Abstract
The sex determining gene is divergent among different animal species. However, sox9 is up-regulated in the male gonads in a number of species in which it is the essential regulator of testis determination. It is therefore often discussed that the sex determining gene-sox9 axis functions in several vertebrates. In our current study, we show that sox9b in the medaka (Oryzias latipes) is one of the orthologues of mammalian Sox9 at syntenic and expression levels. Medaka sox9b affects the organization of extracellular matrices, which represents a conserved role of sox9, but does not directly regulate testis determination. We made this determination via gene expression and phenotype analyses of medaka with different copy numbers of sox9b. Sox9b is involved in promoting cellular associations and is indispensible for the proper proliferation and survival of germ cells in both female and male medaka gonads. Medaka mutants that lack sox9b function exhibit a seemingly paradoxical phenotype of sex reversal to male. This is explained by a reduction in the germ cell number associated with aberrant extracellular matrices. Together with its identified roles in other vertebrate gonads, a testis-determining role for Sox9 in mammals is likely to have been neofunctionalized and appended to its conserved role in germ cell maintenance.
Introduction
Sox9 is a member of the Sry-related HMG box (Sox) gene family and is conserved in vertebrates. Among the mammalian Sox family, Sox9 has been extensively analyzed and is known to be critical for many aspects of cell differentiation such as chondrocyte specification, neural crest differentiation, heart valve development and male sex determination [1]–[4]. Many of these functions are achieved through the role of sox9 in the extracellular matrix and this has been confirmed in various vertebrates. This suggests that sox9 is conserved both structurally and functionally.
During the initial events in sex determination, many vertebrates employ a species-specific sex determining gene. In mammals, the sex determining gene, Sry, is on the Y chromosome and directly upregulates the transcription of Sox9 in the supporting cells of the XY gonad only. Once Sox9 expression is established in the XY supporting cells, it is both functionally required and sufficient for testis determination [5]–[9]. In other higher animals, such as the chicken, alligator and turtle, sox9 is also up-regulated exclusively in the male gonad [10]–[12]. In lower vertebrates, the role of sox9 in the gonad is not yet known. It has been often described that a sex determining gene-sox9 axis may constitute a conserved component of the sex determination system in many vertebrates.
The medaka (Oryzias latipes) is a species of teleost fish and represents a good model system for studying the conserved mechanisms of sex determination and the differentiation of gonads across different vertebrate species [13]. Male sex determination in the medaka is initiated in the supporting cells via the expression of the DM-domain gene on the Y chromosome, DMY/dmrt1bY [14], [15]. However, the involvement of the medaka sox9 homologue in DMY/dmrt1bY-involving testis determination has not yet been functionally addressed.
As a result of teleost-specific genome duplication [16], most genes in teleosts are present in two copies. Two sox9 genes have also been reported in the medaka genome, sox9a and sox9b/sox9a2 (hereafter referred to as sox9b) [17]–[19]. Among these two copies, sox9a is not expressed in the gonadal supporting cells essential for sex determination, but is expressed in oocytes of the adult ovary [19]. In contrast, sox9b initiates its expression in the gonadal precursor cells that develop into the supporting cells [20]. It is therefore thought that the expression of sox9b in the supporting cells of the medaka parallels the role of mammalian Sox9 in the gonads.
Our previous examinations have revealed that unlike mammalian gonads, sox9b expression in the medaka is maintained in a few supporting cells in the developing ovary. In addition, the histological units of sox9b-expressing supporting cells in medaka have been recently identified as ovarian niche structures (known as the germinal cradles) that contain germline stem cells [21]. These expression patterns led us to speculate that the role of medaka sox9b may differ from that of mammalian Sox9 during testis determination.
Using syntenic and gene expression analysis in our current study, we first reconfirmed that medaka sox9a and sox9b are co-orthologues of mammalian Sox9 and that sox9b, but not sox9a, is expressed in the supporting cells responsible for initiation of medaka testis determination [17], [22]. Using both transgenic and chimeric sox9b medaka mutants, we show that medaka sox9b is required for germ cell proliferation and survival, but not for testis determination. The expression of components of the extracellular matrix was found to be largely disorganized in sox9b mutant medaka gonads. In addition, our results show that zebrafish sox9a is also expressed in the ovarian supporting cells. These findings collectively challenge the discussion along with the mammalian Sox9 function in the gonads and suggest that a testis determining role is an appended function during vertebrate evolution.
Results
Syntenic analysis and expression study of medaka sox9 genes
To confirm the phylogenetic relationships between medaka sox9a and sox9b with other vertebrate sox9 genes, we first examined the sox9 synteny among representative vertebrates using the Ensemble genomic information (Fig. S1A). As expected from previous analysis [17], the genomic region around sox9 is conserved in the mouse, chick and frog (Xenous tropicalis) and is duplicated in the teleosts stickleback, medaka and zebrafish. The two regions in the teleosts contain either sox9a or sox9b. Other than these two genes, no other sox9-like gene could be found using whole genome scanning. In addition to these observations, the main Sox9 expression domains in mammalian embryos have been shown to correspond to both or either sox9a and/or sox9b in teleost embryos. Hence, sox9a and sox9b in teleosts are very likely to be the only co-orthologues of the sox9 gene in other vertebrates [17], [22].
Our current expression analysis demonstrated that medaka sox9a is not expressed in the somatic cells during gonadal differentiation (sox9a, XY n = 5, XX n = 5). Sox9b was only detected in the supporting cells surrounding the germ cells (sox9b, XY n = 5, XX n = 5) (Fig. S1B), as expected from previous reports [18]–[20]. These results may be supportive of the conventional view that medaka sox9b has a conserved role in sex determination in the supporting cells within the medaka sex determining gene pathway. However, unlike mammalian Sox9 which is expressed in male supporting cells only, medaka sox9b is also expressed in the female developing gonads [20]. Additionally, in the adult ovary, sox9b-expressing supporting cells constitute niche structures harboring germline stem cells [21].
To evaluate whether the female expression of sox9 is specific to the medaka, we examined the gonadal sox9a and sox9b expression patterns of a phylogenetically distant teleost, the zebrafish. The previous reports indicate that zebrafish sox9a is expressed in the male supporting cells in adult testes (Fig. S2C) [22], [23]. Our present in situ hybridization analyses revealed that zebrafish sox9a expression occurs in some populations of somatic cells surrounding the small germ cells in the adult ovaries (Fig. S2A, S2B) while zebrafish sox9b was found to be expressed in the oocytes of adult ovaries but not in the testes (Fig. S2D, S2E). The expression analysis, suggests that there is functionally conserved similarity between medaka sox9b and zebrafish sox9a in gonads. This, together with our syntenic analysis, supports the previous report by Klüver et al that medaka sox9a and sox9b are an example of lineage-specific subfunctionalization and have arisen from duplication of the ancestral proto-chromosome 2 [17]. We next conducted functional analysis of the medaka sox9b gene to reveal a possible conserved role in teleost gonads.
