSCs divide to generate two vCSCs (Figure 3B, D) or one vCSC and one committed progeny (Figure 3C, E, F). These ways of divisions are observed also in cardiac niches (Figure 4A, B). Proliferated and differentiated cells (Figure 4C-F) eventually leave niches and migrate in order to replace old and/or damaged cells for spontaneous regeneration.
Figure 3.
Symmetric and asymmetric divisions of human cardiac stem cells. (A) Daughter cells of symmetrically dividing mCSCs (asterisks) do not express GATA4 (white), whereas one daughter cell of asymmetrically dividing mCSCs (arrows) shows GATA4 (arrowhead). (B and C) Mitotic vCSC (arrows) show uniform (B) and nonuniform (C) distribution of α-adaptin (green), documenting symmetric and asymmetric division, respectively. (D-F) Daughter cells of symmetrically dividing vCSCs (asterisks) do not express Ets1 (D, blue), whereas one daughter cell of asymmetrically dividing vCSCs (arrows) show Ets1 (E, blue; arrowhead) or GATA6 (F, yellow; arrowhead) [2].
Figure 4.
Human cardiac stem cell division and commitment in niches. (A and B) Mitosis (phospho-H3, magenta; arrows) in c-kitPOS cells; α-adaptin (A, white) and Numb (B, yellow) show a uniform (A) and nonuniform (B) localization in the mitotic c-kitPOS cells. (C-F) c-kitPOS cells show in nuclei Nkx2.5 (C, white; arrowheads) and MEF2C (D, magenta; arrowheads) and in the cytoplasm α-SA (E, red; arrow) and α-cardiac actinin (F, red; arrows) [1].
When mCSCs and vCSCs are cultured in a condition favoring their differentiation, both cells commit to multiple lineages: cardiomyocytes, SMCs, and ECs. This documents a fundamental property of stem cells as mentioned above. Importantly, however, they have distinct tendencies; while mCSCs form about 6-fold more myocytes than vCSCs, vCSCs form nearly 6-fold more SMCs as well as 5-fold more ECs with respect to mCSCs (Figure 5) [2].
Figure 5.
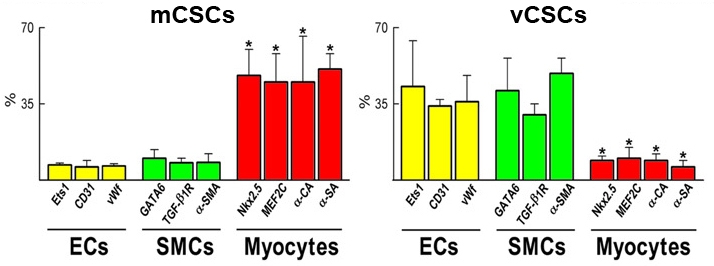
Percentage of mCSCs and vCSCs differentiating into endothelial cells (ECs), smooth muscle cells (SMCs), and myocytes. *P<0.05 vs. ECs and SMCs [2].
Myocardial regeneration by human mCSCs
When human mCSCs are injected at the border zone of infarcted myocardium created in an immunosuppressed rat, viable myocardium is formed at the center of the wall, and cardiac function is improved (Figure 6A). Here, Alu is a specific DNA sequence for primates. Therefore, regenerated human myocardium can be distinguished from surviving rat tissue. At a higher magnification, Alu-positive regenerated human myocytes form gap junctions with surviving rat myocytes (Figure 6B).
Figure 6.
