Abstract
SDF-1α/CXCR4 signaling is important for endogenous processes, including organogenesis and hematopoeisis, as well as in response to tissue injury. The secretion of SDF-1α acts as a chemoattractant to facilitate the homing of circulating CXCR4 positive cells as well as other stem cells to the site of injury for the initiation organ regeneration and repair. In the case of cardiovascular disease, and particularly myocardial infarction, this signaling axis is implicated in many of these processes, and has an additional role in providing trophic support for cells and utilizing paracrine mechanisms to enhance cell survival, promote angiogenesis, and stimulate differentiation. Current research is focused on elucidating these complex events, and so far have produced promising results that have led to the development of cell therapies that can more effectively repair cardiac tissue following ischemic injury than currently used treatments. Despite these advancements, much remains to be discovered so that in the future, new treatments will be better able to regenerate tissue and recover function.
Keywords: SDF-1α/CXCR4, progenitor/stem cells, cell mobilization, myocardial infarction, endothelial cells
Introduction
SDF-1α is a cytokine of the CXC chemokine family, initially cloned from the murine bone marrow stromal cell lines ST-2 and PA6 [1] and purified from supernatant from the murine MS-5 cell line [2]. Its signaling effects are mediated through CXCR4, its G-protein linked receptor, through which it carries out many downstream signaling cascades and cellular responses. Both SDF-1α and CXCR4 are constitutively expressed in a variety of tissues and cell types and play a pivotal role in cell mobilization, migration, proliferation, and survival [3]. In addition to its role in proper organogenesis and the normal homeostasis of bone marrow stem cell environment, SDF-1α/CXCR4 axis is also important in many pathological conditions of tissue injury and stress. For example, in the case of cardiovascular disease and myocardial infarction, this signaling axis is implicated in the recruitment of CXCR4 positive progenitor cells to the ischemic area, which serves to provide the injured tissue with a source of progenitor/stem cells and paracrine factors for tissue repair [4].
Research from the past several years has contributed to our knowledge of the many functions of SDF-1α/CXCR4 in many endogenous cellular processes. In addition, manipulation of this signaling axis so that it is overexpressed or abolished in different cells and tissue types has provided insight into its associations with cellular response to pathology, and ways in which cell therapies can be used to enhance beneficial effects and diminish harmful ones. Here we will discuss the many ways in which the SDF-1α/ CXCR4 signaling axis is crucial to both normal tissues as well as in response to cell injury and death, with a focus on its importance in cardiovascular disease. We will also provide perspective on the current and future applications of stem cell therapies that take advantage this signaling axis, and the implications of these promising treatment alternatives.
Function and significance of SDF-1α and CXCR4
As a chemokine and receptor pair, SDF-1α and CXCR4 are unique because of the lack of redundancy in their ligand-receptor interaction; many other signaling pairs exhibit pleiotropism and cross react with multiple different factors [5]. Additionally, rather than being genetically clustered with the other CXC subfamily chemokines, SDF-1α has a unique chromosomal location and is highly conserved between species [6-8]. This knowledge suggests the functional importance of the SDF-1α/CXCR4 signaling axis, as has been confirmed by multiple studies.
Interference with SDF-1α/CXCR4 leads to deficiencies in embryogenesis, organogenesis, hematopoiesis, as well as maintenance of other homeostatic functions. A functional role of SDF-1α and CXCR4 in embryonic development is supported by the fact that SDF-1-/- and CXCR4-/-mice exhibit a cardiac ventricular septal defects, abnormal formation of the large vessels supplying the gastrointestinal tract and suppressed hematopoiesis in the bone marrow [9, 10]. Proper delivery of hematopoietic stem cells to the marrow is impaired [11, 12], and B-cell lymphopoiesis and myelopoiesis are both severely affected. These are most often lethal to the embryo, and accordingly no known pathologies involve complete SDF-1/CXCR4 deficiencies. Furthermore, SDF-1α is expressed by several different types of tissues and organ systems, particularly bone marrow, lymph nodes, liver, kidney, and the central nervous system [13-15], and has been linked to GC-rich binding sites of transcription factors that control the expression of housekeeping genes [6] suggesting its significance in very fundamental processes.