Identification of sox9b mutants
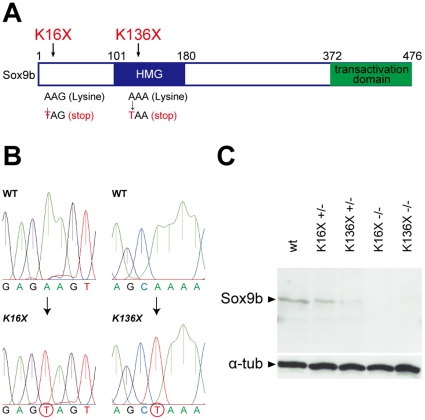
To address the function of sox9b in medaka gonads, we isolated two nonsense mutant alleles, sox9bK16X and sox9bK136X from the medaka tilling library (Fig. 1A, 1B) [24]. These variants have nonsense mutations in the 5′ coding region and HMG box domain, respectively. Western blotting analysis further revealed that the sox9b protein product levels are absent in the homozygous mutants (Fig. 1C), indicating that both of the nonsense alleles are functionally null. The heterozygous mutants of both alleles were found to be viable and to reach sexual maturity, although their reproductive ability was low. However, both the sox9bK16X and sox9bK136X homozygous mutants died by 20 dph (days post hatching) which is approximately 28 dpf (days post fertilization).
Figure 1. Identification and characterization of two sox9b mutant alleles in medaka.
(A) Schematic representation of the sox9b medaka protein and the locations of mutations. (B) Genomic sequences of wild-type sox9b, and of the sox9bK16X (−/−) and sox9bK136X (−/−) mutants. (C) Western blotting analysis of sox9b and alpha-tubulin demonstrating the absence of expression in the homozygous mutants, confirming that both alleles are null.
Sox9b is not required for testis determination
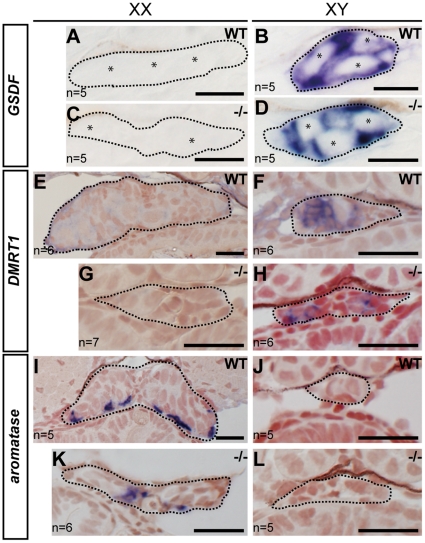
In order to address the possible involvement of sox9b in sex determination, the expression of sex markers were examined in early stages of gonadal development. GSDF [25] and DMRT1 [20] are typical of male supporting cell markers. In our current experiments, both markers were found to be expressed in all of the homozygous XY gonads examined, but not in the XX gonads (Fig. 2A–H). The female marker, aromatase, is known to be expressed only in female somatic gonadal cells including female-specific theca cells [26]. In contrast to GSDF and DMRT1, aromatase was detectable only in XX gonads (Fig. 2I–L). Hence, the genes involved in early sex differentiation in medaka are not regulated by sox9b, and sex differentiation therefore proceeds normally at these stages in the sox9b mutants.
Figure 2. Early stage sex differentiation processes are unaffected in the sox9b mutant gonads.
(A–L) GSDF (A–D), DMRT1 (E–H) and aromatase (I–L) expression (blue) were detected by in situ hybridization analysis of st. 39 (A–D) and 10 dph (E–L) medaka embryos, respectively. Consistent with the pattern found in wild-type (WT) medaka gonads, GSDF and DMRT1 were found to be expressed in XY gonads of the homozygous sox9b mutants, whilst aromatase was expressed in XX gonads of the homozygous sox9b mutants. More than five gonads were examined in each experiment. Dotted lines (A–L), gonadal outlines; asterisks in (A–D), germ cells. Scale bar, 10 µm (A–D); 20 µm (E–L).
In the adult heterozygous mutants, the size of ovaries and testes is decreased due to the reduced numbers of germ cells (Fig. S3A–C). We often found the gonads without germ cells (Fig. S3D), revealing a loss of germline maintenance. Interestingly, heterozygous XX mutants often exhibited male secondary sex characteristics (Fig. S3B and S3C) with gonads morphologically comparable to wild-type testes. In these sex-reversed mutants, a testis-marker gene, P45011β, is upregulated in the gonad while the expressions of ovarian marker genes, foxl2 and aromatase, were not detected by PCR. Other gonadal markers, which express in both testis and ovary, were detected normally as those in the wild-type gonad (Fig. S3E). The sex reversal phenotype was completely rescued by one copy of sox9b transgene in the heterozygous mutant background (Table 1). The heterozygous mutants with female secondary sex characteristics possessed the gonads with ovarian structures having the reduced number of germ cells (Fig. S3B and S3C). We did not observe the sex reversal event that was anticipated given the homology between mammalian Sox9 and medaka sox9b. We also observed no feminization of heterozygous XY mutants (a half-dose of functional sox9b) and no masculinization of transgenic XX medaka with an additional sox9b-transgene (Table 1), clearly supporting that sox9b is not involved in masculinization.
Table 1. Sox9b mutations lead to a discordance between the genetic and phenotypic sex in the medaka.
| K16X allele | +/+ | +/− | +/−/sox9b tg2 | +/+/sox9b tg | ||||
| male1 | female1 | male | female | male | female | male | female | |
| XX | 0 | 15 | 7 | 5 | 0 | 14 | 0 | 10 |
| XY | 11 | 0 | 7 | 0 | 8 | 0 | 18 | 0 |
The terms ‘male’ or ‘female’ are defined as secondary sex characteristics and adult gonad morphology (testis or ovary). See also Figure S3.
Sox9b tg; one additional copy of sox9b transgene.
Very interestingly the compound mutant mice with Sox8 and Sox9 revealed a phenotype of gradual loss of germ cells [27]. Therefore with suspect of the conserved sox9 function on germ cell regulation, we focused on the germ cells in the medaka sox9b mutants.
Reduced proliferation and survival of germ cells in sox9b mutants
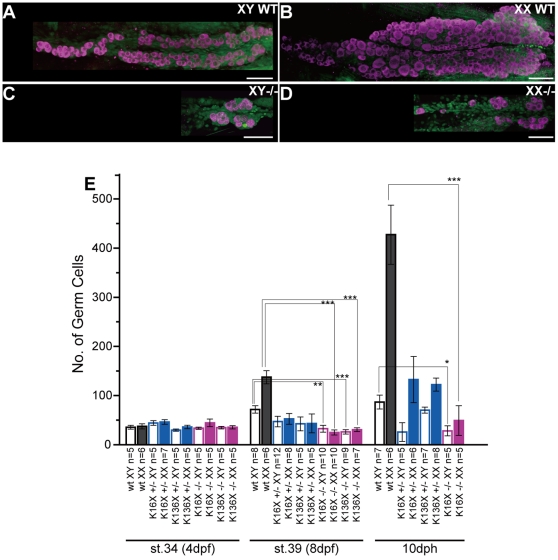
In wild-type medaka, the gonads exhibit proliferation of the germ cells with more of these cells found in females as sex differentiation proceeds [28], [29]. At stage 34 (4 dpf) when the gonadal primordium is established with germ cells, no differences were found in the total numbers of germ cells in both wild-type and sox9bK16X and sox9bK136X mutant medaka (Fig. 3E and Fig. S4). However, the germ cells in the sox9bK16X and sox9bK136X mutant gonads did not increase much at the later stages in either sex. This difference is detectable as early as stage 39 (8 dpf) and becomes more apparent at 10 dph (Fig. 3). The total numbers of germ cells were significantly reduced in these mutants when compared with the wild-type medaka (Fig. 3E). The sexual differences in the germ cell number typically seen in wild-type gonads were also observed in the heterozygous mutants (Fig. 3E and Fig. S4). This indicates that germ cell proliferation and/or survival is affected in sox9b mutants.
