SUMMARY
In Pogonomyrmex californicus harvester ants, an age-associated division of labor occurs in the worker caste, in which young workers perform in-nest tasks and older workers forage for food. Here, we tested whether this behavioral division is age based or age flexible, and whether it coincides with differential expression of systemic hormones with known roles in behavioral regulation. Whole-body content of juvenile hormone (JH) and ecdysteroids was determined in workers from (1) age-typical colonies, in which a typical age structure is maintained and workers transition across behaviors naturally, and (2) single-cohort colonies, which are entirely composed of same-aged workers, facilitating the establishment of age-independent division of labor. Foragers from both colony types had higher JH and lower ecdysteroid content than workers performing in-nest tasks, suggesting that age is not the sole determinant of worker behavior. This association between hormone content and behavior of P. californicus workers is similar to that previously observed in founding queens of this species. Because these hormones are key regulators of development and reproductive behavior, our data are consistent with the reproductive ground plan hypothesis (RGPH), which posits that the reproductive regulatory mechanisms of solitary ancestors were co-opted to regulate worker behavior.
KEY WORDS: behavior, division of labor, ecdysteroids, endocrine, Formicidae, juvenile hormone
INTRODUCTION
The evolution of increasing social complexity in insects culminates in colonial species exhibiting a reproductive division of labor (Wilson, 1971). In addition, a further division of tasks within the non-reproductive worker caste often exists (Hölldobler and Wilson, 2009). The regulatory mechanisms by which such complexity is produced have received considerable attention. Much of this work has focused on the honey bee, Apis mellifera (e.g. Robinson, 1992; Ben-Shahar et al., 2002; Amdam et al., 2004; Rueppell et al., 2004; Amdam et al., 2006; Hunt et al., 2007; Ihle et al., 2010). Emerging from these studies is support for a hypothesis that social behavior evolved through the co-option of regulatory factors that originally coordinated the progression of female reproductive physiology and behavior in solitary ancestors – the reproductive ground plan hypothesis (RGPH) (West-Eberhard, 1987; West-Eberhard, 1996; Amdam et al., 2004). Specifically, this hypothesis predicts that several non-reproductive behavioral traits that are observed in social insect societies are regulated by mechanisms normally associated with reproductive development and activity. While there is support for this argument in a few species of bees and wasps, testing in other social insect taxa is necessary to assess the broader application of the RGPH (Amdam and Page, 2010). Here, we present the first such study for ants, a major taxon that independently evolved (Brady et al., 2006; Moreau et al., 2006) a level of social complexity comparable to that of A. mellifera (Hölldobler and Wilson, 1990). In addition, our investigations provide basic proximate information on how hormones correlate with behavioral transitions in ants.
Numerous evolutionary scenarios have been posited for the division of labor between ant workers (Hölldobler and Wilson, 2009), but few studies have investigated connections between physiology, specifically of the reproductive system, and the regulation of behavioral differentiation. We recently showed that changes in the behavior of nest-founding queens (female reproductives) of the California harvester ant, Pogonomyrmex californicus, are coordinated with their endocrine state. This finding suggests that divergent behavioral phenotypes could be produced by differential expression of endocrine factors normally associated with reproduction (Dolezal et al., 2009), and is consistent with the predictions of the RGPH. If the RGPH is broadly applicable, then the behavior of P. californicus workers should be underpinned by the same reproductive physiology that was observed in founding queens. Task performance in these workers is naturally age related (Hölldobler and Wilson, 2009) (A.G.D., personal observation), with younger individuals performing in-nest tasks such as brood care and nest maintenance, and older individuals leaving the nest to perform foraging-related tasks; this temporal polyethism is one of the key factors contributing to the major ecological success of social insects (Hölldobler and Wilson, 2009). For many species exhibiting these behavioral transitions, it is possible to decouple behavior from age by manipulating the environment of the workers (Nelson, 1927; Hölldobler and Wilson, 2009), providing a useful tool for verifying the role of putative behavioral regulators.
The primary regulators of both behavior and ovarian activity in insects are the systemic endocrine factors juvenile hormone (JH) and ecdysteroids. Both have organizational, priming and/or coordinating effects (reviewed by Raikhel et al., 2005). JH has been associated with division of labor among workers of the honey bee (reviewed by Robinson and Vargo, 1997), the bumble bee (Bloch et al., 2000a), the queenless ant Streblognathus peetersi (Brent et al., 2006) and Polistes wasps (Giray et al., 2005). Although no causal route from ecdysteroid content to division of labor has been demonstrated (Hartfelder et al., 2002), this group of hormones is suspected of having priming effects on honey bee worker behavior (Velarde et al., 2009; Wang et al., 2009; Amdam and Page, 2010), and has links to adult reproductive activity (Robinson et al., 1991). Ecdysteroids act via effects on the axis of brain/fat body/ovaries (Wang et al., 2010; Yamazaki et al., 2011), and ecdysteroid production is often linked to insect ovarian activation (Raikhel et al., 2005; Dong et al., 2009). These hormones are also implicated as a behavioral regulator in S. peetersi (Brent et al., 2006), paper wasps (Röseler et al., 1985) and bumble bees (Bloch et al., 2000b). In P. californicus founding queens, we found more JH in foragers compared with those performing in-nest tasks, while ecdysteroids had no apparent behavioral association (Dolezal et al., 2009). Queen ovarian activity (egg production) co-varies with JH and behavior, while the ovaries, a major source of ecdysteroids in insects (Raikhel et al., 2005), are invariably intact and presumably functional (A.G.D., unpublished data). Nurse workers of P. californicus have functional ovaries that produce nutritional eggs; in contrast, foragers usually have degraded and presumably non-functional ovaries (Hölldobler and Wilson, 2009) (A.G.D., unpublished data).