Cell homing and trafficking
A key function of SDF-1α and CXCR4 signaling is progenitor/stem cell homing. This homing is heavily influenced by the hematopoietic stem cell “niche,” which is defined as the microenvironment in which bone marrow cells reside that is in turn responsible for their function and behavior, including homing to a particular site of need [16]. For example, stromal cells in the bone marrow secrete SDF-1α, which consequently anchors progenitor/stem cells to endosteal osteoblasts [16]. There is also evidence that hematopoietic stem cells respond specifically to SDF-1α/CXCR4 signaling [17] and the loss of this signaling results in the disassociation of stem cells from the bone marrow [18], which is consistent with its central role embryonic hematopoiesis. In this way, the SDF-1α / CXCR4 axis is crucial for managing bone marrow homeostasis [19].
It was first discovered that the secretion of SDF-1α was involved in the signaling and trafficking of a small group of stem cells that included CD34+ cells and murine stem cells, and subsequently enhanced their survival and proliferation [20]. Studies in the last several years have also expanded CXCR4 expression to a variety of tissue specific stem cells including neural [21], liver [22], and skeletal muscle stem cells [23]. This expression of CXCR4 in a wide range of cells is also consistent with the widespread expression of SDF-1α and its control of several basic developmental processes.
Injury and tissue repair
Even more significantly, this signaling axis appears to be crucial for purposes of response to injury and tissue repair; cardiovascular injury is one prime example. SDF-1α expression is increased in heart tissue following myocardial infarction [24]. The involvement of this signaling axis is also significant in other diseases, including leukemia and other cancers [5], as well as rheumatoid arthritis [5]. SDF-1α expression is also increased in liver [25] and kidney disease [26] in animal models and was found to improve the transplantation of stem cells expressing CXCR4.
Additionally, CXCR4 expression is regulated by various factors, including cytokines, chemokines, stromal cells, adhesion molecules, and proteolytic enzymes [3]. Several transcription factors positively regulate CXCR4 expression, many of which are involved with hypoxia, tissue stress and damage: NF-κB, hypoxia-inducible factor-1α, glucocorticoids, lysophosphatidylcho-line [4], TGF-β1 [27], VEGF [3], IFN-α [28], STAT3 [19], and several interleukins (IL-2, IL-3, and IL-7) [29]. In the case of myocardial infarction, SDF-1α expression is significantly increased in heart tissue, particularly in the peri-infarct zone [30, 31]. This release of SDF-1α is needed to create a suitable environment for the signaling and homing of circulating progenitor cells such that they are brought specifically to the injured tissue for the initiation of repair processes [4]. Additionally, SDF-1α is secreted by hematopoietic stem/progenitor cells and is involved in autocrine/paracrine regulation of their development and survival via activation of calcium flux, focal adhesion components such as proline-rich kinase 2 (pyk-1), Crk-associated substrate (p130Cas), focal dhesion kinase (FAK), paxillin, Nck, Crk, Crk-L, protein kinase C, phospholipase C-γ (PKC γMPK ps42/44-ELK-1 and PI-2K-Akt-NF-κB axes [3].
Our current knowledge of the expression and function of the SDF-1α/CXCR4 axis suggests that, this signaling axis is a central component to many crucial processes and is also involved in endogenous responses to tissue damage. The clinical implications of SDF-1α/CXCR4 signaling are significant, and future therapies that involve this axis are particularly promising in cardiovascular disease.
SDF-1α/CXCR4 as a therapeutic target in cardiovascular disease
Heart disease a leading cause of death globally. However, it is challenging from a clinical point of view because cardiac tissue does not have the capacity to regenerate. Since cardiomyocytes are considered terminally differentiated, therapies have typically revolved around helping patients managed symptoms and prevent further stress or damage to the heart. However, recent evidence has demonstrated the ability of cardiac tissue to activate endogenous repair mechanisms following injury like myocardial infarction, and thus have hinted at the potential for future therapies to effectively target and induce these reparative processes to regenerate infarcted tissue and recover normal function. Many such therapies have already been demonstrated in vivo and in vitro, and have also shown promise in clinical studies [32-37].