Figure 3. Gonadal morphology and germ cell number in the sox9b mutant medaka.
(A–D) Ventral images of the medaka gonad at 10 dph obtained by confocal microscopy. Germ cells and nuclei were stained with an anti-OLVAS antibody (purple) and DAPI (green), respectively. The tissue structures are formed but the germ cell numbers are reduced in the mutant gonads. (E) Number of germ cells in wild-type and sox9b heterozygous and homozygous mutant medaka during gonad differentiation. *P<0.05, ** P<0.01, *** P<0.001, Student's t test. All values are the mean ± SEM. Scale bars, 50 µm.
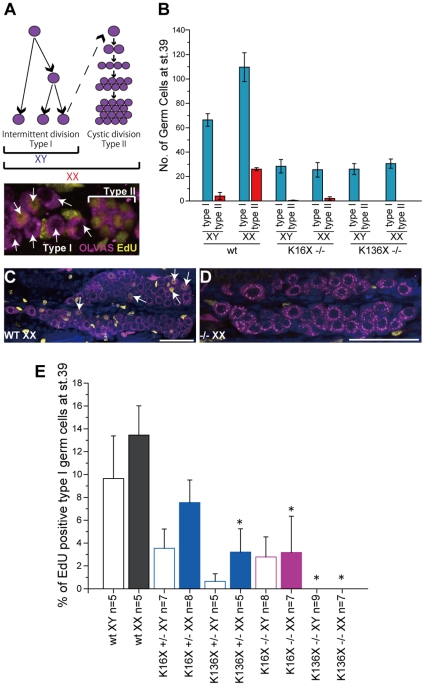
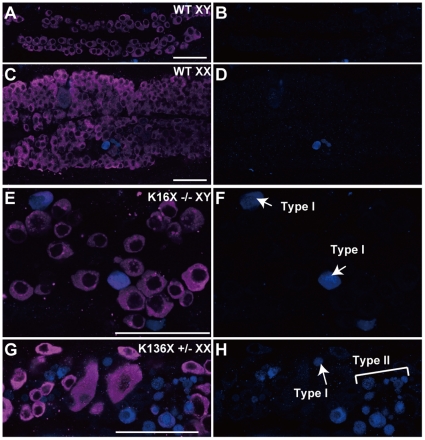
We next characterized the germ cell proliferation modes in the sox9b medaka mutants. During the early stages of sex differentiation in the wild-type medaka, there are two modes of germ cell proliferation in operation. The type I mode ensures germ cell maintenance (including stem cell proliferation) which is histologically indicated by the presence of isolated germ cells. Type II proliferation occurs in germ cells that are committed to gametogenesis and involves successive cell divisions. This mode of proliferation results in tightly packed cyst-forming germ cells (Fig. 4A). The female-specific increase in germ cells after the initiation of DMY/dmrt1bY expression is the consequence of a sub-population of germ cells shifting from type I to type II proliferation [29].
Figure 4. Germ cell proliferation is impaired in the sox9b mutant medaka.
(A) Medaka exhibit two modes of germ cell division during the early stages of gonadal differentiation. Intermittent divisions (type I) lead to germ cell maintenance and occur in both males and females, whilst synchronous and successive divisions (type II) form germ cell-cysts that are committed to gametogenesis. Type II divisions occur in developing female gonads and cause a female-specific rapid increase in germ cell number. Germ cells undergoing type I or II divisions are identifiable by the presence of isolated (arrows) or packed germ cells (brackets), respectively. (B) In the sox9b mutants, germ cells undergoing both type I and II divisions were reduced in number. Cysts containing more than two germ cells were counted as undergoing type II divisions. (C and D) Representative images of EdU labeling experiments in wild-type and mutant medaka gonads at stage 39 are shown. Note that the nuclei of type I germ cells are positively labeled by EdU (yellow) in wild-type (arrows) but not in mutant medaka. Germ cells were stained with an anti-OLVAS antibody (purple). (E) The percentage of EdU-positive type I germ cells was calculated, and type I divisions responsible for germ cell maintenance found to be significantly impaired in the mutants. All values are the mean ± SEM. *P<0.05 student's t test (each value was compared with wild-type XY and XX, respectively). Scale bar, 50 µm.
The numbers of isolated and cystic germ cells were found not to be increased in the sox9bK16X and sox9bK136X medaka mutants during sex differentiation (Fig. 4B). Importantly, we observed that the commitment of germ cells to gametogenesis was not impaired in these mutants as clusters of germ cells undergoing type II division, although quite rare though, were detectable (Fig. 4B). The reduced proliferative activity of type I germ cells was confirmed in the mutants using an EdU incorporation experiment (Fig. 4C–E). Moreover, type I and type II germ cells in the mutant gonads showed frequent apoptosis, as detected by cleaved-caspase3 expression (Fig. 5 and Table 2). Hence, the germ cells in sox9bK16X and sox9bK136X mutants demonstrate reduced survival and proliferation. A similar tendency but less severe phenotype was observed in the heterozygous mutants (Fig. 3E, Fig. 4E and Fig. S4).
Figure 5. Both type I and II germ cells are eliminated by apoptosis in the sox9b mutant medaka.
(A–H) Ventral views of 10 dph medaka gonads immunostained with anti-OLVAS (germ cell marker, purple) and anti-cleaved caspase 3 (blue). Merged images (A, C, E and G) and cleaved caspase 3 signals (B, D, F and H) are shown. Low levels of germ cell apoptosis only were evident in wild-type XY and XX medaka at 10 dph. However, type I and type II germ cells (type I, arrows; type II, a bracket) were eliminated by apoptosis (Table 2) in the homozygous (E and F) and heterozygous (G and H) mutants. Scale bar, 50 µm.
