Abstract
Progenitor/stem cell (PSC) has shown great promise for generation in failing heart. Advances in PSC biology have greatly enhanced our understanding of how PSC self-renewal, migration, maintenance of stemness, and cell-fate commitment depend on the balance of complex signals in their microenvironment. Endogenous PSC exists within structural and functional units known as PSC niches, which play important roles in directing PSC behavior. Recent years have witnessed great progress in our understanding of the PSC niche in cardiovascular biology. PSC based therapy could lead to successful cardiac regeneration or repair. Realizing the potential of therapeutic strategies is based on 1) differentiation of the PSC into all of the cellular constituents of the heart; 2) release of paracrine/ autocrine factors from the PSC; 3) fusion of the PSC with the existing constituents of the heart; and 4) stimulation of endogenous repair (regeneration of PSC niches). Importantly, cardiac PSC niches contain supporting cells and these cell-cell interactions have crucial regulatory roles in PSC based therapy. These findings have important implications for heart development, bioengineering, and furthermore elucidate a broader dimension of PSC control within the niche toward cardiomyocyte phenotype.
Keywords: Progenitor/stem cell, myocardial infarction, SDF-1α/CXCR4 axis, growth factors, progenitor/stem cell niches
Introduction
Ischemic heart disease remains a leading cause of mortality and morbidity in the developed world [1]. The infarcted myocardium is unable to efficiently replace myocytes lost secondary to myocardial infarction and heals itself by fibrosis [2]. Ultimately these changes lead to worsening cardiac performance and heart failure.
Endogenous cardiac stem cells, as PSC reservoirs, exist within structural and functional units known as PSC niches [3-4]. Cell based therapy could lead to successful cardiac regeneration or repair by mechanisms that stimulate endogenous repair by regulation of PSC niches [5]. PSC niches provide a regulatory structure for PSC, thereby controlling and balancing self-renewal, manipulating cell migration and differentiation. In addition, cardiac PSC niches contain supporting cells and cell-cell interactions have crucial regulatory roles [5]. Adult mammalian cardiomyocytes are considered terminally differentiated and incapable of proliferation [6]. However, recent reports have shown that adult cardiac myocytes can be induced to reenter the cell cycle with periostin [7], a p38 MAP kinase inhibitor [8], neuropeptide [9], and transforming growth factor-β (TGF-β) [10]. Although the adult heart has limited capacity for cardiomyocyte proliferation, these new discoveries raise the possibility that increasing the number of the differentiated cardiomyocytes derived from PSC by activating their proliferative potential and changing the microenvironment of PSC niches could repair damaged myocardium.
CXCR4 is a major regulator of PSC activities [11]. The importance of the SDF-1α/CXCR4 signaling is documented in CXCR4 knockout mice which die in utero, thereby indicating a fundamental developmental role for this receptor-ligand axis [12]. Resident PSC existing in the cardiac injured area can change the properties of the microenvironment by secreting cytokines that contribute to cell homing, proliferation, and differentiation [13-15]. Therefore, the cardiac PSC niche is valuable in enhancing the cardio-myocyte proliferation, cell migration, and differentiation from PSC into cardiomyocytes for cardiac repair. Effective cell based therapy could lead to restoration of these PSC niches. This review discusses current understanding of the role of endogenous and exogenous progenitor cell niche in the treatment of ischemic heart failure.
Endogenous stem cell niche in heart
Translation of PSC biology from bench to bedside has proceeded at a rapid pace in cardiovascular medicine. PSC cell-based therapy can regenerate injured myocardium by three major mechanisms: 1) differentiation of PSC into cardiomyocyte-like cells; 2) fusion of PSC with cardiomyocytes; and 3) secretion of paracrine factors to nourish the myocardium [16].
Adult PSC also participate in tissue generation, maintenance, and repair by virtue of their surrounding structures, referred to as the PSC microenvironment, or the PSC niche [17] (Figure 1).
Figure 1.