The genus Pogonomyrmex is a very suitable model species for studying the endocrinological parameters involved in the division of labor between nurses and foragers. Pogonomyrmex californicus is, in particular, relatively easy to culture in the laboratory under controlled conditions. In the current study, we anticipated that P. californicus worker division of labor would be associated with endocrine content, and that associations could be predicted from the physiology of queens, as suggested by the RGPH. Accordingly, we expected JH to be elevated in foragers, while ecdysteroids should be influenced by worker ovarian integrity, and therefore be reduced in foragers. To test these predictions, we examined the behavior and corresponding endocrine patterns in the monomorphic workers and foragers from both age-typical colonies (those possessing a normal distribution of workers at all ages and stages of development) and single-cohort colonies. The similarly aged workers of the single-cohort colonies must divide labor between nest tasks and foraging, removing the influence of any age-based behavioral bias. Although this robust approach has been widely used in honey bee research (Nelson, 1927; Robinson et al., 1989; Huang and Robinson, 1995), single-cohort experiments are rare in ant research (Gronenberg et al., 1996; Haight, 2006; Haight, 2008).
Our analysis, which provides one of the most complete investigations between ant division of labor and hormone physiology, reveals significant associations between ant reproductive endocrine physiology and social task performance independent of chronological age effects. The results highlight the behavioral and endocrine plasticity of worker ants and provide support for the RGPH in a non-Apis eusocial insect species with an independent evolutionary origin of sociality.
MATERIALS AND METHODS
Division of labor among workers from colonies with a typical age structure
Mature colonies of P. californicus, Buckley 1867, were maintained in the laboratory at the School of Life Sciences, Arizona State University. Four colonies were chosen for the experiment, each reared under laboratory conditions (natural light cycle, ∼25°C) for at least 3 years and stably exhibiting normal social structure. Late-stage pupae from these and five other laboratory-maintained colonies were carefully observed. When callow (young, light-colored) workers emerged, all individuals that eclosed on the same day were marked by tying a small colored wire around the petiole (modified from Tschinkel, 2006). Approximately 350 newly emerged workers were identified, marked, and introduced in several pulses to the four experimental colonies between 15 July 2007 and 28 October 2007. The new workers were readily accepted, and because they were marked with colors corresponding to their day of emergence, it was possible to determine their age at the point at which their behavior transitioned.
Between 15 July 2007 and 13 May 2008 the colonies were observed 1-3 times per week, for 15-30 min per observation. After 15 days, we collected marked workers that had performed nursing behaviors; these were individuals that had not been observed outside of the inner nest and were collected from the vicinity of the brood pile. No marked workers had begun foraging at this time. Colony observation continued and the day on which marked individuals were first observed to forage was noted. Ants handling food items or water outside the nest were defined as foraging. New foragers were marked with a small amount of colored fluorescent powder, to allow for repeated identification. The powder is normally observable for several days. When an individual worker had been observed foraging 3 times, it was designated as a confirmed forager. Although the marking powder may have been groomed off prior to confirmation of forager status, this would only result in some workers being observed foraging more than 3 times. For both identified nest workers and foragers, 89 individuals were surveyed for age and collected to determine hormone content.
Division of labor among workers from single-cohort colonies
Local field colonies of P. californicus were partially excavated in the summer and autumn of 2008, and as many callow workers as possible were collected from each nest. Cuticle pigmentation was used as a marker to identify workers of close to identical adult age. Only the lightest colored, and thus youngest, workers were collected. The workers were brought back to the laboratory, and approximately 200 were added to each of six experimental colonies, where they were readily accepted. The host colonies were 1.5-2.5 years of age, with approximately 300 workers each. After 2 days, all of the original members of the colony were removed, leaving only the queen, eggs, larvae and 200 young, same-aged workers. Four out of the six colonies recovered successfully from the disturbance while the remaining two failed and were excluded from the experiment.
The four single-cohort colonies were observed 3-7 days per week, 2-3 times per day, for 5-10 min per observation. Foraging individuals were marked as described above, with fluorescent powder. Once a worker had been observed foraging on three occasions, she and an in-nest worker from the same nest were collected. These collections continued for the duration of the experiment, with all workers collected between the ages of 13 and 50 days. The experiment was ended after 50 days, at which point the population of each colony had dropped below approximately 25 workers, and foraging events were rare.
Sampling and hormone assays
The measurement of whole-body hormone content was necessitated by the small size of the ants, but facilitated by the monomorphic (Johnson, 2000) bodies of these workers. Five workers from within the same behavioral group (i.e. foraging or nursing) and colony type (i.e. age-typical or single-cohort colony) were pooled to form each biological sample for hormone analysis. Workers were pooled between the four replicate colonies of each type, so each biological sample provided a representative cross-section of the sample material. Animals were collected directly into 0.5 ml of cold methanol (Sigma-Aldrich, St Louis, MO, USA) to minimize the effect of handling stress on their endocrine state.