Table 2. Increased apoptotic activity in the germ cells of sox9b mutant medaka.
| No. of gonads harboring cleaved-Casp3+ germ cells/No. of gonads examined at 10 dph | |
| wt XY | 0/7 (0%) |
| wt XX | 1/6 (17%) |
| sox9bK16X +/− XY | 0/5 (0%) |
| sox9bK16X +/− XX | 2/6 (33%) |
| sox9bK16X −/− XY | 3/5 (60%) |
| sox9bK16X −/− XX | 2/5 (40%) |
| sox9bK136X +/− XY | 0/7 (0%) |
| sox9bK136X +/− XX | 4/8 (50%) |
Cellular associations are impaired in sox9b mutants
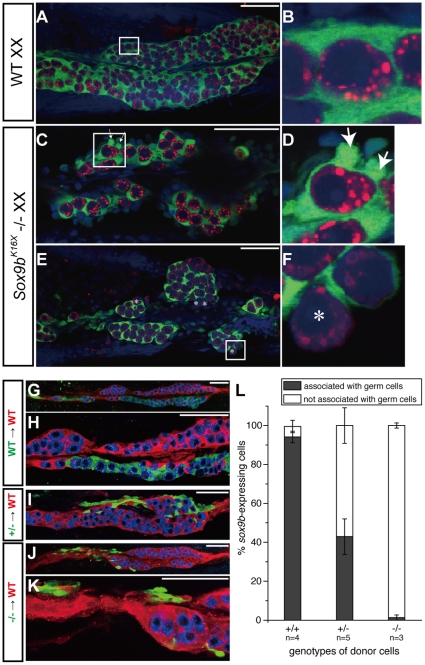
We often observed that the sox9b-expressing supporting cells in the sox9bK16X and sox9bK136X mutants exhibited aberrant shapes with frequent blebs and incomplete ensheathment (Fig. 6A–F), suggesting an impairment of cell to cell associations leading to abnormal germ cell homeostasis. To further evaluate these effects, chimeric gonads containing both wild-type and mutant cells were generated via transplantation. In contrast to chimeric gonads between wild-type cells, mutant sox9b-expressing supporting cells tended to be expelled from the chimeric gonads (Fig. 6G–K). The frequency of contact between sox9b-expressing supporting cells and germ cells was found to be dramatically decreased (Fig. 6L). In addition, homozygous mutant cells exhibited a more severe phenotype than heterozygous mutant cells, indicating that this severity depends on the functional sox9b gene dose per supporting cell. Since mutant sox9b-expressing supporting cells retain the ability to form gonads, the phenotype of the chimeric gonads can be attributed to a reduced capability of the mutant sox9b-expressing supporting cells to associate with each other, but not to a loss of identity of the supporting cells.
Figure 6. Mutant sox9b-expressing cells demonstrate a reduced cellular association.
(A–F) The morphologies of wild-type (A and B) and mutant (C–F) medaka gonads. Ventral views of XX gonads at 8 dpf (A–D) and 10 dph (E and F) are shown. Green, sox9b-expressing cells were immunostained with anti-GFP; red, germ cells were stained with anti-OLVAS; blue, nuclei were counterstained with DAPI. Medaka germ cells are completely surrounded by sox9b-expressing supporting cells and demonstrate a smooth surface (B), whilst mutant sox9b-expressing cells have cytoplasmic protrusions (D, arrows). Some isolated germ cells were not completely surrounded by sox9b-expressing cells in the mutants (F, asterisks). This was not seen in wild-type animals. (G–K) Representative images of somatic chimera. Green, donor-derived sox9b-EGFP expressing cells; red, host sox9b-DsRed positive cells; blue, germ cells stained with anti-OLVAS. (L) Calculated ratio of donor-derived sox9b-EGFP expressing cells associated with germ cells (black) to those not associated with germ cells (white). Scale bar, 50 µm.
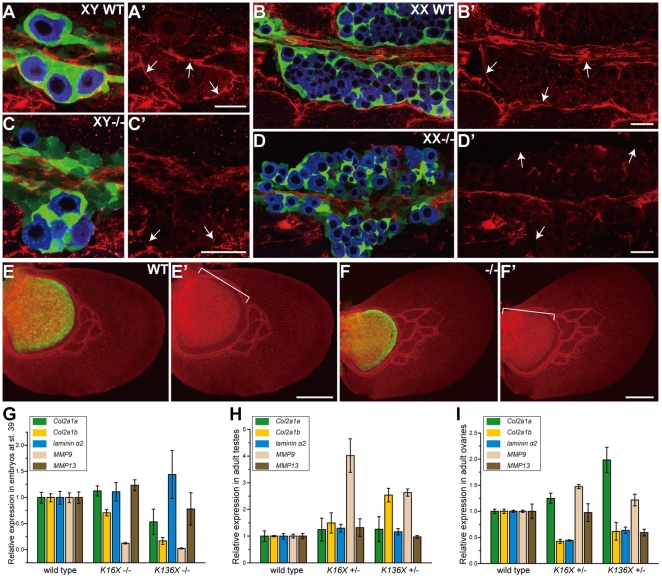
A number of studies have attributed many aspects of Sox9-involving phenomena to the regulation of extracellular matrices [1], [4], [30]. In medaka, we observed that laminin deposition was abnormal in the sox9b mutant gonad (Fig. 7A–D) whereas the tissues expressing both sox9a and sox9b do not show this defect (Fig. 7E, 7F). Furthermore, the expression of other extracellular matrix components, such as collagen genes and MMP genes, were found to be altered in mutant embryos, testes and ovaries (Fig. 7G–I). Hence, the observed impairment of cellular associations via the disorganization of extracellular matrix components is consistent with the failure of germ cell maintenance in medaka sox9b mutants, although further analysis needs to be done to fully elucidate these pathways.
Figure 7. The deposition of ECM components is altered in sox9b medaka mutants.
(A–D′) Ventral views of wild-type and sox9bK16X−/− medaka gonads at stage 39. Laminin (red), sox9b-EGFP (green) and germ cells detected by OLVAS antibody (blue) are shown. Note that the laminin deposits detected in wild-type sox9b-expressing cells are disorganized in the mutant cells (A′, B′, C′ and D′, arrow). (E–F′) The wild-type and sox9bK16X−/− pectoral fin at stage 39. Laminin (red) and sox9b-EGFP (green) signals are shown. The laminin expression pattern (brackets in E′ and F′) is unchanged in the mutant pectoral fin, in which both sox9a and sox9b are expressed. (G–I) The relative mRNA expression levels of extracellular matrix (ECM) components and matrix metalloproteases (MMP) in stage 39 medaka embryos (G), and the adult testis (H) and ovary (I) determined by qRT-PCR. The intensity of each band was normalized to EF1α and the relative expression levels compared with wild type are shown. Note that some ECM components and MMP are upregulated or downregulated in mutants compared with wild type. Each value represents the mean ± s.e.m. (n = 4). Scale bars, 20 µm and 100 µm (E–F′).
