Abstract
Survival of bacterial infection is the result of complex host-pathogen interactions. An often-overlooked aspect of these interactions is the circadian state of the host. Previously, we demonstrated that Drosophila mutants lacking the circadian regulatory proteins Timeless (Tim) and Period (Per) are sensitive to infection by S. pneumoniae. Sensitivity to infection can be mediated either by changes in resistance (control of microbial load) or tolerance (endurance of the pathogenic effects of infection). Here we show that Tim regulates resistance against both S. pneumoniae and S. marcescens. We set out to characterize and identify the underlying mechanism of resistance that is circadian-regulated. Using S. pneumoniae, we found that resistance oscillates daily in adult wild-type flies and that these oscillations are absent in Tim mutants. Drosophila have at least three main resistance mechanisms to kill high levels of bacteria in their hemolymph: melanization, antimicrobial peptides, and phagocytosis. We found that melanization is not circadian-regulated. We further found that basal levels of AMP gene expression exhibit time-of-day oscillations but that these are Tim-independent; moreover, infection-induced AMP gene expression is not circadian-regulated. We then show that phagocytosis is circadian-regulated. Wild-type flies exhibit up-regulated phagocytic activity at night; Tim mutants have normal phagocytic activity during the day but lack this night-time peak. Tim appears to regulate an upstream event in phagocytosis, such as bacterial recognition or activation of phagocytic hemocytes. Interestingly, inhibition of phagocytosis in wild type flies results in survival kinetics similar to Tim mutants after infection with S. pneumoniae. Taken together, these results suggest that loss of circadian oscillation of a specific immune function (phagocytosis) can have significant effects on long-term survival of infection.
Author Summary
Circadian rhythms, or daily oscillations in physiological functions, are nearly ubiquitous among animals. Though immune parameters are known to vary with time of day, circadian regulation of the immune system is not well understood. Studies of circadian biology and innate immunity are well established in the genetically tractable model organism, the fruit fly. We use this model to investigate the mechanisms underlying circadian-regulated immunity. We and others have found that flies infected at different times of day exhibit different mean survival times. There are three main components of fly immune defense against bacterial pathogens: antimicrobial peptide synthesis, reactive oxygen species generation, and phagocytosis, or the ingestion of microbes by host immune cells. Of these three, we found that only the phagocytic activity of host immune cells is circadian-regulated and peaks at night, during the rest phase. This night-time peak of phagocytosis correlates with the time of day of maximal resistance to infection by the bacteria Streptococcus pneumoniae. Mutants that lack circadian regulation also lack this night-time peak in phagocytosis. Inhibition of phagocytosis sensitized wild type flies to infection but did not further sensitize circadian mutants. These results suggest that this night-time peak in immune system function has significant effects on survival of infection.
Introduction
Survival of bacterial infection is the result of complex host-pathogen interactions. An often-overlooked aspect of these interactions is the circadian state of the host. Most metazoans undergo daily, dynamic changes in their physiology that are regulated by well-characterized circadian machinery. At its core, the circadian clock is composed of four transcriptional regulators paired as two distinct heterodimers. In Drosophila, Period and Timeless form one heterodimer and Clock and Cycle form the other. These two heterodimers engage in an autoregulatory negative feedback loop that causes circadian oscillations in the expression levels of target genes [1]. Microarray analyses of different vertebrate tissues, such as heart, liver, or spleen-derived macrophages, have shown that approximately 5–10% of total gene expression in each tissue is circadian-regulated [2], [3]. These oscillations in transcription are thought to underlie circadian changes in the organism's physiology and behavior.
Reflecting the pervasiveness of circadian regulation, the Drosophila circadian mutants Timeless (Tim) and Period (Per) have pleiotropic phenotypes. They exhibit loss of circadian rhythms in locomotor activity, eclosion, male courtship, and oxidative stress response [4], [5], [6]. These circadian mutants are also sensitive to infection by at least two bacterial pathogens and resistant to infection by another [7], [8]. The circadian-regulated mechanisms underlying these changes in immunity were not known.
Two types of mechanisms can affect survival after infection: resistance and tolerance [9]. Resistance mechanisms control microbial growth, while tolerance mechanisms allow the host to endure the pathogenic effects of infection, including the host's response to infection. In Drosophila, known resistance mechanisms to control microbial growth in the hemolymph include antimicrobial peptide synthesis, reactive oxygen species generation, and phagocytosis by immune cells. Previously, microarray analysis comparing wild type and circadian mutants suggested that expression of immune signaling molecules that induce resistance mechanisms such as Imd (Immune deficient) may be circadian-regulated [10], [11]. Here we test the hypothesis that circadian mutants are sensitive to infection due to changes in mechanisms of resistance and investigate specific resistance mechanisms for circadian regulation.
It has been recently shown that in mice, many immune cell functions, such as cytokine secretion by macrophages and natural killer (NK) cells, undergo oscillatory time-of-day variation [3], [12]. Phagocytosis by immune cells such as macrophages and neutrophils has been reported to change with time of day, though it is not clear if this oscillation is diurnal (due to photoperiod) or circadian (regulated by circadian proteins) [13], [14], [15], [16]. Phagocytosis of photoreceptors by pigment cells in the vertebrate retina has also been shown to be circadian-regulated [17]. Thus we further hypothesized that phagocytosis of bacteria by immune cells in Drosophila is also circadian-regulated and that decreased phagocytic activity may significantly contribute to the sensitivity of circadian mutants to certain types of bacterial infection.