Hormone extraction and purification then followed the same processes utilized in P. californicus queens (Dolezal et al., 2009) (modified from Shu et al., 1997). JH was extracted from the homogenate using hexane and was then purified via elution through aluminium oxide columns with hexane, 10% ethyl ether-hexane and 30% ethyl ether-hexane (Sigma-Aldrich). The JH was derivatized using a solution of methyl-d alcohol and trifluoroacetic acid, then resuspended and eluted through aluminium oxide columns with 30% ethyl ether and ethyl acetate-hexane. JH was quantified using an Agilent 6890 Series GC (Hewlett Packard, Palo Alto, CA, USA) equipped with a 30 m×0.25 mm Carbowax Econo-Cap GC column (Alltech, Fresno, CA, USA) coupled to an Agilent 5973N inert mass-selective detector/detection software (MSD/DS).
After JH had been extracted, the remaining homogenate in methanol was analyzed using a radioimmunoassay to determine ecdysteroid content. Standard competition curves were generated for each sample set using 20-hydroxyecdysone stock (Sigma-Aldrich). Duplicates of each sample were incubated overnight at 4°C on an orbital shaker with 100 μl of (3H)-20-hydroxyecdysone stock (1 mg ml-1, NEN, PerkinElmer, Waltham, MA, USA) in borate buffer, and 100 μl of a polyclonal ecdysteroid antiserum (H-22 antibody, L. Gilbert, University of North Carolina at Chapel Hill). Subsequently, samples received 20 μl of cleaned protein A solution (Pansorbin; CalBiochem, San Diego, CA, USA) and were incubated for 1 h at room temperature. Samples were then centrifuged and washed with borate buffer. Microlabel incorporation was determined by a 2450 MicroBeta2 scintillation counter (Perkin-Elmer, Waltham, MA, USA) and ecdysteroid concentrations were estimated via non-linear regression (Brent et al., 2006).
Statistics
The data for initiation of foraging, JH content and ecdysteroid content in the single-cohort workers showed a general lack of normality, and did not pass the assumption of homogeneity of variances (Levene's test; P<0.05). Therefore, non-parametric Mann-Whitney U-tests were used to determine whether there were significant differences in foraging age, JH content and single-cohort ecdysteroid content between nest workers and foragers. The ecdysteroid content of the age-typical group met the homogeneity assumption (Levene's test, P>0.05), but did not fit a normal distribution as determined by a normality plot on the residuals. The data conformed to the assumption of normality after log transformation and were subsequently analyzed with the parametric Student's t-test. A non-parametric Kruskal-Wallis one-way ANOVA was performed to identify any intercolonial differences in hormone levels. Spearman rank tests were used to determine whether there were correlations between age and hormone levels under each colony condition. An alpha value of 0.05 was used for acceptable significance in all tests. The analyses were performed using Statistica 7.0 (StatSoft, Tulsa, OK, USA).
RESULTS
Timing and age of behavioral transitions in naturally aging and single-cohort colonies
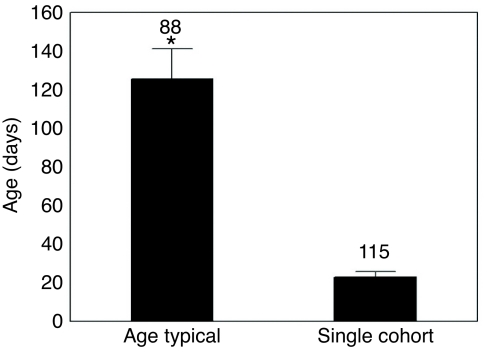
The observations of confirmed foraging by age-typical and single-cohort workers showed significant differences in age at foraging confirmation. On average, single-cohort (sc) workers initiated foraging 5 times earlier than the marked workers (at) in age-typical colonies (Mann-Whitney U-test: U=0.0, Nat=88, Nsc=115, P<0.001; Fig. 1). In addition, the variance in age at foraging initiation was reduced in single-cohort colonies (Varat=4600.506, Varsc=141.75; Levene's test, F=164.76, P<0.001), reflecting the compressed timescale for the transition to foraging behavior.
Fig. 1.
Age of confirmed foraging of age-typical and single-cohort colonies. Single-cohort workers initiated foraging significantly earlier, as indicated by the asterisk (Mann-Whitney U-test, P<0.05). Sample sizes are indicated above the bars.
Age-typical colonies: hormone activity
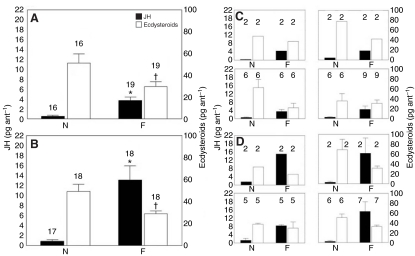
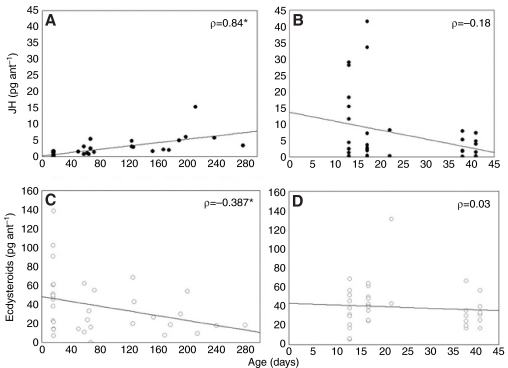
Significant differences were observed in JH content between nest workers and foragers. Relative to nest workers, foragers from age-typical colonies contained 6 times the JH (Mann-Whitney U-test: U=18, Nnest=16, Nforager=19, P<0.001; Fig. 2A) and half the ecdysteroids (Student's t-test: Nnest=16, Nforager=19, F=1.363, P=0.043; log transformed for normalization; Fig. 2A). There were no significant differences in JH (Kruskal-Wallis ANOVA: N=35, H=0.299, P=0.96) or ecdysteroid (Kruskal-Wallis ANOVA: N=35, H=3.938, P=0.268) content between the colonies. While each replicate colony had a sample size that was too small for robust statistics, the data trend of each colony was the same as the overall result (Fig. 2C). Analysis also showed that there was no significant correlation between JH and ecdysteroid content (Spearman rank correlation: Nnest=16, Nforager=19, ρ=-0.19, P>0.05). In the age-typical colonies, however, there was a significant correlation between hormone content and age when all individuals were considered together. Increased age was correlated with increased JH (Spearman rank correlation: Nnest=16, Nforager=19, ρ=0.84, P<0.05; Fig. 3A) and decreased ecdysteroids (Spearman rank correlation: Nnest=16, Nforager=19, ρ=-0.387, P<0.05; Fig. 3C).