The increasing number of germ cells rescues the masculinization phenotype in sox9b mutants
The seemingly paradoxical masculinization of the medaka sox9b mutants is consistent with the germ cell-loss phenotype found in adult mutant gonads because our previous analysis has indicated that gonadal somatic cells, in the absence of germ cells, are predisposed to male development (Fig. S3F) [31], [32]. In contrast, the hotei homozygous mutant has a defect in the gene expressing the type II receptor for the anti-Müllerian hormone (amhrII) and shows continuous proliferation of germ cells [33]. Since the receptor and the ligand (anti-Müllerian hormone) are both expressed in the supporting cells but not in the germ cells, the impairment of anti-Müllerian hormone system consequently causes the germ cell-excessive phenotype. The homozygous hotei mutants show the male to female sex reversal phenotype but the sex reversal is not observed in the heterozygous hotei mutants. In this context, the number of germ cells in the gonad affects the proper sex differentiation directed by the sex chromosomes, and wild-type XX medaka without germ cells develop male secondary sex characteristics with a male gene expression profile (Fig. S3F).
To further obtain the solid evidence that the masculinization in sox9b mutants is a secondary effect of the decreasing number of germ cells, we crossed a sox9b mutant with a hotei heterozygous mutant to recover the germ cell number. We found that the number of germ cells is recovered and that the female to male sex reversal phenotype of the sox9b heterozygous XX mutants was abolished completely in the hotei and sox9b compound heterozygous mutants (Table 3). This result indicates that the female to male sex reversal phenotype of the sox9b mutants is caused by a decreased number of germ cells and not by a direct impairment of sox9b-expressing supporting cells. Taken together with the gene expression analysis and the analysis of medaka containing different doses of functional sox9b gene, we conclude that medaka sox9b is not required for testis determination or the subsequent processes of early testis differentiation.
Table 3. Female to male sex reversal phenotype is rescued in sox9b and hotei compound heterozygous mutants.
| amhrIIhot +/− | sox9bK16X+/−;amhrIIhot +/− | |||
| male | female | male | female | |
| XX | 0 | 4 | 0 | 14 |
| XY | 9 | 0 | 9 | 0 |
Discussion
We find in our current study that the medaka sox9b gene does not directly regulate testis determination and differentiation in this teleost species but is involved in germline maintenance and survival in both female and male gonads. The combined expression domains of medaka and zebrafish sox9a and sox9b largely match those of mouse Sox9, suggesting that the conserved sox9 roles among vertebrates are divided between two sox9 genes in medaka as a result of teleost-specific genome duplication. Medaka heterozygous sox9b mutants are viable and a few homozygous mutants can survive until 20 dph. This is in contrast to the embryonic lethality seen in the Sox9-disrupted mouse embryo. The discrepancy in these survival outcomes between medaka and mouse could be attributable to the redundant expression of sox9a and sox9b in the medaka.
A testis-determining role of mammalian Sox9 is likely to be neofunctionalized
During chondrogenesis, mammalian Sox9 directly regulates the expression of Col2a1, the extracellular matrix component type II collagen gene [34]. In mouse chimeras, Sox9 −/− mesenchyme cells are excluded from wild-type cells during cartilage formation, possibly due to a disorganized extracellular matrix [35]. It is intriguing that a gradual loss of germ cells has been reported in the compound Sox8 and Sox9 mutant mouse and concluded to be the result of functionally defective supporting cells caused by the loss of Sox8 and 9 redundant activity [27]. This is a representative example of the germ cell maintenance role of Sox9 in mammalian gonads. Our present results show that the germ cell-loss in our medaka sox9b mutants are likely to have been caused by reduced cellular associations. The results of these studies are therefore collectively supportive of an evolutionally conserved role of sox9 in the regulation of germ cell maintenance and survival. In this context, both medaka sox9a and sox9b seem to have a redundant role on the regulation of extracellular matrix because the laminin deposition in the pectoral fin was not impaired in the sox9b mutants (Fig. 7E, 7F).
Interestingly, medaka sox9b mutants show a female to male sex reversal phenotype, which is the opposite effect of the masculinizing function of Sox9 in the mammalian gonads. Our current results demonstrate that the masculinization caused by the loss of sox9b function is accounted for by a decreased number of germ cells. Recent comparative genomic approaches have revealed that testis-specific enhancer of Sox9 core element (TESCO), which is a binding region for Sry and is critical for the male-specific expression of Sox9 in supporting cells in the mouse, is conserved in amniotes and amphibia (Xenopus tropicalis), but not in the medaka or zebrafish [36]. This provides a fascinating view that TESCO-mediated regulation was utilized by the evolutional stem from amphibia and was then recruited to mediate the testis-determining function of Sox9 in parallel with the emergence of Sry in mammals. This possibility is consistent with our current data showing that a sex determining gene-sox9 axis does not underpin sex determination in medaka and suggests that Sox9 is likely to play a neofunctionalized role in testis determination in mammals, which is appended to the more conserved sox9 role in germ cell maintenance.
It is also of interest that the loss of Sox9 expression in mammalian female supporting cells may be related to the lack of premeiotic germ cells in the adult ovary. This would explain why germline stem cells are either absent or very few in number in the mammalian ovary and also the species-specific configuration of gonadal differentiation.
In conclusion, the molecular pathways in medaka that start with DMY/dmrt1bY represent a unique system of testis determination and differentiation and should prompt a reconsideration of the discussion regarding the sox9-dependent testis determination system in many vertebrates.
Materials and Methods
Ethics Statement
All the treatments of animals in this research followed the guideline of National Institute for Basic Biology and were approved by the Institutional Animal Care and Use Committee of National Institutes of Natural Sciences. The approval IDs by the committee are 11A094 and 10A023.
Medaka strains and isolation of tilling medaka
The wild-type cab strain, Sox9b-EGFP/DsRed transgenic medaka [20], [37] and hotei mutants [33] were used in this study. Isolation of tilling medaka was performed as previously described by Taniguchi et al. [24]. The region encompassing the first and second exons of the medaka sox9b gene, including the initial ATG, was screened for mutations using the primers 5′-AACTCTTGGACGCAGAAAGG-3′ (forward) and 5′-TCAGGGTGCAAACGGATAAC-3′ (reverse). The PCR products were treated with ExoSAP-IT (GE Healthcare) and sequenced using the forward primer and a 3730xl 96-capillary DNA analyzer (Applied Biosystems). Two different alleles (K16X and K136X) were identified, as shown in Figure 1A. Medaka that are homozygous for the K16X or K136X alleles die by 20 dph (days post hatching).