SDF-1α/CXCR4 expression in the ischemic heart
Following coronary artery occlusion, SDF-1α is upregulated in heart tissue and acts to recruit various progenitor/stem cells to the infarcted area [24]. This increase in SDF-1α in the ischemic region and the border appears to be involved in the recruitment of progenitor cells to the damaged tissue, which is consistent with its role in cell homing and trafficking [4]. SDF-1α/ CXCR4 signaling can also be stimulated with hypoxic preconditioning [38]. Factors, including G-CSF [39] and hypoxia inducible factor-1α (HIF) -1α [40] have also demonstrated the ability to mediate an increase in SDF-1α which leads to targeting of the CXCR4 expressing cells to the infarcted area in a rabbit ischemia model. The use of a CXCR4 receptor antagonist, AMD3100, removes the protective effects found with the increased levels of SDF-1α [39].
Researchers have performed manipulations of this signaling axis to induce greater therapeutic responses and improve functional outcomes following in infarcted tissue. Direct injections of SDF-1α into peri-infarct regions improve cardiac function, as well as overexpression of SDF-1α with adenoviral delivery; AMD3100 abolishes these benefits [41]. It is also known that despite the increased levels of SDF-1α following MI, those levels are not sustained for more than 7 days [30]. This is due to the result of inactivation by CD26/dipeptidyl peptidase IV (DPP-IV) [42, 43]. Consequently, efforts have also been made to prolong the effect of SDF-1α in the infarcted heart, thus stabilizing expression as a means of stimulating greater repair. One such technique utilized a cell patch engineered from mesenchymal stem cells (MSCs) overexpressing CXCR4 with concurrent treatment with DPP-IV; results demonstrated migration of the MSCs into the heart tissue with additional functional improvement in DPP-IV treated cells [44]. The overexpression of CXCR4 through adenoviral vector delivery into different stem cell types also enhances vascularization in the damaged myocardium and thus SDF-1α/CXCR4 seems to be particularly important in progenitor cell chemotaxis, homing, engraftment, and retention in the damaged myocardium. SDF-1α has also been overexpressed via adenoviral transfection into MSCs, and has improved the rate of c-kit+ homing and improved LV function after MI [45]. This is significant because c-kit+ cells play an important role in cardiac repair and restoration of heart function and produce high levels of VEGF, which in turn stimulates their differentiation into endothelial cells for angiogenesis [46].
SDF-1α/CXCR4 and downstream signaling pathways
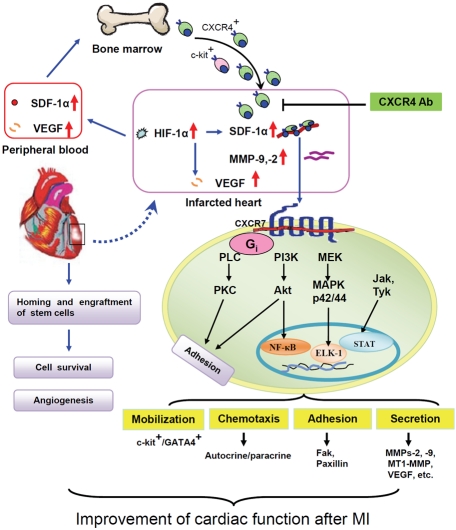
As for the direct effect of the interaction between SDF-1α and CXCR4, binding stimulates many signaling pathways, including those involved in adhesion, migration, motility, and gene expression [3, 47]. SDF-1α binding leads to dimerization of the receptor, stimulating G-protein signaling [48], which in turn activates various other signaling cascades [19]. Additionally, while CXCR4 is generally considered the single receptor of SDF-1α, there is additional binding to CXCR7, which appears to stimulate MAPK, PI3K, and Jak/STAT signaling [49]. MSCs overexpressing CXCR4 release anti-fibrotic enzymes (MMP-2 and-9) under hypoxic conditions [30], which can aid these cells in crossing the infarcted area, thus leading to reduced scar formation and improvement in cardiac function. SDF-1α is also thought to activate endothelial progenitor cell integrins involved in the extravasation process. The binding of SDF-1α to CXCR4 leading to the activation of several signaling pathways is important in cardiac repair as summarized in Figure 1. SDF-1α also causes accumulation of these endothelial progenitor cells (EPCs) in ischemic areas and promotes angiogenesis [24]; this has also been confirmed in a hind-limb ischemic injury model [3].