Fig. 2.
Mean ± s.e.m. content (pg ant-1) of juvenile hormone (JH) and ecdysteroids of (A) age-typical workers transitioning naturally from nest (N) to foraging (F) stages, and (B) single-cohort workers performing nest (N) or foraging (F) tasks. Significant differences in juvenile hormone and ecdysteriod content between nurses and foragers are indicated by asterisks and daggers, respectively. Age-typical ecdysteroids: Student's t-test, P<0.05; all others: Mann-Whitney U-test, P<0.05 between groups. In addition, JH content of single-cohort foragers is significantly higher than JH content of age-typical foragers (Mann-Whitney U-test, P<0.05). For both the age-typical (C) and single-cohort (D) colonies, each component colony has too low a sample size for robust statistical analyses; however, the general trend in each colony is the same as the overall result (A and B, respectively). Sample sizes are indicated above the bars.
Fig. 3.
Scatter plot showing distribution of hormone content in relation to age. There is a significant positive association between JH content and age in age-typical colonies (A), but no such correlation exists for single-cohort colonies (B). Similarly, a significant negative correlation is observed between ecdysteroid content and age in age-typical colonies (C), but not in single-cohort colonies (D). This relationship illustrates that the association between hormone content and behavior is not necessarily age associated, as the relationship disappears in single-cohort colonies. Asterisks denote significant correlations, and Spearman coefficients (ρ) are indicated (Spearman rank correlation, P<0.05).
Single-cohort colonies: hormone activity
Relative to nest workers, same-aged foragers from the single-cohort colonies contained 10 times more JH (Mann-Whitney U-test: U=3, Nnest=17, Nforager=18, P<0.001; Fig. 2B) and 50% less ecdysteroids (Mann-Whitney U-test: U=74, Nnest=18, Nforager=18, P=0.005; Fig. 2B). There were no significant differences in JH (Kruskal-Wallis ANOVA: N=35, H=0.313, P=0.9576) or ecdysteroid levels (Kruskal-Wallis ANOVA: N=35, H=3.814, P=0.2822) between the colonies. While statistics were not calculated for each replicate colony, the data trends were consistent with the overall result (Fig. 2D). Unlike the age-typical colonies, single-cohort colonies exhibited a significant negative correlation between JH and ecdysteroid content (Spearman rank correlation: Nnest=17, Nforager=18, ρ=-0.569, P<0.05), and no correlation between age and JH content (Spearman rank correlation: Nnest=17, Nforager=18, ρ=-0.18, P>0.05; Fig. 3A vs 3B) or ecdysteroid content (Spearman rank correlation: Nnest=17, Nforager=18, ρ=0.03, P>0.05; Fig. 3C vs 3D). In addition, the JH content of foragers from single-cohort colonies was significantly higher than that of foragers from the age-typical colonies (Mann-Whitney U-test: U=48, Nat=18, Nsc=18, P<0.005).
DISCUSSION
Our observations of foraging onset times in age-typical and single-cohort colonies show that P. californicus worker behavior is very flexible, and can be accelerated substantially by modified colony demography (Fig. 1). This acceleration is well described in honey bees (Nelson, 1927; Robinson et al., 1989; Huang and Robinson, 1995), and similar experiments in ants have shown that worker behavior can change quickly as task requirements change (Ehrhardt, 1931). However, our manipulation of P. californicus worker age demography provides new evidence that extensive behavioral plasticity is possible in these ants. Workers in single-cohort colonies exhibited remarkably accelerated behavioral maturation and initiated foraging an average of 100 days earlier than those in age-typical colonies (Fig. 1).
Regardless of whether the workers were raised in age-typical or single-cohort colonies, there was an association between endocrine patterns and behavioral phenotypes. JH content was consistently higher in foragers from both groups relative to the content in the in-nest workers (Fig. 2A,B). Although age was correlated with JH content in the age-typical colonies (Fig. 3A), there was no correlation in the single-cohort colonies (Fig. 3B). This suggests that, while age may influence endocrine state to indirectly affect behavior, JH is the principal correlate of foraging activity. Another notable difference between these colony types was that JH was higher in single-cohort foragers than in age-typical foragers (Fig. 2A vs 2B). Perhaps the very young workers of the single-cohort colonies have a higher threshold for foraging (Beshers and Fewell, 2001) and, correspondingly, more circulating JH may be required for foraging onset to occur. The finding that JH was elevated in foraging workers is similar to results for P. californicus founding queens (Dolezal et al., 2009) and parallels information on the behavioral physiology of honey bees, where elevated JH corresponds to foraging activity in workers (e.g. Jaycox et al., 1974; Robinson, 1987; Sullivan et al., 2000). While there is evidence that JH is not required for foraging activity in honey bees (Huang and Robinson, 1995; Sullivan et al., 2000) and P. californicus queens (Dolezal et al., 2009), collectively the data support the hypothesized role of JH as a behavioral reinforcer during and following the transition from nest tasks to field tasks (Amdam and Omholt, 2003), changing its role from a regulator of reproductive status and behavioral dominance in more primitive groups (Hartfelder, 2000).