Genotyping
The sex of the fish (XY or XX) was determined by PCR genotyping using a previously described method to detect polymorphisms between dmy and DMRT1 [20]. To genotype the sox9b alleles (+/−, +/− or −/−), direct sequencing and/or SNP genotyping assays were performed. For direct sequencing, genomic regions encompassing the mutation sites were amplified by PCR using the primers; sox9b tilling F 5′-GGGCTCCAACTCTTGGACGC-3′ and sox9b tilling R 5′-CAATAAAACCTCGTGCGCCG-3′. After removing dNTPs by ExoSAP-IT (USB), amplified fragments were used as templates and subjected to sequencing by PCR with the forward or reverse primers described above. Sample sequences are shown in Figure 1B. Genotyping of sox9b mutant alleles was also performed using a custom SNP genotyping assay (Applied Biosystems) and a real time PCR system (StepOne; Applied Biosystems). The following primers and TaqMan probes were used for the SNP genotyping assay:
Sox9bK16X
seq_sox9b_F 5′-CCTCGATCCATACCTGAAGATGACA-3′
seq_sox9b_R 5′-ACTGGGAGCGTCGGAGT-3′
seq_sox9b_VIC 5′-VIC-AAGAACAGGAGAAGTGTC-3′ (wild type)
seq_sox9b_FAM 5′-FAM-AAGAACAGGAGTAGTGTC-3′ (mutant)
Sox9bK136X
seq_sox9b_F 5′-CCAATACCCGCATTTGCACAAC-3′
seq_sox9b_R 5′-GGGCTTACCTCCAAAGTTTTCCA-3′
seq_sox9b_VIC 5′-VIC-AGCTCAGCAAAACT-3′ (wild type)
seq_sox9b_FAM 5′-FAM-CAGAGCTCAGCTAAACT-3′ (mutant)
Western blotting
Western blotting was performed as described previously [38]. Briefly, after SNP genotyping using the fins of sox9bK16X and sox9bK16X offspring at stage 39, each embryo (+/+, +/− and −/−) was crushed and boiled in SDS sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS). Following centrifugation, the supernatant was used for western blotting. Anti-Sox9b serum was raised in rabbits immunized with a sox9b peptide (RAQYDYSDHQNSANS). Anti-sox9b serum (rabbit, 1/500) and an alpha tubulin antibody (mouse, 1/4000; Sigma) were used as the primary antibodies. The secondary antibodies used included anti-rabbit HRP (1/2000; Zymed) and anti-mouse HRP (1/2000; Nacalai).
In situ hybridization, immunohistochemistry and histology
In situ hybridization (ISH), immunohistochemistry (IHC) and histology were performed as previously described [39]. Sox9bK16X and sox9bK16X siblings were collected and fixed with 4% PFA at stage 34, stage 39 and 10 dph. For ISH, a medaka GSDF clone (NCBI accession number; FS532259) was obtained from the NBRP Medaka cDNA library (http://www.shigen.nig.ac.jp/medaka/top/top.jsp) and used as the RNA probe. RNA probes for DMRT1 and aromatase were prepared as described previously [20], [26]. Zebrafish sox9a and sox9b probes were gifts from Dr. C. Chung and were prepared as described [22]. Genetic sex and sox9b genotypes were determined after ISH. Transverse plastic sections (4 µm) were also prepared as described [39]. For IHC, anti-GFP (1/100, rat; Nacalai or 1/100, mouse; Clontech), anti-DsRed (1/100, rabbit; Clontech), anti-OLVAS (1/100, rat) [38], anti-cleaved caspase3 (1/100, rabbit; Cell Signaling Technology) and anti-laminin (1/100, rabbit; SIGMA) were used as the primary antibodies followed by incubation with Alexa 488, 568 or 647 coupled secondary antibodies (1/100, goat; Molecular Probes).
EdU incorporation experiments
Germ cell proliferation was assessed using the Click-iT EdU labeling kit (Invitrogen). Embryos of sox9bK16X and sox9bK16X offspring at stage 39 were exposed to 500 µM EdU/BSS (Balanced Salt Solution) for 1 h and then fixed in 4% PFA. Detection of EdU was performed according to the manufacturer's instructions. After genotyping, IHC using anti-OLVAS (rat, 1/100) and anti-rat Alexa 488 (1/100; Molecular Probes) was performed. Embryos were counterstained with DAPI and EdU positive germ cells/all germ cells ratios were determined in both the wild type and mutants.
Generation of chimeric medaka
Medaka embryos from wild-type sox9b-DsRed and the sox9bK16X (+/−)/sox9b-EGFP incross were used as the host and donor, respectively. To mark the donor cells, 1% fluorescent dextran (Fluroruby; Molecular Probes) was microinjected into donor embryos at the one cell stage. Donor and host embryos at the mid-blastula stage were dechorionated in a solution containing hatching enzyme (obtained from NBRP medaka) for 30 min. Pipettes for transplantation were prepared by pulling 1 mm glass capillary tubes using a PC-10 puller (Narishige). Using a pipette installed on an oil-driven manipulator (CellTram Vario; Eppendorf), the labeled donor cells were transferred into the dechorionated mid-blastula stage host embryos on an 0.8% agar plate filled with 0.9% BSS (balanced salt solution). One day after transplantation, donor embryos were genotyped to distinguish +/+, +/− or −/−. Chimeric embryos were incubated up to stage 39 and fixed in 4% PFA. IHC was performed using anti-GFP (rat, 1/100; Nacalai), anti-DsRed (rabbit, 1/100; Clontech) and anti-OLVAS (rat, 1/100) antibodies.
Sox9b rescue experiments
The sox9b coding sequence lacking its stop codon (Sox9b CDS) was inserted into the SalI site of the pBLSK+ PTV1-2A-mCherry vector [40], (a gift of Dr. Hibi; Nagoya University), upstream of the 2A peptide. The resulting sox9b CDS- PTV1-2A -mCherry DNA fragment was then inserted by homologous recombination into a previously described BAC clone containing the entire sox9b gene [20], [37]. This modified BAC was then injected into fertilized eggs of the OKcab strain and two independent stable lines were obtained. One line was used for rescue experiments by crossing with sox9b mutants. The Sox9b transgene copy numbers were determined by qPCR with THUNDERBIRD SYBR qPCR Mix (TOYOBO) using primers for sox9b and dmy (one copy control). The following primers were used for qPCR;
q-sox9b-F, 5′-TTCCTTACGCACGATCCTCA-3′;
q-sox9b-R, 5′-TTCGATCTTTCACTGGTTTGC-3′;
q-dmy-F, 5′-CTCCGGTAAATTGACGCACA-3′;
q-dmy-R, 5′-GTCTGACTTTCCGGTCAAAGG-3′.
RT-PCR analysis of heterozygous mutant gonads
RT-PCR was performed as previously described [38]. Briefly, total RNA from the ovaries or testes of wild type or sox9b heterozygous mutants was isolated by ISOGEN (Nippon Gene). After removing genomic DNA by treatment with DNase I (Ambion), single stranded cDNA was synthesized from 300 ng total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen). The PCR reactions were performed using the primers described above [31].
qRT-PCR analysis of ECM and MMP genes
Total RNA was prepared from wild-type or sox9b mutant embryos, adult testes and ovaries using ISOGEN (Nippon Gene) or RNAqueous (Ambion). After removal of the genomic DNA by treatment with DNase I (Ambion), cDNA was prepared from a 300 ng aliquot of total RNA using SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) and used for subsequent qPCR analysis. qPCR. was performed using the Thunderbird SYBR qPCR mix (Toyobo) and StepOne (ABI) and the primers col2a1a F, 5′- GGCAACAGCCGCTTTACTTA -3′ and col2a1a R, 5′- AATGTCCACAATGGGCAAAC -3′; col2a1b F, 5′- GGTAACAGCCGCTTCACCTA -3′ and col2a1b R, 5′- AATGTCCATGGGAGCAATGT -3′; laminin α2 F, 5′- CCCAATCTACGTGGGAGGAT -3′ and laminin α2 R, 5′- GTCTTTGACGCCTTGGTGAT -3′; MMP13 F, 5′- AGGTCGATGCTGCTGCTTAC -3′ and MMP13 R, 5′- GCATTCAAGGATGGAGTTGG -3′; MMP9 F, 5′- TTGACAAAGGCTACCCCAAG -3′ and MMP9 R, 5′- CCGCCAGTAGAATTGGTCAC -3′; EF1α F, 5′- CATGGTTGTGGAGCCTTTCT -3′ and EF1α R, 5′- CTTTCTCTGCAGCCTTGGTC -3′.