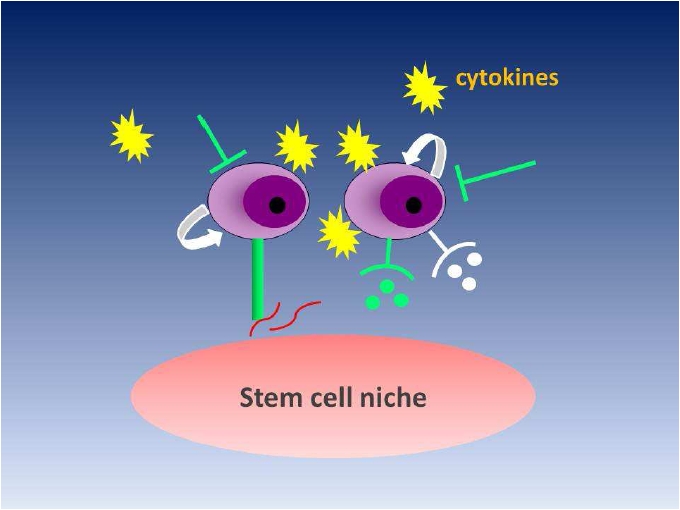
Schematic representation of stem cell niche. Niches are special microenvironments interacting with stem cells to regulate stem cell fate. Interactions between stem cells and adhesion molecules, extracellular matrix components, the oxygen tension, cytokines, and physiochemical nature of the environment including(PH, ionic strength etc.) are all coordinated to enforce quiescence (Green signals) and to tightly regulate self-renewal(White signals).
It has been discovered that PSC niches are present in heart [18], skin [19], bone marrow [20] and other organs. For instance, in a porcine model of myocardial infarction, allogeneic mesenchymal stem cells stimulated substantial improvement in the ejection fraction, reduction of infarct size, and the growth of a rim of new cardiac tissue in the region into which the mesenchymal stem cells were injected [21]. These effects occurred in the absence of definitive cardiogenic differentiation. After myocardial infarction, porcine hearts exhibit evidence of cardiac myocytes that have reentered the cell cycle, angiogenesis, and reduced levels of apoptosis. These data, in addition to new insights regarding the presence of endogenous cardiac stem cells, strongly support the concept that the heart contains PSC niches.
Another group led by Annarosa Leri has identified the components of cardiac niches, the contribution of symmetric division of cardiac stem cells (CSCs) to cardiac homeostasis and the growth kinetics of CSCs by BrdU-retaining assays [18].
Previously, cardiomyocytes have been thought to be incapable of renewing after the postnatal period. However, through integration of the carbon isotope 14C (carbon-14) into DNA to establish the age of cardiomyocytes in humans, a recent report demonstrated that the capacity to generate cardiomyocytes still exists in the adult human heart. The analysis suggests that cardiomyocytes renew at a very slow rate (1% and 0.45% annually at ages 25 and 75 respectively), with fewer than 50% of the cardiomyocytes in the adult heart generated after birth. This result is at odds with previous studies (largely in rodent models) that have concluded that adult cardiomyocytes retain a high mitotic renewal rate in the post-natal period.
However, the identification of even a low level of cardiomyocyte turnover in the adult human heart is important, since this process could be therapeutically exploited to slow or reverse various forms of cardiac dysfunction. Further, manipulating of the cardiac niche to stimulate significant cardiomyocyte turnover might be a future strategy for endogenous cardiac repair.