Unlike JH, ecdysteroid content did not follow the same pattern as found in P. californicus queens, in which no clear differences were observed between foragers and non-foragers (Dolezal et al., 2009). Under age-typical (Fig. 4A) and single-cohort (Fig. 4B) circumstances, the onset of worker foraging behavior corresponded with both increased JH and, unlike in P. californicus queens, ecdysteroid content. As we speculated, ecdysteroid content was associated with the general trend in ovarian integrity and was thereby consistently elevated in nest workers compared with foragers (Fig. 2A,B). Active ovaries are the primary source of ecdysteroids in adult insects (Raikhel et al., 2005), and although P. californicus nest workers do not reproduce directly, their ovaries produce sterile nutritive eggs that are fed to developing larvae (A.G.D., personal observation). Because ovarian activation is often linked to ecdysteroid changes (Raikhel et al., 2005; Dong et al., 2009), we suggest that this activity is related to the elevation in the workers' ecdysteroid level. As workers transition to foraging, they no longer produce nutritive eggs and their ovaries gradually degenerate (A.G.D., unpublished data) (Hölldobler and Wilson, 2009). We suggest that these changes are related to reduction in ovarian activity and ecdysteroid levels. Unlike workers, queens forage for only a short period before returning to large scale egg production in the nest, and during foraging the functionality of queen ovaries is maintained (A.G.D., unpublished data). These factors explain why ecdysteroid levels remain steady in queens instead of declining, as they do in workers.
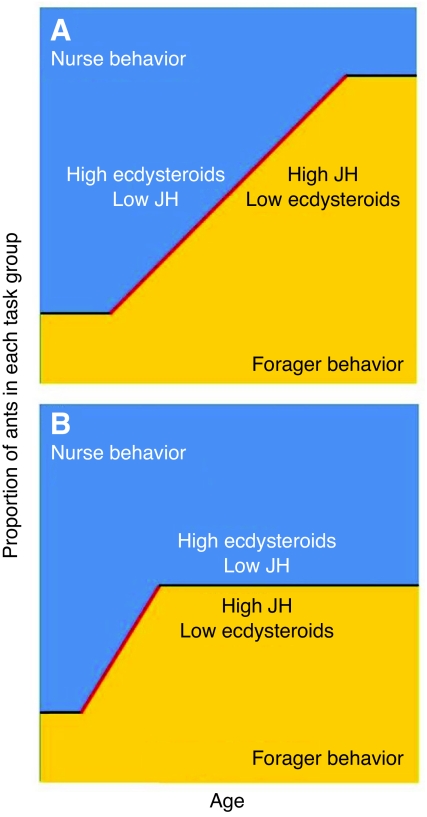
Fig. 4.
A summary of the hypothesized relationship between JH, ecdysteroids, age and division of labor in P. californicus workers. Age is defined as the age of any given worker cohort, from adult emergence until death. That is, as a group of workers age (x-axis), the proportion of workers performing different tasks changes (y-axis). High JH content corresponds to foraging activities, and a high ecdysteroid content with nest tasks; the bisecting line represents the proportion of workers performing nurse (blue) or foraging (yellow) tasks, and demarks the different hormone levels. (A) In age-typical colonies, the vast majority of young workers have low JH levels and high ecdysteroid levels, and are inside the nest performing nursing tasks. As they age, more workers initiate foraging, which is a behavioral transition that is associated with high JH and low ecdysteroids. (B) In single-cohort colonies, foraging onset begins at a much earlier age, and proceeds faster, until the colony has achieved the necessary balance between nurses and foragers. In both colony types, the onset of foraging coincides with increased JH and decreased ecdysteroid levels.
Despite a body of evidence suggesting that ecdysteroids may have lost their behavioral role in adults during the evolution of eusocial insect taxa (Hartfelder et al., 2002), there are examples of divergent ecdysteroid titers being associated with behavioral castes (Röseler et al., 1985; Bloch et al., 2000b; Brent et al., 2006), and there is emerging evidence of a link between ecdysteroids and foraging onset in worker honey bees (Velarde et al., 2009; Wang et al., 2010). Whether ecdysteroids impact the behavior of P. californicus workers remains to be determined. Lower levels in foragers might only reflect changes in ovarian physiology, and not be robustly tied to JH – the primary endocrine correlate of behavior. This lack of association is supported by our results; although JH and ecdysteroid levels were significantly correlated in single-cohort colonies, they were not in age-typical colonies (Fig. 3). Correlation in single-cohort colonies could be a consequence of the compressed transition that is taking place. Ecdysteroid-related processes are being downregulated at the same time that JH-related processes are being upregulated, when they would otherwise be on different schedules. While ecdysteroids may not directly influence the expression of foraging behavior in P. californicus workers, their role in ovarian activity makes them likely endocrine facilitators of nurse behavior. Ovarian ecdysteroids can stimulate the production of egg yolk precursors (vitellogenins) from the insect fat body – a tissue that is functionally homologous to the vertebrate liver and white adipose tissue (Raikhel et al., 2005). This stimulatory effect on yolk production implies that ecdysteroids are not only markers of ovarian activity but also a functional requirement for nutritive egg production, a nurse-specific trait in many ants, including other Pogonomyrmex species (Wilson and Eisner, 1957; Hölldobler and Wilson, 1990). Whether ecdysteroids influence the rate of nutritive egg production in workers of P. californicus can be addressed in future experiments.