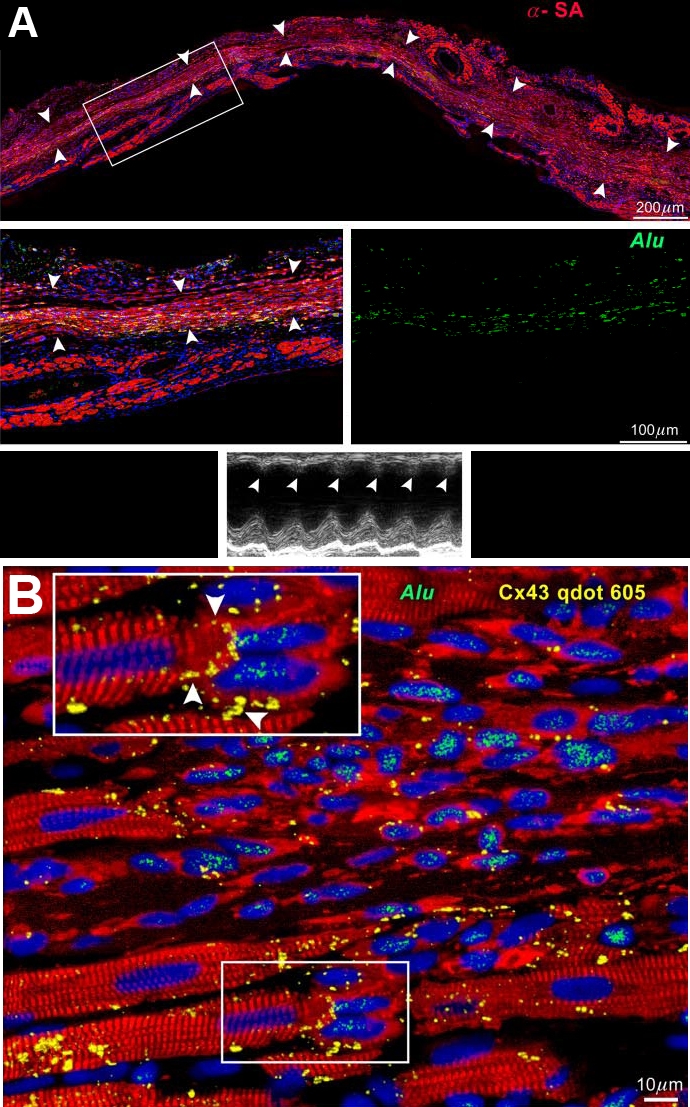
Myocardial regeneration in a treated rat. (A) Human myocardium (arrowheads) is present within the infarct. The area in the rectangle is shown at higher magnification. Human myocytes are α-SA- (red) and Alu-(green) positive. Echocardiogram shows contraction in the infarcted wall (arrowheads). (B) Connexin 43 (Cx43, yellow) is present between human myocytes (α-SA, red; Alu, green) and spared rat myocytes (α-SA, red; Alu-negative). See Inset for higher magnification [1].
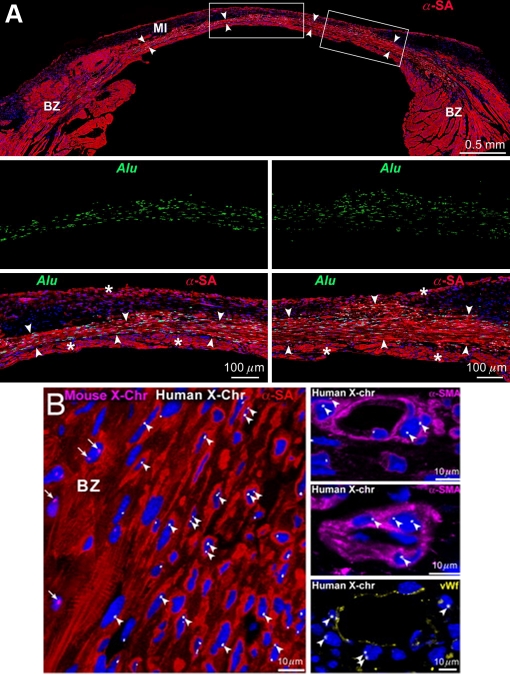
Similar findings are observed in mice. Alu probe defines the regenerated human myocardium (Figure 7A). Alternatively, muscles and vessels derived from human cells are recognized by human X-chromosomes, and clearly distinguished from remaining mouse cells (Figure 7B). The regenerated human myocytes and coronary arteries are structurally and functionally integrated with host myocytes and vessels. This large reconstitution of the in-farcted myocardium results in a significant improvement of ventricular remodeling and cardiac performance.
Figure 7.