SDF-1α/CXCR4: the key axis in progenitor/stem cell homing
SDF-1α, also known as CXCL12α, is involved in hematopoietic stem cells (HSCs) migration to ischemic myocardium (Figure 2). SDF-1α/ CXCR4 axis has been well characterized as a key player in bone marrow derived-MSC homing due to its significant involvement in HSC homing. Nevertheless, BM-MSCs share different properties with HSC regarding to the cell surface expression of CXCR4, showing instability following ex vivo expansion. Previously, it has been shown that a subtype of BM-MSCs maintain cell surface expression of CXCR4 [22, 23], but the expression gradually declines after ex vivo expansion, reaching undetectable level on 4-5 passages. However, even after several passages, CXCR4 expression in BM-MSCs is maintained at a high level [24]. Furthermore, short-term exposure of human Flk1+ BM-MSCs to the combination of cytokines (Flt-3 ligand, stem cell factor (SCF), IL-6, hepatocyte growth factor (HGF) and IL-3) have been shown to induce a significant up-regulation of both cell surface and intracellular CXCR4 [24]. This phenotype of BM-MSCs is related to an upregulated migratory capacity ex vivo in response to SDF-1α, and an increase in the capacity to home to the bone marrow of irradiated mice [24]. Therefore, it is hypothesized that cytokines modulate the expression and distribution CXCR4. Correspondingly, ectopic transgenic overexpression of CXCR4 in rat BM-MSCs resulted in an increase in their homing to infarcted myocardium following intravenous injection [25]. However, blockade of CXCR4 did not affect the intramyocardial migration of murine BM-MSCs to ischemic areas in mice [26]. These results suggest that the SDF -1α/CXCR4 axis is involved but not the only mediator for the recruitment of BM-MSCs to the ischemic myocardium. Other researcher investigated whether circulatory SDF-1α level is positively correlated with the number of PSC recruited to the infarcted heart. Surprisingly, there is no association between serum SDF-1α levels and the number of circulating mesenchymal stem cells in patients with ischemic heart diseases. Therefore, although the role of the SDF-1α/CXCR4 axis in HSCs has been well recognized, the role of this signaling axis in the homing of BM-MSCs remains to be elucidated. Recently, Smith-Berdan et al. [27] show that Roundabout 4 (Robo4), a neuronal guidance molecule, regulates engraftment and mobilization and, in cooperation with CXCR4, localizes HSCs to the niche. These findings give rise to an exciting new line of investigation in stem/niche cell interactions and raise many questions to be answered in future work. One of the key questions is whether the pattern of Robo4 expression in human HSCs mimics that in mouse models and whether pharmacologic approaches targeting Robo4 could be a useful strategy for mobilization of HSCs. Mechanistically, the reciprocal loss of Robo4 and the upregulation of the CXCl12/CXCR4 axis remain to be defined. Is there a point where the two pathways intersect in their downstream signaling? Given that Robo4 is expressed in endothelium and functions in vascular sprouting upon activation by its ligand Slit2, it will be interesting to determine if Robo4 in this context acts via Slit2 and if there is an additional co-receptor. Activated Robo4 also stabilizes the vascular network through inhibition of endothelial permeability [28]. Thus, how loss of Robo4 affects the endothelial function will be an important topic to address in future studies. Finally, where are the Robo4+ HSC in the BM normally localized and to where do they home? Would Knockdown Robo4 promote BM stem cells homing to the infarcted myocardium and promote angiomyogenesis?
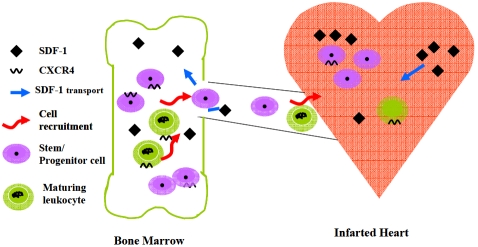
Figure 2.
Stem cell homing mediated by upregulated SDF-1α can be seen as a model for communication between the injured heart and bone marrow. Endothelial cells of the blood vessels translocate SDF-1α from the damaged heart via the circulation into the bone marrow in a CXCR4-dependent manner. Presentation of the translocated SDF 1α by bone marrow endothelial and other stromal cells recruits CXCR4-expressing immature progenitors and stem cells as well as maturing leukocytes to the injured organ as part of host defense and organ repair.