Interestingly, the function and makeup of nutritive eggs in these ants bears a striking resemblance to secretions by the hypopharyngeal head glands of honey bees (Wilson and Eisner, 1957; Amdam et al., 2003). During the nest stage, these glands produce royal jelly that can be mixed with other secretions, nectar and pollen as a general food source for honey bee larvae and adult colony members, including foragers (Crailsheim and Stolberg, 1989). The hypopharyngeal glands use and store vitellogenin, the yolk protein that is essential for egg production. Metabolic consumption of vitellogenin by the bees' hypopharyngeal glands has been causally linked to their production of proteinaceous food secretions (Amdam et al., 2003). Thereby, both ants with nutritive eggs and honey bees have evolved mechanisms for exploiting vitellogenin in social nourishment. Such nourishment is crucial to colony integrity and development and has been much studied in honey bees (Engels and Imperatriz-Fonseca, 1990; Naiem et al., 1999; Amdam et al., 2003), while less work has been done in ants (Hölldobler and Wilson, 1990; Gobin and Ito, 2000; Khila and Abouheif, 2008). Thus, further understanding of the role of non-reproductive worker egg production in P. californicus can allow for richer comparisons into how the reproductive infrastructure could be exploited to evolve and sustain eusocial societies.
Although much remains to be clarified, our findings can be interpreted as supporting the view that the mechanisms underlying the worker division of labor may have been derived from regulatory networks of reproductive development. The RGPH suggests that the co-option of such networks may be a common route from which insect societies evolved complex social behaviors (Amdam et al., 2004). The finding that JH in P. californicus correlates with the foraging behavior of sterile workers in a manner similar to that of reproductive, colony-founding queens is consistent with this hypothesis. The inference that presumably ovarian-produced ecdysteroids may facilitate nurse behavior is also in line with the model of social co-option of solitary reproductive mechanisms. While neither of these associations has been causally linked to behavior, the correlations described here provide important additional information for understanding relationships between reproductive physiology and complex social behavior. A more robust evaluation of the RGPH would be made possible by future development of protocols for endocrine and functional genetic manipulation of ants. The ability to push forward with these investigations becomes more feasible as a result of the increasing number of tools available for ant researchers (Smith et al., 2009). For example, the growing number of annotated ant genomes (Bonasio et al., 2010; C. R. Smith et al., 2011; C. D. Smith et al., 2011; Suen et al., 2011; Wurm et al., 2011), including a closely related Pogonomyrmex species (C. R. Smith et al., 2011; C. D. Smith et al., 2011), opens up more ant systems to studies of the molecular genetics of behavioral regulation.
ACKNOWLEDGEMENTS
We thank Joshua Johnson and Kelly A. Dolezal for help in preparing the manuscript and Kevin Haight for assistance with developing a wire-marking protocol for these ants. We also appreciate the constructive comments provided by two anonymous reviewers.
FOOTNOTES
FUNDING
Support was provided to G.V.A. by the Research Council of Norway [grant nos 180504, 185306 and 191699], the National Institute on Aging [NIA P01 AG22500], and the PEW Charitable Trust, and to A.G.D. by the Arizona State University Graduate and Professional Students Association research grant. Deposited in PMC for release after 12 months.
REFERENCES
- Amdam G. V., Omholt S. W. (2003). The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J. Theor. Biol. 223, 451-464 [DOI] [PubMed] [Google Scholar]
- Amdam G. V., Page R. E. (2010). The developmental genetics and physiology of honeybee societies. Anim. Behav. 79, 973-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam G. V., Norberg K., Hagen A., Omholt S. W. (2003). Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 100, 1799-1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam G. V., Norberg K., Fondrk M. K., Page R. E. (2004). Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl. Acad. Sci. USA 101, 11350-11355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam G. V., Csondes A., Fondrk M. K., Page R. E. (2006). Complex social behavior derived from maternal reproductive traits. Nature 439, 76-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y., Robichon A., Sokolowski M. B., Robinson G. E. (2002). Influence of gene action across different time scales on behavior. Science 296, 741-744 [DOI] [PubMed] [Google Scholar]
- Beshers S. N., Fewell J. H. (2001). Models of division of labor in social insects. Annu. Rev. Entomol 46, 413-440 [DOI] [PubMed] [Google Scholar]
- Bloch G., Borst D. W., Huang Z.-Y., Robinson G. E., Cnaani J., Hefetz A. (2000a). Juvenile hormone titers, juvenile hormone biosynthesis, ovarian development and social environment in Bombus terrestris. J. Insect Physiol. 46, 47-57 [DOI] [PubMed] [Google Scholar]