Figure 1.
Schematic depiction of signaling transduction pathways activated by the SDF-1α/CXCR4 interaction for restoration of heart function after MI. In infarcted heart tissue, increased expression of SDF-1α, HIF-1α, and VEGF stimulate repair mechanisms. SDF- 1α and VEGF levels are also increased in the peripheral blood and enhance recruitment of progenitor cells to the injured tissue. Binding of SDF-1α to CXCR4 activates G protein-coupled receptor kinases, which are responsible for many signaling transduction pathways in cells. The PI3-K-stimulated activation of extracellular signal-related kinase (ERK)-1/2 has been implicated in SDF-1α-induced chemotaxis, cell proliferation, and regulation of integrin activity. Increased nuclear translocation of NF-κB and DNA-binding activity also occurs. SDF-1α promotes the association of CXCR4 with Janus kinases (JAK) followed by tyrosine phosphorylation. Phosphorylated JAKs induce the activation and nuclear translocation of signal transducers and transactivators of transcription (STAT). Activation of these pathways regulates mobilization via phosphorylation of focal adhesion proteins including focal adhesion kinase or paxilin by both PI-3K and PKC, chemotactic responses via the release of autocrine/paracrine factors, adhesion, secretion of anti-fibrotic enzymes (MMP-2, -9, and MT1-MMP) to enhance progenitor cell engraftment, and synthesis of angiopoietic factors by the progenitor cells. In addition, pretreatment of progenitor cells with hypoxic preconditioning or overexpression of pro-survival genes like Akt or HIF-1α can reduce ischemia-induced cell death. SDF-1α also contributes to mobilization of progenitor cells from the bone marrow, including mesenchymal stem cells and c-kit+ cells, as well as c-kit+/GATA4+ cells from the heart. SDF-1α/CXCR4 signaling enhances cell survival, proliferation, and angiogenesis, thereby enhancing heart tissue repair following ischemic injury.
Mechanisms of ischemic tissue repair
As seen thus far, adenoviral delivery of SDF-1α and CXCR4 into various stem cell types that are then transplanted into infarcted tissue have demonstrated efficacy in improving tissue repair and function. However, the exact mechanisms by which SDF-1α/CXCR4 signaling repairs infarcted myocardium remain unclear. While it is known that recruitment of progenitor/stem cells allows for the increased cardiomyocyte regeneration, evidence of differentiation is limited. Studies have shown that SDF-1α is able to recruit endogenous cardiac stem cell-like cells that depolarize in vivo, which may contribute to increased contractile function even in the absence of maturation into a mature cardiac myocytes [50-52]. Another separate mechanism is the function of SDF-1α as a strong chemoattractant for CXCR4+ endothelial progenitor cells which protect the ischemic area through what seems to be both paracrine factors [53] as well as regeneration [8, 54]. C-kit positive cells are also recruited, and participate in VEGF secretion to stimulate differentiation into endothelial cells [46].
The link between SDF-1α/CXCR4 signaling and VEGF is important, and increased angiogenesis confirmed by measurement of capillary density plays a key role in improving function in an infarcted heart. It is also thought that stem cells expressing CXCR4 secrete certain factors that are likely involved in processes like angiogenesis and cardioprotection; for example, direct injection of SDF-1α activates anti-apoptotic pathways and angiogenesis [41]. CXCR4 activation in some cell types also results in Akt activation and stimulation of cell proliferation and survival, and cells overexpressing SDF-1α display an increased capacity for cellular growth and protection against interleukin-4-induced apoptosis [3]. Thus, SDF-1α/CXCR4 likely acts in multiple ways to produce positive effects, including stem cell homing for regeneration, cardioprotection, neoangiogenesis, as well as providing paracrine factors to facilitate all processes.