Oxygen and reprogramming of induced pluripotent stem cells
In 2006, Takahashi and Yamanaka published their hallmark strategy to reprogramme mammalian fibroblasts into induced pluripotent stem cells (iPSCs) by overexpression of four transcription factors (Oct3/4, Sox2, Klf4, and c-Myc) [29, 30]. The efficiency of reprogramming into iPSCs is quite low in face of normxia. Because 1) early stages of embryogenesis takes place in an hypoxic environment, and 2) several PSC lineages resides in oxygen-poor niches; and 3) DNA damage in reprogramming occursed through the generation of ROS, the role of hypoxia in determining stem cell state was examined in iPSC generation [31]. The researcher tranduced the four pluripotent factors into MEF and human somatic cells by retrovirus transduction in normoxic (21% O2) and hypoxic (1% and 5% O2) conditions, and evaluate the efficiency of iPSCs generation by Nanog-GFP reporter. The percentage of GFP-positive colonies was significantly higher in 5% O2, compared to normoxic and 1% O2 condition, and GFP-positive cells arose earlier when cultured in hypoxia, indicating mild hypoxia could promote reprogramming of iPSC. Furthermore, hypoxia can reduce the number of transduced pluripotent factors (only Oct3/4 and Klf-4) [31]. These discoveries were consistent with the poor oxygen tension in ESC microenvironment, implying the crucial role of oxygen in retaining stem cell pluripotency. Since upregulation of hypoxia inducible factors (HIF) is the key transcriptional event for stem cells under hypoxia, the link between HIFs and stemness factors may provide the hint for enhanced reprogramming potency in anoxia. Previously, it was reported that in ESCs, HIF-2α transactivates Oct4 expression, leading to maintenance of the stemness and the formation of ESC-derived teratoma [32]. Moreover, culture at 20% oxygen, resulted in a significantly reduced expression of SOX2, NANOG, OCT4, and silencing HIF2α and HIF-3α (but not HIF-1α) was able to duplicate this effect [33], implying that NANOG, Oct4, and SOX2 are downstream targets of HIF-2α/HIF-3α. It is speculated that hypoxia is involved in regulating PSC state via modulating HIF-2α/HIF-3α expression, thus profiling of genomic binding sites of HIF-2a/HIF-3a in hypoxia by ChIP-seq (Chromatin immunoprecipitation combined deep sequencing) may provide more information on the role of anoxia in maintaining stemness and pluripotency.
Optimizing mobilization by modulating progenitor/stem cell niche
The anchoring of PSC depends mainly on adhesion via integrins to stromal cells and to extracellular matrix proteins such as (e.g.VLA-4/ fibronectin). This multiple-step process has involved many different adhesion molecules (e.g. VLA-4/VCAM-1) that are activated by a range of modifiers, which may explain for selective migration of stem cells/progenitor cells to specific places.
Within the bone marrow, PSC are anchored to stromal cells and ECM via integrin binding to cell adhesion molecules or ECM proteins, and via interaction with cell surface- or matrix-bound cytokines (e.g. SDF-1α/CXCR4; SCF/c-kit) [34]. Disruption of this interaction showed the underlying mechanism of several currently applied mobilization strategies, e.g. by the CXCR4 inhibitor AMD3100, or by colony-stimulating factors (CSFs) such as granulocyte or granulocyte-monocyte colony-stimulating factor (G-CSF, GM-CSF, respectively) [35, 36]. The second underlying mechanism of stem cell/progenitor liberation is the guidance of progenitor/stem cells in the blood by chemotactic agents in a gradient-dependent manners. A variety of growth factors have been used to elevate circulating progenitor cell levels, among them VEGF and erythropoietin (Epo) [37, 38]. G-CSF, currently the most widely used agent in clinical trials for stem cells mobilization, affects both mechanisms of PSC mobilization, i.e. the interruption of anchoring mechanisms (by down-regulation of SDF-1α expression, activation of the protease CD26 (Dipeptidyl peptidase-IV, DPP-IV) which cleaves SDF-1α N-terminus, thereby rendering it unable to bind CXCR4, and by attenuating β1 integrin function), as well as increasing serum levels of further cytokines and growth factors [39]. Consistently, G-CSF treatment provides a restorative benefit in ischemic animal models, but on the contrary, experimental studies in mice and early -phase clinical trials in patients with coronary artery disease suggest that G-CSF may promote atherosclerosis with the potential for adverse outcomes in these patients [40-42].
Reconciliation between mobilization and recruitment
Bone marrow stem cell liberation from bone marrow is the first step in stem cell recruitment to the heart. The dual role of SDF-1α (retension in bone marrow as well as recruitment to the heart) implies that interrupting anchoring/ adhesion molecules and reducing homing efficiency are both sides of the “sword” when liberating stem cell from bone marrow. Currently, the clinical trials administrating granulocyte colony-stimulating factor (G-CSF) for stem cell mobilization showed negative result in ameliorating heart function [43, 44]. The underlying reason might be attribute to the interruption of G-CSF with SDF-1α/CXCR4 signaling, impairing the homing and engraftment of the mobilized stem cell to the infarcted heart [45].