- Bloch G., Hefetz A., Hartfelder K. (2000b). Ecdysteroid titer, ovary status, and dominance in adult worker and queen bumble bees (Bombus terrestris). J. Insect Physiol. 46, 1033-1040 [DOI] [PubMed] [Google Scholar]
- Bonasio R., Zhang G. J., Ye C. Y., Mutti N. S., Fang X. D., Qin N., Donahue G., Yang P. C., Li Q. Y., Li C., et al. (2010). Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 329, 1068-1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. G., Schultz T. R., Fisher B. L., Ward P. S. (2006). Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc. Natl. Acad. Sci. USA 103, 18172-18177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent C. S., Peeters C., Dietemann V., Crewe R., Vargo E. L. (2006). Hormonal correlates of reproductive status in the queenless Ponerine ant, Streblognathus peetersi. J. Comp. Physiol. A 192, 315-320 [DOI] [PubMed] [Google Scholar]
- Crailsheim K., Stolberg E. (1989). Influence of diet, age and colony condition upon intestinal proteolytic activity and size of the hypopharyngeal glands in the honeybee (Apis mellifera L). J. Insect Physiol. 35, 595-602 [Google Scholar]
- Dolezal A. G., Brent C. S., Gadau J., Holldobler B., Amdam G. V. (2009). Endocrine physiology of the division of labour in Pogonomyrmex californicus founding queens. Anim. Behav. 77, 1005-1010 [Google Scholar]
- Dong S. Z., Ye G. Y., Guo J. Y., Hu C. (2009). Roles of ecdysteroid and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae). Gen. Comp. Endocrinol. 160, 102-108 [DOI] [PubMed] [Google Scholar]
- Ehrhardt S. (1931). Über Arbeitsteilung bei Myrmica-und Messor-Arten. Zeitschrift für Morphologie und Ökologie der Tiere 20, 755-812 [Google Scholar]
- Engels W., Imperatriz-Fonseca V. L. (1990). Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees. In Social Insects: an Evolutionary Approach to Castes and Reproduction (ed. Engels W.). Berlin: Springer-Verlag; [Google Scholar]
- Giray T., Giovanetti M., West-Eberhard M. J. (2005). Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proc. Natl. Acad. Sci. USA 102, 3330-3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin B., Ito F. (2000). Queens and major workers of Acanthomyrmex ferox redistribute nutrients with trophic eggs. Naturwissenschaften 87, 323-326 [DOI] [PubMed] [Google Scholar]
- Gronenberg W., Heeren S., Holldobler B. (1996). Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011-2019 [DOI] [PubMed] [Google Scholar]
- Haight K. L. (2006). Defensiveness of the fire ant, Solenopsis invicta, is increased during colony rafting. Insectes Sociaux 53, 32-36 [Google Scholar]
- Haight K. L. (2008). Ontogeny of the defensive stinging behavior of the fire ant, Solenopsis invicta. J. Insect Behav. 21, 147-152 [Google Scholar]
- Hartfelder K. (2000). Insect juvenile hormone: from 'status quo' to high society. Braz. J. Med. Biol. Res. 33, 157-177 [DOI] [PubMed] [Google Scholar]
- Hartfelder K., Bitondi M. M. G., Santana W. C., Simões Z. L. P. (2002). Ecdysteroid titer and reproduction in queens and workers of the honey bee and of a stingless bee: loss of ecdysteroid function at increasing levels of sociality? Insect Biochem. Mol. Biol. 32, 211-216 [DOI] [PubMed] [Google Scholar]
- Hölldobler B., Wilson E. O. (1990). The Ants. Cambridge MA: Belknap Press of Harvard University Press; [Google Scholar]
- Hölldobler B., Wilson E. O. (2009). The Superorganism: the Beauty, Elegance, and Strangeness of Insect Societies. New York: W. W. Norton; [Google Scholar]
- Huang Z.-Y., Robinson G. E. (1995). Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. J. Comp. Physiol. B 165, 18-28 [DOI] [PubMed] [Google Scholar]
- Hunt G. J., Amdam G. V., Schlipalius D., Emore C., Sardesai N., Williams C. E., Rueppell O., Guzman-Novoa E., Arechavaleta-Velasco M., Chandra S., et al. (2007). Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften 94, 247-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle K. E., Page R. E., Frederick K., Fondrk M. K., Amdam G. V. (2010). Genotype effect on regulation of behaviour by vitellogenin supports reproductive origin of honeybee foraging bias. Anim. Behav. 79, 1001-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaycox E. R., Skowronek W., Guynn G. (1974). Behavioral changes in worker honey bees (Apis mellifera) induced by injections of a juvenile hormone mimic. Ann. Entomol. Soc. Am. 67, 529-534 [Google Scholar]
- Johnson R. A. (2000). Seed-harvester ants (Hymenoptera: Formicidae) of North America: An overview of ecology and biogeography. Sociobiology 36, 89-122 [Google Scholar]
- Khila A., Abouheif E. (2008). Reproductive constraint is a developmental mechanism that maintains social harmony in advanced ant societies. Pro. Natl. Acad. Sci. USA 105, 17884-17889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C. S., Bell C. D., Vila R., Archibald S. B., Pierce N. E. (2006). Phylogeny of the ants: diversification in the age of angiosperms. Science 312, 101-104 [DOI] [PubMed] [Google Scholar]
- Naiem E. S., Hrassnigg N., Crailsheim K. (1999). Nurse bees support the physiological development of young bees (Apis mellifera L.). J. Comp. Physiol. B 169, 271-279 [Google Scholar]
- Nelson F. C. (1927). Adaptability of young bees under adverse conditions. Am. Bee J. 67, 242-243 [Google Scholar]