Issues of clinical applications of SDF-1α/CXCR4
Evidence of the benefit of SDF-1α/CXCR4 manipulation in the treatment of myocardial infarction has been expanding, and continuing research has provided insight into the various ways in which future therapies can take advantage of the processes regulated by SDF-1α/ CXCR4 signaling. Transplantation of stem cells into infarcted tissue has already been tested clinically. However, many important pieces of our knowledge remain to be discovered before we will be able to successfully implement these treatment alternatives. For example, the issue of the type of stem cell that should be used for transplantation purposes has yet to be officially determined. In addition, several studies have shown inconclusive results regarding the exact effects of signaling. For example, in 2006, Pyo et al. reported that SDF-1α signaling actually resulted in a decrease in cardiac contractility and function [55]; this was speculated to be the result of the direct effect off SDF-1α on cells immediately local to the site of expression [19]. Other studies have found no significant improvement in cardiac function [56] or even detrimental effects [57]. The aforementioned antagonist of CXCR4, AMD3100, which is hypothesized to decrease the positive effects of CXCR4 was found to increase the amount of c-kit+ cardiac progenitor cells, instead of impairing function as was expected [58]. This is thought to be due to the role of SDF-1α/CXCR4 in promoting the differentiation and commitment as a means of increasing tissue replacement [58]. Researchers also recently engineered mice that were deficient only in cardiac CXCR4 expression so as to isolate its role in heart function without inducing other systemic deficiencies. Their results suggested no difference in CXCR4 deficient mice with respect to baseline cardiac function or function following acute myocardial infarction [59]. Despite these surprising findings, the study does not explore the effect of CXCR4 deficiency in the presence of stem cell engraftment, which may explain their findings.
SDF-1α/CXCR4 in vascular damage
SDF-1α/CXCR4 has also been studied with respect to vascular physiology and atherosclerosis. Given the effects that signaling has on stem cell recruitment and homing to sites of injury, it is not difficult to imagine that this process might be overstimulated and have deleterious effects. It is known that damage to vessel walls will stimulate repair through neointimal thickening, and it is often smooth muscle cells (SMCs) which are derived from activated SMC precursors that are responsible for this process [60]. SDF-1α levels appear to be important in response to wire injury to vessels, which induces apoptosis of damaged SMCs in the intima and recruits SMC progenitors [61]. SDF-1α blockage was also associated with reduction of cell recruitment and vessel wall thickness following injury [60]. A similarly diminished effect was exhibited in mice that did not express CXCR4 [60]. However, long term overexpression of SDF-1α has been reported to cause chronic allograft deterioration associated with development of transplant arteriosclerosis [24].
In the example of transplant arteriosclerosis, vascular progenitor cells have been shown to be experimentally recruited to injured vessels [60, 62], and this is thought to be an SDF-1α mediated process [63]. Increased SDF-1α expression in injured vasculature may also accelerate neointima formation leading to obstructive vascular disease, such as restenosis after percutaneous interventions in coronary or peripheral artery disease and transplant arteriosclerosis. Moreover, a study of single nucleotide polymorphisms demonstrated that a SDF-1α gene variation has an influence on SDF-1α levels and circulating EPC number, and that plasma SDF-1α levels are a predictor of EPC number, which may help to understand the mechanism in the pathogenesis of atherosclerosis and other cardiovascular disease [64]. Alternatively, the use of both SDF-1α and CXCR4 may be operative in patients with cardiovascular diseases. Blocking the SDF-1α/CXCR4 signaling axis using CXCR4 antagonists might be a valuable strategy to treat transplant vasculopathy or re-stenosis. However, given that there is still much unknown regarding the role of SDF-1α in atherosclerosis, the actual effect of therapeutic administration is uncertain.
These various inconsistencies and gaps remain in our understanding of SDF-1α/CXCR4, and will need to be sufficiently explored before the development of widespread cell based therapies that incorporate SDF-1α/CXCR4.