A recent clinical study shows that timing of administration is a critical point for enhanced stem homing [46]. In one study, authors compared the short- and long-term effect of CXCR4 blockade, showing that CXCR4 inhibitor AMD3100 carried dual effect on enhanced PSC liberation from the bone marrow, and their recruitment to the infarcted heart, beneficially affecting cardiac vascularity and survival in a mouse model of myocardial infarction (MI). Nevertheless, continuous CXCR4 blockade increased PSC liberation from bone marrow, but reduced their engraftment and stimulated cardiac remodeling [46]. Hence, targeting both cell liberation and homing might be a more efficient strategy in regenerating myocardium and improving cardiac performance post-myocardial infarction [47].
Recently, Zaruba et al. demonstrated that synergy between retarding the degradation of SDF-1α and mobilization of stem cells by G-CSF enhanced homing of CD34+/CXCR4+cells to ischemic heart and attenuated subsequent ischemic cardiomyopathy [48]. This dual targeting linked DPP-IV during G-CSF treatment and prevented cleavage SDF-1α, while G-CSF induced progeitor/stem cell liberation. Dual administration of G-CSF and DPP-IV inhibitor (sitagliptin/diprotin A) showed increased homing of mobilized stem cell from bone marrow to the injured myocardium, improved myocardial regeneration after MI and enhanced survival in the pre-clinical setting [47].
Convincing data also showed that the combination of MSC patch (transgenic overexpressing CXCR4) with DIP pretreatment inhibits cell death post-myocardial infarction, increases tissue angiogenesis, and enhances cell engraftment, leading to improved LV mechanical function after MI [49]. These findings have changed the design of clinical trials since the key issue of all therapeutic stem cell approaches is stem cell homing. More recently, a clinical trial called SITAGRAMI (sitagliptin plus granulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction) has tested this hypothesis and a recent report has reported the feasibility and safety of this approach [50].
In a pre-clinical study, the combination of G-CSF and HGF had a significant synergistic effect, suggesting that mobilization of bone marrow derived stem cells to peripheral circulation combining their recruitment to the ischemic area could potentiate angiogenesis and vasculogenesis[51].
Future perspective
We can manipulate cell fate forwards (PSC differentiation into somatic cells) or backwards (reversing the fate of a differentiated cell to embryonic stem cells) just by “playing” with a couple of transcription factors. Whether fate switches from stem to progenitor cells are reversible in normal homeostasis of adult vertebrate tissues, such as cardiac tissue? Is still unknown if dedifferentiation happen naturally in adult myocardium to maintain the normal homeostasis of residential cardiac progenitor cells pool? These questions remain to be answered in future work.
Many patients resorting to regenerative medicine and stem cell-based therapies are older individuals suffering from ischemic heart diseases. Therefore, one of pivotal questions that we need to address is how aging influences stem cells and the niche. Is niche aging insufficient to replenish these patients with “robust” stem cells? Do we need to co-transplant niche with stem cell in aging patients? A deeper understanding of the mechanism of niche aging will open a new avenue for cardiac regeneration.
For successful tissue regeneration by endogenous PSC homing, it is necessary to direct progenitor/stem cells to find their way home and provide homed PSC with a local environment of artificial ECM (where they can proliferate and differentiate efficiently). Advances in biomaterial design and engineering are converging to enable a new generation of instructive materials that bear complex information coded in their physical and chemical structures. Release technology often enhances the in vivo stability of information cues and related molecules (e.g., homing factors) and prolongs the maintenance of biological functions for the efficiency of stem cell homing and tissue regeneration. Elucidating the molecular complexity of cell chemotaxis, identifying both the essential molecules that dictate stem cell trafficking and their dosing criteria, improving the pharmacokinetics and bio -distribution of bioactive cues released from an implanted biomaterial, and developing products tailored to different pathologies are but a few of the challenges in the design of medical devices that conduct sufficient PSC homing and robust tissue regeneration, thereby, improving the benefit to individuals suffering from severely injured tissues. Addressing each of these issues will lead to a future in which navigational cues and growth factors will be delivered solely to the place where they are needed and only at the levels and time at which they are required, thereby creating an innovative, biologically-based generation of clinical treatments that utilize endogenous cell homing to regenerate tissues.