- Raikhel A. S., Brown M., Belles X. (2005). Hormonal control of reproductive processes. In Comprehensive Molecular Insect Science (ed. Gilbert L., Gill S., Iatrou K.) pp. 433-491 Boston, MA, USA: Elsevier Press; [Google Scholar]
- Robinson G. E. (1987). Regulation of honey bee age polyethism by juvenile hormone. Behav. Ecol. Sociobiol. 20, 329-338 [Google Scholar]
- Robinson G. E. (1992). Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637-665 [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Vargo E. L. (1997). Juvenile hormone in adult eusocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 35, 559-583 [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Page R. E., Strambi C., Strambi A. (1989). Hormonal and genetic control of behavioral integration in honey bee colonies. Science 246, 109-111 [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Strambi C., Strambi A., Feldlaufer M. F. (1991). Comparison of juvenile hormone and ecdysteroid hemolymph titers in adult worker and queen honey-bees (Apis mellifera). J. Insect Physiol. 37, 929-935 [Google Scholar]
- Röseler P. F., Röseler I., Strambi A. (1985). Role of ovaries and ecdysteroids in dominance hierarchy establishment among foundresses of the primitively social wasp, Polistes gallicus. Behav. Ecol. Sociobiol. 18, 9-13 [Google Scholar]
- Rueppell O., Pankiw T., Page R. E. (2004). Pleiotropy, epistasis and new QTL: the genetic architecture of honey bee foraging behavior. J. Heredity 95, 481-491 [DOI] [PubMed] [Google Scholar]
- Shu S. Q., Park Y. I., Ramaswamy S. B., Srinivasan A. (1997). Hemolymph juvenile hormone titers in pupal and adult stages of southwestern corn borer [Diatraea grandiosella (Pyralidae)] and relationship with egg development. J. Insect Physiol. 43, 719-726 [DOI] [PubMed] [Google Scholar]
- Smith C. D., Zimin A., Holt C., Abouheif E., Benton R., Cash E., Croset V., Currie C. R., Elhaik E., Elsik C. G., et al. (2011). Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl. Acad. Sci. USA 108, 5673-5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. R., Dolezal A. G., Eliyahu D., Holbrook C. T., Gadau J. (2009). Ants (Formicidae): models for social complexity. CSH Protocols, Vol. 3, issue 7 [DOI] [PubMed] [Google Scholar]
- Smith C. R., Smith C. D., Robertson H. M., Helmkampf M., Zimin A., Yandell M., Holt C., Hu H., Abouheif E., Benton R., et al. (2011). Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc. Natl. Acad. Sci. USA 108, 5667-5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen G., Teiling C., Li L., Holt C., Abouheif E., Bornberg-Bauer E., Bouffard P., Caldera E. J., Cash E., Cavanaugh A., et al. (2011). The genome sequence of the leaf-cutter ant Atta cephalotes reveals insights into its obligate symbiotic lifestyle. PLoS Genetics 7, 1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. P., Jassim O., Fahrbach S. E., Robinson G. E. (2000). Juvenile hormone paces behavioral development in the adult worker honey bee. Hormon. Behav. 37, 1-14 [DOI] [PubMed] [Google Scholar]
- Tschinkel W. R. (2006). The Fire Ants. Cambridge, MA: The Belknap Press of Harvard University Press; [Google Scholar]
- Velarde R. A., Robinson G. E., Fahrbach S. E. (2009). Coordinated responses to developmental hormones in the Kenyon cells of the adult worker honey bee brain (Apis mellifera L.). J. Insect Physiol. 55, 59-69 [DOI] [PubMed] [Google Scholar]
- Wang Y., Amdam G. V., Rueppell O., Wallrichs M. A., Fondrk M. K., Kaftanoglu O., Page R. E. (2009). PDK1 and HR46 gene homologs tie social behavior to ovary signals. PLoS One 4, e4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kaftanoglu O., Siegel A. J., Page R. E., Amdam G. V. (2010). Surgically increased ovarian mass in the honey bee confirms link between reproductive physiology and worker behavior. J. Insect Physiol. 56, 1816-1824 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J. (1987). Flexible strategy and social evolution. In Animal Societies: Theories and Facts (ed. Itô Y., Brown J. L., Kikkawa J.), pp. 35-51 Tokyo: Japan Science Society Press; [Google Scholar]
- West-Eberhard M. J. (1996). Wasp societies as microcosms for the study of development and evolution. In Natural History and Evolution of Paper Wasp (ed. Turillazzi S., West-Eberhard M. J.), pp. 290-317 New York: Oxford University Press; [Google Scholar]
- Wilson E. O. (1971). The Insect Societies. Cambridge, MA: Belknap Press of Harvard University Press; [Google Scholar]
- Wilson E. O., Eisner T. (1957). Quantitative studies of liquid food transmission in ants. Insectes Sociaux 4, 157-166 [Google Scholar]
- Winston M. L. (1987). The Biology of the Honey Bee. Cambridge, MA: Harvard University Press; [Google Scholar]
- Wurm Y., Wang J., Riba-Grognuz O., Corona M., Nygaard S., Hunt B. G., Ingram K. K., Falquet L., Nipitwattanaphon M., Gotzek D., et al. (2011). The genome of the fire ant Solenopsis invicta. Proc. Natl. Acad. Sci. USA 108, 5679-5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y., Kiuchi M., Takeuchi H., Kubo T. (2011). Ecdysteroid biosynthesis in workers of the European honeybee Apis mellifera L. Insect Biochem. Mole. Biol. 41, 283-293 [DOI] [PubMed] [Google Scholar]