Conclusion
SDF-1α/CXCR4 signaling is crucial to normal homeostasis as well as response to injury. The many fundamental processes that are tied to this axis - hematopoiesis, organogenesis, embryogenesis, cell mobilization, trafficking, ne-ovascularization - are evidence in themselves of its important role. Both SDF-1α and CXCR4 are both potential targets that can be manipulated and incorporated into cell therapies to achieve a desired effect. Understanding the molecular mechanism regulating CXCR4 expression and activation in various CXCR4 positive cells is crucial to developing therapeutic strategies.
Despite the promise of such therapies, much work remains to be done before alternative treatments will be widely available to patients with cardiovascular disease. The challenge in future years will lie in identifying the exact mechanisms, most likely multiple, that are responsible for tissue response to SDF-1α/ CXCR4. Researchers will need to exercise caution in targeting this axis, given that it is a double-edged sword, as seen in the example of transplant arteriosclerosis. While excess signaling might be harmful, blockage of SDF-1α/ CXCR4 with antagonists [4] or inactivation of CXCR4 [65] might be a valuable strategy for treatment of vasculopathy or restenosis. Such is the balance that must be attained in future research. Continued investigation of SDF-1α and CXCR4 in the context of cardiovascular disease will be central to identifying ideal means of targeting this chemokine and its receptor to enhance cardiac tissue repair and functional recovery.
Acknowledgments
This work was supported by NIH grants, HL089824, HL081859, and HL110740 (Y. Wang).
References
- 1.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1994;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 3.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 4.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt M, Schabath R. SDF-1 (CXCL12) in haematopoiesis and leukaemia: impact of DPP IV/CD26. Front Biosci. 2008;13:1774–1779. doi: 10.2741/2798. [DOI] [PubMed] [Google Scholar]
- 6.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human SDF-1 gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 7.Knaut H, Werz C, Geisler R, Nusslein-Vollard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 1998;393:591–4. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- 8.Molyneauz KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehnmann R. The chemokine SDF1/CXCL12 and is receptor CXCR4 regulate mouse germ clel migration and survival. Development. 2003;130:4279–86. doi: 10.1242/dev.00640. [DOI] [PubMed] [Google Scholar]
- 9.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 10.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 11.Moepps B, Braun M, Knopfle K, Dillinger K, Knochel W, Gierschik P. Characterization of a Xenopus laevis CXC chemokine receptor 4: Implications for hematopoietic cell development in the vertebrate embryo. Eur J Immunol. 2000;30:2924–2934. doi: 10.1002/1521-4141(200010)30:10<2924::AID-IMMU2924>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 13.Zou YR, Kottmann AH, Kuroda M, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/ progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 15.Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for SDF-1/CXCR4 chemokine receptor system in adult brain: Isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 17.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghadge SK, Mühlstedt S, Özcelik C, Bader M. SDF-1α as a therapeutic stem cell homing factor in myocardial infarction. Pharmacology & Therapeutics. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A, Ratajczak MZ. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453–466. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- 21.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 22.Hatch H, Zheng D, Jorgensen ML, Petersen BE. SDF-1α/CXCR4: A mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 23.Pituch-Noworolska A, Majka M, Janowska-Wieczorek A, Baj-Krzyworzeka M, Urbanowicz B, Malec E, Ratajczak MZ. Circulating CXCR4-positive stem/progenitor cells compete for SDF-1 positive niches in bone marrow, muscle and neural tissues: An alternative hypothesis to stem cell plasticity. Folia Histochem Cytobiol. 2003;41:13–21. [PubMed] [Google Scholar]