Although several reports [52-55] have indicated that cardiomyocytes derived from iPSCs improve heart function, and reverse cardiac remodeling, the risk for cancer is still a key concern for translational medicine39. To address safety problem, several other approaches have been devised to generate iPSCs, including non-integrating adenoviral approaches [57, 58], the piggyBac transposon system (removals of the transgenes from established iPSC lines after inducing pluripotency [59, 60], the Cre/loxP recombination system [61], non-integrating “episomal” vectors to create iPSCs free of vector and transgene DNA [62], and zinc finger nuclease technology [63]. All of these methods, however, are based on the transfer of foreign DNA into the target cell and still carry the risk of carcinogenesis. Recently, protein-based methods have been successfully developed and implemented [64, 65]. However, so far, there have been no reports of methods that involve purely niche-reprogrammed iPSCs. Future work should address this issue and verify this finding as fact or artifact since purely niche-reprogrammed iPSCs would be of tremendous clinical value.
In conclusion, the niche has attracted more and more attention in cardiac regenerative medicine; unraveling its internal organization and integration could contribute greatly to not only mechanistic insight of stem cell biology, but also stem cell-based therapies in the clinical setting.
Acknowledgments
AcknowledgementsThis work was supported by NIH grants, HL089824, HL081859, and HL110740 (Y. Wang). National Natural Science Foundation of major international cooperation projects in China (No. 81120108003 to Yu XY).
References
- 1.Lafeber M, Spiering W, Singh K, Guggilla RK, Patel V, Webster R. The cardiovascular polypill in high-risk patients. Eur J Cardiovasc Prev Rehabil. 2011 doi: 10.1177/1741826711428066. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Smart N, Risebro CA, Clark JE, Ehler E, Miquerol L, Rossdeutsch A, Marber MS, Riley PR. Thymosin beta4 facilitates epicardial neovascu-larization of the injured adult heart. Ann N Y Acad Sci. 2010;1194:97–104. doi: 10.1111/j.1749-6632.2010.05478.x. [DOI] [PubMed] [Google Scholar]
- 3.Shah VK, Shalia KK. Stem Cell Therapy in Acute Myocardial Infarction: A Pot of Gold or Pandora's Box. Stem Cells Int. 2011 doi: 10.4061/2011/536758. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc. 2009;84:876–892. doi: 10.4065/84.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 6.Ueno H, Perryman MB, Roberts R, Schneider MD. Differentiation of cardiac myocytes after mitogen withdrawal exhibits three sequential states of the ventricular growth response. J Cell Biol. 1988;107:1911–1918. doi: 10.1083/jcb.107.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 8.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang D, Ashraf M, Zhao T, Huang W, Ashraf A, Balasubramaniam A. Combining neuropeptide Y and mesenchymal stem cells reverses remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;298:H275–286. doi: 10.1152/ajpheart.00765.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell. 2007;18:2264–2273. doi: 10.1091/mbc.E06-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Overexpression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. JMCC. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells. Biol Cell. 2005;97:133–146. doi: 10.1042/BC20040069. [DOI] [PubMed] [Google Scholar]
- 13.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 15.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 16.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20–28. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marban E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–71. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 21.Zimmet JM, Hare JM. Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Res Cardiol. 2005;100:471–481. doi: 10.1007/s00395-005-0553-4. [DOI] [PubMed] [Google Scholar]
- 22.Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn U, Bellan-tuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 23.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 24.Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/ SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarc-ted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta 1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, Li D, Garcia-Ojeda ME, Hinck L, Forsberg EC. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CA, London NR, Chen H, Park KW, Sau-vaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, Gerhardt H, Zhang K, Li DY. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 35.Fujita J, Mori M, Kawada H, Ieda Y, Tsuma M, Matsuzaki Y, Kawaguchi H, Yagi T, Yuasa S, Endo J, Hotta T, Ogawa S, Okano H, Yozu R, Ando K, Fukuda K. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25:2750–2759. doi: 10.1634/stemcells.2007-0275. [DOI] [PubMed] [Google Scholar]