Myocardial regeneration in a treated mouse. (A) Mouse heart 21 days after infarction and injection of mCSC. Human myocardium (arrowheads) is present within the infarct (MI). BZ, border zone. Areas in rectangles are shown at higher magnification below. Human myocytes are α-SA- (red) and Alu- (green) positive. Asterisks indicate spared mouse myocytes. (B) Human myocytes and vessels show, at most, two human X-chromosomes (X-Chr, white dots; arrowheads). Mouse X-Chr (magenta dots; arrows) are present in myocytes at BZ [1].
The multipotentiality of each stem cell in vivo has also been demonstrated utilizing genetic tagging approach [4]. Importantly, in order to improve cardiac function, both muscles and vessels need to be regenerated but not either one alone; muscles without vessels do not work due to persisting ischemia, and vessels without muscles do not generate contraction force.
Formation of large conductive artery by human vCSCs
Although mCSCs are able to create cardiomyocytes and vascular cells, according to the in vitro results it is reasonable to assume that vCSCs are better equipped with the potential to regenerate vascular structures in vivo compared to mCSCs. In fact, when human vCSCs are injected to the close proximity of the critical stenosis created in the dog coronary artery, large conductive arteries are formed. Since cell implantation in a region without damage results in a high rate of apoptosis [5], vCSCs need to be treated with hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF-1) prior to injection. One month later, regenerated coronary arteries with various sizes are identified. Newly formed vessels are recognized by the EGFP fluorescence, which is used to label injected vCSCs, as well as by Alu DNA sequences specific for primates (Figure 8A). The inner lumen reaches up to 1.5 mm in diameter (Figure 8B), and red blood cells are eventually found in these regenerated vessels. Many arterioles and capillaries are also formed, and tissue perfusion is improved by this therapeutic intervention [2]. Therefore, the regenerated vessel serves as a “biological coronary bypass".
Figure 8.
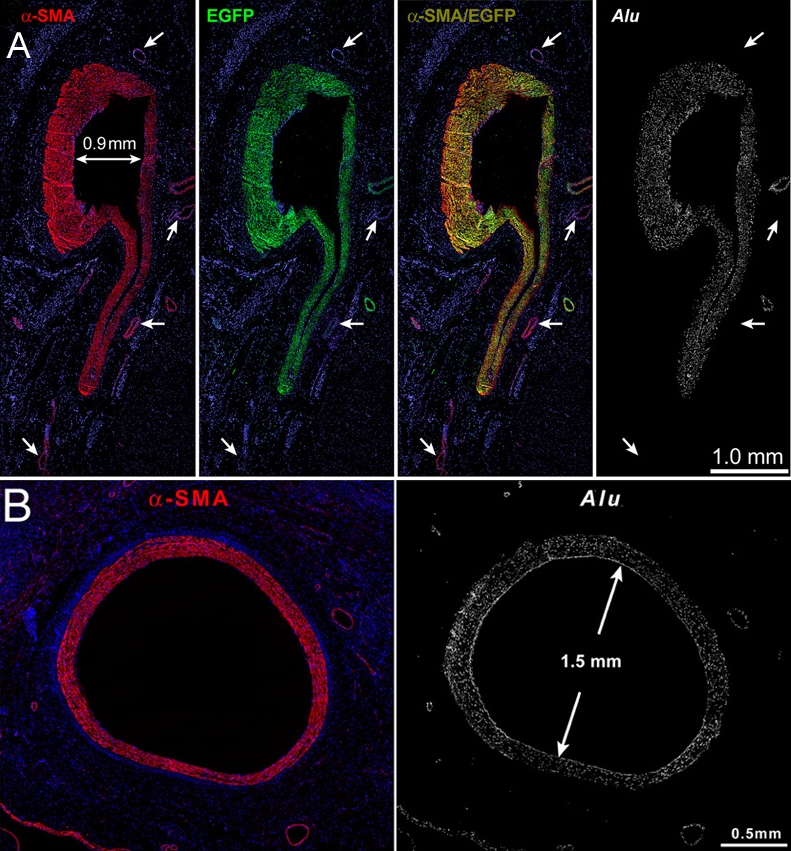
Regeneration of large coronary arteries. (A) All smooth muscle cells in the newly formed human vessel are positive for α-smooth-muscle-actin (α-SMA, red), EGFP (green), and Alu sequences (white). Preexisting coronary vessels (arrows) are α-SMA positive but EGFP and Alu negative. (B) A regenerated human coronary artery with a lumen of 1.5 mm in diameter [2].