- 24.Wang Y, Haider H, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol. 2006;41:478–487. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 25.Kollet O, Shivtiel S, Chen YQ, Siriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 27.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 28.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 29.Tan W, Martin D, Gutkind JS. The Galpha13-Rho signaling axis is required for SDF-1 -induced migration through CXCR4. J Biol Chem. 2006;281:39542–9. doi: 10.1074/jbc.M609062200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Overexpression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 32.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:11247–11256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 33.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JL, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 34.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 35.Beltrami AP, Barlucchi L, Torell D, Bake M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 37.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 38.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misao Y, Takemura G, Arai M, Ohno T, Onogi H, Takahashi T, Minatoguschi S, Fujiwara T, Fujiwara H. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–465. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Ceradini D, Kulkarni A, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Gali-ano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 41.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 43.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Huang W, Dai B, Zhao T, Ashraf A, Millard RW, Ashraf M, Wang Y. Genetically manipulated progenitor cell sheet with diprotin A improves myocardial function and repair of infarcted hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1339–47. doi: 10.1152/ajpheart.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ZhangG, Nakamura Y, Wang X, Hu Q, Suggs U, Zhang J. Controlled release of stromal cell -derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–71. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 46.Fazel S, Cimini M, Liwen Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong D, Korz W. Translating an antagonist of chemokine receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 48.Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, Bouvier M, Heveker N. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 49.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki T, Fukazawa R, Ogawa S, Kanno S, Nitta T, Ochi M, Shimizu K. Stromal cell- derived factor-1α improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49:966–971. doi: 10.1111/j.1442-200X.2007.02491.x. [DOI] [PubMed] [Google Scholar]
- 51.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell like cells that depolarize in vivo. Cell Transplant. 2007;16:879–86. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 52.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 53.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A, Ratajczak MZ. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells. 2001;19:453–466. doi: 10.1634/stemcells.19-5-453. [DOI] [PubMed] [Google Scholar]
- 54.Zhao T, Zhang D, Millard RW, Ashraf M, Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1α delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–986. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, Tunstead J, Logothestis DE, Hijjar RJ, Schecter AD. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–844. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koch KC, Schaefer WM, Liehn EA, Rammos C, Mueller D, Schroeder J, Dimassi T, Stopinski T, Weber C. Effect of catheter-based tran-sendocardial delivery of stromal cell-derived factor 1α on left ventricular function and per-fusion in a porcine model of myocardial infarction. Basic Res Cardiol. 2006;101:69–77. doi: 10.1007/s00395-005-0570-3. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, Kingery J, Prabhu SD, Bolli R, Rokosh G. Chronic AMD3100 antagonism of SDF-1α-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49:587–97. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal U, Ghalayini W, Feng D, Weber K, Zou Y, Rabbany SY, Rafii S, Penn MS. Role of Cardiac Myocyte CXCR4 Expression in Development and Left Ventricular Remodeling After Acute Myocardial Infarction. Circ Res. 2010;107:667–676. doi: 10.1161/CIRCRESAHA.110.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 61.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Merickskay M, Gierschik P, Biessen EA, Weber C. SDF-1a/ CXCR4 axis is instrumental in neointimal hy-perplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 62.Hillebrands JL, Klatter FA, Rozing J. Origin of vascular smooth muscle cells and the role of circulating stem cells in transplant arteriosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:380–387. doi: 10.1161/01.ATV.0000059337.60393.64. [DOI] [PubMed] [Google Scholar]
- 63.Sakihama H, Masunaga T, Yamashita K, Hashimoto T, Inobe M, todo S, Uede T. SDF-1 and CXCR4 interaction is critical for development of transplant arteriosclerosis. Circulation. 2004;110:2924–2930. doi: 10.1161/01.CIR.0000146890.93172.6C. [DOI] [PubMed] [Google Scholar]
- 64.Xiao Q, Ye S, Oberhollenzer F, Mayr A, Jahangiri M, Willeit J, Kiechl S, Xu Q. SDF1 gene variation is associated with circulating SDF1α level and endothelial progenitor cell number: the Bruneck Study. PLoS One. 2008;3:e4061. doi: 10.1371/journal.pone.0004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coffield VM, Jiang Q, Su L. A genetic approach to inactivating chemokine receptors using a modified viral protein. Nat Biotechnol. 2003;21:1321–1327. doi: 10.1038/nbt889. [DOI] [PMC free article] [PubMed] [Google Scholar]