- 36.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T. Vascular endothelial growth factor (165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 38.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Choi U, Liu PC, Whiting-Theobald NL, Linton GF, Malech HL. Diprotin A infusion into nonobese diabetic/severe combined immunodeficiency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem Cells Dev. 2007;16:361–370. doi: 10.1089/scd.2007.9997. [DOI] [PubMed] [Google Scholar]
- 40.Cella G, Marchetti M, Vignoli A, Randi ML, Saggiorato G, Pasetto L, Pagnan A, Barbui T, Falanga A. Blood oxidative status and selectins plasma levels in healthy donors receiving granulocyte-colony stimulating factor. Leukemia. 2006;20:1430–1434. doi: 10.1038/sj.leu.2404271. [DOI] [PubMed] [Google Scholar]
- 41.Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- 42.Tura O, Crawford J, Barclay GR, Samuel K, Hadoke PW, Roddie H, Davies J, Turner ML. Granulocyte colony-stimulating factor (G-CSF) depresses angiogenesis in vivo and in vitro: implications for sourcing cells for vascular regeneration therapy. J Thromb Haemost. 2010;8:1614–1623. doi: 10.1111/j.1538-7836.2010.03900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, Schoenberg SO, Steinbeck G, Franz WM. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 44.Engelmann MG, Theiss HD, Theiss C, Henschel V, Huber A, Wintersperger BJ, Schoenberg SO, Steinbeck G, Franz WM. G-CSF in patients suffering from late revascularised ST elevation myocardial infarction: final 1-year-results of the G-CSF-STEMI Trial. Int J Cardiol. 2010;144:399–404. doi: 10.1016/j.ijcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 45.Yin Y, Huang L, Zhao X, Fang Y, Yu S, Zhao J, Cui B. AMD3100 mobilizes endothelial progenitor cells in mice, but inhibits its biological functions by blocking an autocrine/ paracrine regulatory loop of stromal cell derived factor-1 in vitro. J Cardiovasc Pharmacol. 2007;50:61–67. doi: 10.1097/FJC.0b013e3180587e4d. [DOI] [PubMed] [Google Scholar]
- 46.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, Ito A, Misener S, Tanaka T, Klyachko E, Kobayashi K, Tongers J, Roncalli J, Tsurumi Y, Hagiwara N, Losordo DW. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, He H, Yu L, Xia HH, Lin MC, Gu Q, Li M, Zou B, An X, Jiang B, Kung HF, Wong BC. HSF1 down-regulates XAF1 through transcriptional regulation. J Biol Chem. 2006;281:2451–2459. doi: 10.1074/jbc.M505890200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang D, Huang W, Dai B, Zhao T, Ashraf A, Millard RW, Ashraf M, Wang Y. Genetically manipulated progenitor cell sheet with diprotin A improves myocardial function and repair of infarcted hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1339–1347. doi: 10.1152/ajpheart.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theiss HD, Brenner C, Engelmann MG, Zaruba MM, Huber B, Henschel V, Mansmann U, Wintersperger B, Reiser M, Steinbeck G, Franz WM. Safety and efficacy of SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial)-rationale, design and first interim analysis. Int J Cardiol. 2010;145:282–284. doi: 10.1016/j.ijcard.2009.09.555. [DOI] [PubMed] [Google Scholar]
- 51.Ieda Y, Fujita J, Ieda M, Yagi T, Kawada H, Ando K, Fukuda K. G-CSF and HGF: combination of vasculogenesis and angiogenesis synergistically improves recovery in murine hind limb ischemia. J Mol Cell Cardiol. 2007;42:540–548. doi: 10.1016/j.yjmcc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 53.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Germanguz I, Sedan O, Zeevi-Levin N, Shtrichman R, Barak E, Ziskind A, Eliyahu S, Meiry G, Amit M, Itskovitz-Eldor J, Binah O. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2011;15:38–51. doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamp TJ, Lyons GE. On the road to iPS cell cardiovascular applications. Circ Res. 2009;105:617–619. doi: 10.1161/CIRCRESAHA.109.205740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 59.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and trans-gene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B, Meng X, Miller JC, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]