Clinical trial on ischemic heart failure
The first phase 1 clinical trial utilizing autologous endogenous CSCs had been launched in 2009 (ClinicalTrials.gov Identifier: NCT00474461). This SCIPIO (cardiac Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) trial is an open-labeled randomized safety/ efficacy study trial, in which heart failure patients with old myocardial infarct are treated by autologous c-kit-positive CSC infusion. During coronary artery bypass graft (CABG) surgery, the right atrial appendage, which is routinely resected at the cannulation site during on-pump bypass surgeries, is collected and processed for isolation and expansion of CSCs. Importantly, nonviable myocardial segments are also revascularized in order to enable subsequent intracoronary delivery of CSCs into the scarred region. Approximately 4 months after CABG surgery when the beneficial effect of the operation reached maximum, patients with left ventricular ejection fraction (LVEF) less than 40% received CSC infusion to the infarcted region through the bypass graft accompanied by temporal balloon occlusion of the vessel.
Although the study is still ongoing, the initial outcome is very promising and striking. In 14 CSC-treated patients who were analyzed, LVEF increased from 30.3% (SE 1.9) before CSC infusion to 38.5% (SE 2.8) at 4 months after infusion. Moreover, in eight patients the effects of CSCs were even more pronounced at 12 months; LVEF increased by 12.3 EF units (SE 2.1) vs baseline. By contrast, in seven control patients, during the corresponding time interval, LVEF did not change from 30.1% (SE 2.4) at 4 months after CABG to 30.2% (SE 2.5) at 8 months after CABG [6].
Importantly, so far no adverse event has been recorded. This is consistent with other worldwide clinical trials utilizing intracoronary infusions of cells derived from the bone marrow and peripheral blood.
Closing remarks
The adult human heart contains two distinct subpopulations of endogenous stem cells: mCSCs and vCSCs. Previously, the limited magnitude of spontaneous myocardial regeneration following infarct was interpreted as an unequivocal documentation of the inability of the adult heart to undergo tissue repair. However, a similar phenomenon occurs in other solid and non-solid organs including the skin, liver, intestine, and bone marrow. In spite of their well-accepted regenerative potentials, the occlusion of a supplying artery leads to scar formation mimicking cardiac pathology. Thus, although tissue stem cells appear to be equipped to regulate homeostasis of the organ, they do not respond efficiently to ischemic injury or other disease status. Therefore, cellular interventions are required to overcome this constraint.
It is logical to assume that stem cells residing in the heart are more powerful and effective in making new myocardium with respect to stem/ progenitor cells from other organs including the bone marrow [7, 8]. CSCs are programmed to generate cardiac structures and, upon activation, can rapidly form parenchymal cells and coronary vessels possibly rescuing the failing heart.
In the near future, disease-based or even region -oriented customized cell therapy employing various combinations of autologous mCSCs and vCSCs would become available. Cumulative evidence of clinical applications of these cells would guarantee an improved care of patients suffering from heart failure.
References
- 1.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T, Pepe M, Qanud K, Ojaimi C, Bardelli S, D'Amario D, D'Alessandro DA, Michler RE, Dimmeler S, Zeiher AM, Urbanek K, Hintze TH, Kajstura J, Anversa P. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci USA. 2009;106:15885–15890. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 4.Hosoda T, D'Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomized phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Hosoda T, Kajstura J, Leri A, Anversa P. Mechanisms of myocardial regeneration. Circ J. 2010;74:13–17. doi: 10.1253/circj.cj-09-0665. [DOI] [PubMed] [Google Scholar]
- 8.Hosoda T, Rota M, Kajstura J, Leri A, Anversa P. Role of stem cells in cardiovascular biology. J Thromb Haemost. 2011;9(Suppl 1):151–161. doi: 10.1111/j.1538-7836.2011.04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]