Supporting Information
Syntenic analysis and expression study of medaka sox9 genes. (A) Syntenic analysis was performed using Ensembl genome browsers among mouse, chick, frog (Xenous tropicalis), zebrafish, stickleback and medaka. (B) Medaka sox9a (right) and sox9b (left) expression in XY gonads at stage 39. Asterisks indicate germ cells. Scale bars, 20 µm.
(TIF)
Zebrafish sox9a and sox9b expressions in adult ovaries and testes. (A–C) Sox9a expression in zebrafish adult ovaries (A and B) and a testis (C). B is a higher magnification view of the inset in A. Note that sox9a was expressed in the some parts of somatic cells surrounding small germ cells in the adult ovary. (D and E) Sox9b expression in a ovary and a testis. Sox9b is detected only in oocytes but not in testis. Signals are indicated as arrows. Scale bars, 50 µm.
(TIF)
Phenotypes of adult heterozygous mutant medaka. (A–C) Fin shapes (left) and transverse sections of adult gonads (right) in wild-type (A), sox9bK16X +/− (B) and sox9bK136X +/− (C) medaka. Wild-type XY medaka display a jagged dorsal fin and a sharp anal fin, which is typical male secondary sex characteristics. Round-shaped dorsal and anal fins and a developed urinogenital papilla (arrows) are characteristic of wild-type XX medaka. Alleles (K16X or K136X), genetic sex (XY or XX) and phenotypic sex (male or female) are indicated on the left of each panel (B and C). Some XX heterozygous mutants showed female to male sex reversal for both secondary sex characteristics and gonad morphology (middle panels in B and C). (D) A representative image of a germ cell-deficient gonad in an XX heterozygous medaka mutant. This mutant exhibited male secondary sex characteristics. (E) Expression of several sex-related genes assessed by RT-PCR in wild-type and heterozygous mutant gonads. The gene expression patterns in the gonads of XX male heterozygous mutants are consistent with those of the wild-type XY gonads. (F) The sex of the medaka is determined by the presence or absence of the Y chromosome. However sex differentiation requires proper homeostasis of the germ cells. Germ cell-deficient medaka exhibit female to male sex reversal of secondary sex characteristics independently of the genetic sex. Fewer germ cells are inclined to produce a male phenotype whereas hypertrophic germ cells, as in the hotei mutant, cause a male to female sex-reversal phenotype. Female to male sex reversal in heterozygous sox9b mutants is explained by the secondary effects of a reduced number of germ cells but not by the direct effects of sox9b-expressing cell impairment. Scale bar, 500 µm.
(TIF)
Ventral images of medaka wild-type and mutant gonads at different stages. (A–C) Ventral views of medaka gonads at the stage of gonadal primodium, stage 34 (A), the stage of female-specific increase of germ cells, stage 39 (B) and the stage of apparent sexual dimorphism of gonads, 10 dph (C). The germ cells and nuclei were immunostained with OLVAS (purple) and DAPI (green), respectively. Images from wild-type (upper), heterozygous (middle) and homozygous (lower) sox9bK16X medaka are shown. Scale bar, 20 µm (A) and 50 µm (B and C). n, number of gonads examined.
(TIF)
Acknowledgments
We thank Y. Ichikawa and C. Kinoshita for fish maintenance, B. Chung for kindly providing the zebrafish sox9a and sox9b probes, and M. Hibi for generously providing the 2A peptide vector. We are grateful to NBRP medaka for supplying the GSDF cDNA clone and hatching enzyme. We also thank S. Takada for providing us zebrafish ovaries and testes.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas (21116509 and 22132007), for Young Scientists (B) (21770072) and for Scientific Research on Priority Areas (B) (21370101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16:694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Jakob S, Lovell-Badge R. Sex determination and the control of Sox9 expression in mammals. FEBS J. 2011;278:1002–1009. doi: 10.1111/j.1742-4658.2011.08029.x. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama H, Lefebvre V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J Bone Miner Metab. 2011;29:390–395. doi: 10.1007/s00774-011-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal VPI, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 6.Wagner T, Wirth J, Meyer J, Zabel B, Held M, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the Sry-related gene Sox9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 7.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 8.Huang B, Wang SB, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Foster JW, Dominguezsteglich M, Guioli S, Kwok C, Weller P, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an Sry-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 10.Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 11.Western PS, Harry JL, Graves JAM, Sinclair AH. Temperature-dependent sex determination: Upregulation of SOX9 expression after commitment to male development. Dev Dyn. 1999;214:171–177. doi: 10.1002/(SICI)1097-0177(199903)214:3<171::AID-AJA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Barske LA, Capel B. Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev Biol. 2010;341:305–314. doi: 10.1016/j.ydbio.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M. Interaction of Germ Cells and Gonadal Somatic Cells During Gonadal Formation. In: Naruse K, Tanaka M, Takeda H, editors. Medaka: A model for Organogenesis, Human Disease, and Evolution. Heidelberg: Springer; 2011. pp. 219–227. [Google Scholar]
- 14.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 15.Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohara Y, Kasahara M, Naruse K, Sasaki S, Nakatani Y, et al. The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447:714–719. doi: 10.1038/nature05846. [DOI] [PubMed] [Google Scholar]
- 17.Klüver N, Kondo M, Herpin A, Mitani H, Schartl M. Divergent expression patterns of Sox9 duplicates in teleosts indicate a lineage specific subfunctionalization. Dev Genes Evol. 2005;215:297–305. doi: 10.1007/s00427-005-0477-x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto M, Suzuki A, Matsuda M, Nagahama Y, Shibata N. Testicular type Sox9 is not involved in sex determination but might be in the development of testicular structures in the medaka, Oryzias latipes. Biochem Biophys Res Commun. 2005;333:729–736. doi: 10.1016/j.bbrc.2005.05.158. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi H, Kobayashi T, Tanaka M, Nagahama Y, Wakamatsu Y, et al. Sox9 in a teleost fish, medaka (Oryzias latipes): evidence for diversified function of Sox9 in gonad differentiation. Mol Reprod Dev. 2002;63:5–16. doi: 10.1002/mrd.10169. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Aoki Y, Saito D, Kuroki Y, Fujiyama A, et al. Sox9b/sox9a2-EGFP transgenic medaka reveals the morphological reorganization of the gonads and a common precursor of both the female and male supporting cells. Mol Reprod Dev. 2008;75:472–476. doi: 10.1002/mrd.20764. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328:1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 22.Chiang EF, Pai CI, Wyatt M, Yan YL, Postlethwait J, et al. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev Biol. 2001;231:149–163. doi: 10.1006/dbio.2000.0129. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Mari A, Yan YL, Bremiller RA, Wilson C, Canestro C, et al. Characterization and expression pattern of zebrafish Anti-Müllerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr Patterns. 2005;5:655–667. doi: 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi Y, Takeda S, Furutani-Seiki M, Kamei Y, Todo T, et al. Generation of medaka gene knockout models by target-selected mutagenesis. Genome Biol. 2006;7:R116. doi: 10.1186/gb-2006-7-12-r116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata Y, Paul-Prasanth B, Suzuki A, Usami T, Nakamoto M, et al. Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr Patterns. 2010;10:283–289. doi: 10.1016/j.gep.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura S, Kurokawa H, Asakawa S, Shimizu N, Tanaka M. Two distinct types of theca cells in the medaka gonad: germ cell-dependent maintenance of cyp19a1-expressing theca cells. Dev Dyn. 2009;238:2652–2657. doi: 10.1002/dvdy.22068. [DOI] [PubMed] [Google Scholar]
- 27.Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, et al. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327:301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori A, Nagahama Y, Egami N. Development of the tissue architecture in the gonads of the medaka Oryzias latipes. Zool Sci. 1985;2:695–706. [Google Scholar]
- 29.Saito D, Morinaga C, Aoki Y, Nakamura S, Mitani H, et al. Proliferation of germ cells during gonadal sex differentiation in medaka: Insights from germ cell-depleted mutant zenzai. Dev Biol. 2007;310:280–290. doi: 10.1016/j.ydbio.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Matoba S, Hiramatsu R, Kanai-Azuma M, Tsunekawa N, Harikae K, et al. Establishment of testis-specific SOX9 activation requires high-glucose metabolism in mouse sex differentiation. Dev Biol. 2008;324:76–87. doi: 10.1016/j.ydbio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Kurokawa H, Saito D, Nakamura S, Katoh-Fukui Y, Ohta K, et al. Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci USA. 2007;104:16958–16963. doi: 10.1073/pnas.0609932104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka M, Saito D, Morinaga C, Kurokawa H. Cross talk between germ cells and gonadal somatic cells is critical for sex differentiation of the gonads in the teleost fish, medaka (Oryzias latipes). Dev Growth Differ. 2008;50:273–278. doi: 10.1111/j.1440-169X.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 33.Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, et al. The hotei mutation of medaka in the anti-Müllerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci USA. 2007;104:9691–9696. doi: 10.1073/pnas.0611379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 35.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 36.Bagheri-Fam S, Sinclair AH, Koopman P, Harley VR. Conserved regulatory modules in the Sox9 testis-specific enhancer predict roles for SOX, TCF/LEF, Forkhead, DMRT, and GATA proteins in vertebrate sex determination. Int J Biochem Cell Biol. 2010;42:472–477. doi: 10.1016/j.biocel.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura S, Saito D, Tanaka M. Generation of transgenic medaka using modified bacterial artificial chromosome. Dev Growth Differ. 2008;50:415–419. doi: 10.1111/j.1440-169X.2008.01027.x. [DOI] [PubMed] [Google Scholar]
- 38.Aoki Y, Nagao I, Saito D, Ebe Y, Kinjo M, et al. Temporal and spatial localization of three germline-specific proteins in medaka. Dev Dyn. 2008;237:800–807. doi: 10.1002/dvdy.21448. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura S, Kobayashi D, Aoki Y, Yokoi H, Ebe Y, et al. Identification and lineage tracing of two populations of somatic gonadal precursors in medaka embryos. Dev Biol. 2006;295:678–688. doi: 10.1016/j.ydbio.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 40.Nojima H, Rothhamel S, Shimizu T, Kim CH, Yonemura S, et al. Syntabulin, a motor protein linker, controls dorsal determination. Development. 2010;137:923–933. doi: 10.1242/dev.046425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Syntenic analysis and expression study of medaka sox9 genes. (A) Syntenic analysis was performed using Ensembl genome browsers among mouse, chick, frog (Xenous tropicalis), zebrafish, stickleback and medaka. (B) Medaka sox9a (right) and sox9b (left) expression in XY gonads at stage 39. Asterisks indicate germ cells. Scale bars, 20 µm.
(TIF)
Zebrafish sox9a and sox9b expressions in adult ovaries and testes. (A–C) Sox9a expression in zebrafish adult ovaries (A and B) and a testis (C). B is a higher magnification view of the inset in A. Note that sox9a was expressed in the some parts of somatic cells surrounding small germ cells in the adult ovary. (D and E) Sox9b expression in a ovary and a testis. Sox9b is detected only in oocytes but not in testis. Signals are indicated as arrows. Scale bars, 50 µm.
(TIF)
Phenotypes of adult heterozygous mutant medaka. (A–C) Fin shapes (left) and transverse sections of adult gonads (right) in wild-type (A), sox9bK16X +/− (B) and sox9bK136X +/− (C) medaka. Wild-type XY medaka display a jagged dorsal fin and a sharp anal fin, which is typical male secondary sex characteristics. Round-shaped dorsal and anal fins and a developed urinogenital papilla (arrows) are characteristic of wild-type XX medaka. Alleles (K16X or K136X), genetic sex (XY or XX) and phenotypic sex (male or female) are indicated on the left of each panel (B and C). Some XX heterozygous mutants showed female to male sex reversal for both secondary sex characteristics and gonad morphology (middle panels in B and C). (D) A representative image of a germ cell-deficient gonad in an XX heterozygous medaka mutant. This mutant exhibited male secondary sex characteristics. (E) Expression of several sex-related genes assessed by RT-PCR in wild-type and heterozygous mutant gonads. The gene expression patterns in the gonads of XX male heterozygous mutants are consistent with those of the wild-type XY gonads. (F) The sex of the medaka is determined by the presence or absence of the Y chromosome. However sex differentiation requires proper homeostasis of the germ cells. Germ cell-deficient medaka exhibit female to male sex reversal of secondary sex characteristics independently of the genetic sex. Fewer germ cells are inclined to produce a male phenotype whereas hypertrophic germ cells, as in the hotei mutant, cause a male to female sex-reversal phenotype. Female to male sex reversal in heterozygous sox9b mutants is explained by the secondary effects of a reduced number of germ cells but not by the direct effects of sox9b-expressing cell impairment. Scale bar, 500 µm.
(TIF)
Ventral images of medaka wild-type and mutant gonads at different stages. (A–C) Ventral views of medaka gonads at the stage of gonadal primodium, stage 34 (A), the stage of female-specific increase of germ cells, stage 39 (B) and the stage of apparent sexual dimorphism of gonads, 10 dph (C). The germ cells and nuclei were immunostained with OLVAS (purple) and DAPI (green), respectively. Images from wild-type (upper), heterozygous (middle) and homozygous (lower) sox9bK16X medaka are shown. Scale bar, 20 µm (A) and 50 µm (B and C). n, number of gonads examined.
(TIF)