Results/Discussion
Circadian immune phenotypes are pathogen-specific
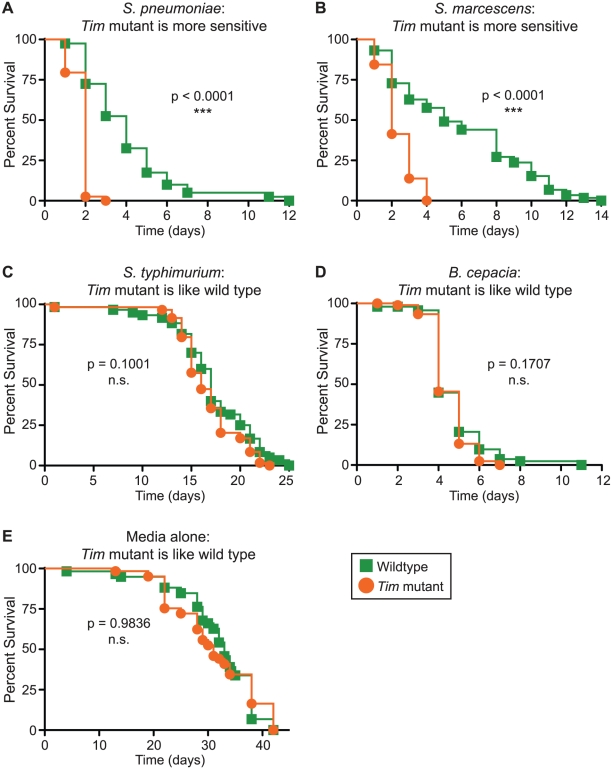
The fly immune response to infection is highly complex and pathogen-specific. Previously, we showed that Timeless (Tim) null mutants die more quickly than wild-type flies when infected with S. pneumoniae [7]. Another group showed that Tim mutants die less quickly than wild-type flies when infected with P. aeruginosa, suggesting a complex immune phenotype that is pathogen-specific [18]. To further understand the immune phenotype of Tim mutants, we infected Tim mutants with three other pathogenic bacteria (S. marcescens, B. cepacia, and S. typhimurium). We compared Tim mutant survival time with wild-type flies and confirmed a complex immune phenotype (Figure 1, A–D). Tim mutants died more quickly than wild-type flies when infected with S. marcescens and died with wild-type kinetics when infected with S. typhimurium and B. cepacia. Tim mutants showed no difference in sensitivity to wounding alone. Thus, these data suggest that Tim activity has different consequences for immunity against different pathogens.
Figure 1. Tim activity has significant consequences for immunity.
Shown here are Kaplan-Meier survival curves comparing Tim null mutants (orange circles) and wild-type control flies (green squares). Tim mutants die more quickly after infection with (A) S. pneumoniae (p<0.0001) or (B) S. marcescens (p<0.0001). Tim mutants die at the same rate as wild-type flies after infection with (C) B. cepacia (p = 0.1001), (D) S. typhimurium (p = 0.1707), or (E) medium alone (p = 0.9836). p-values were obtained by log-rank analysis.
Tim regulates resistance to specific bacterial infection
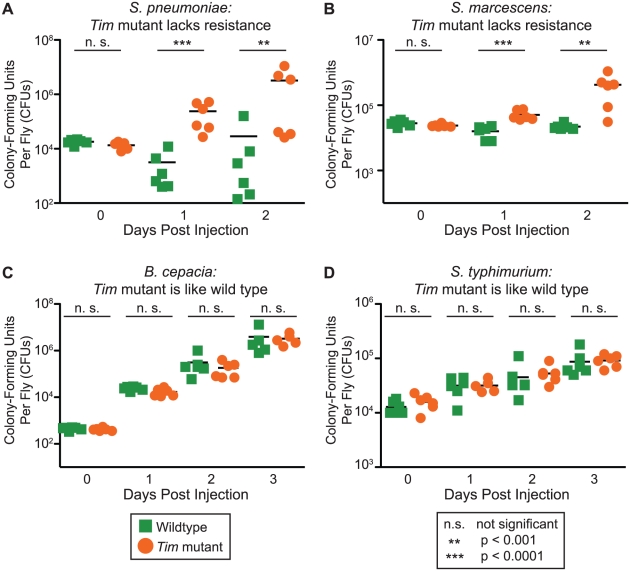
We next asked if Tim regulates mechanisms of resistance or tolerance against specific bacterial pathogens [19]. We functionally distinguished between these two types of immune defense by comparing the bacterial loads of Tim mutant and wild-type flies after infection with four different bacterial pathogens (Figure 2, A–D). We found that loss of Tim protein causes a loss of resistance against two pathogens. Tim mutants die faster than wild-type flies when infected with S. pneumoniae and S. marcescens and have high bacterial loads. This suggests that Tim mutants are less able to control microbial growth and lack resistance to these two pathogens. Tim mutants had no effect on survival or bacterial growth during infection with S. typhimurium and B. cepacia.
Figure 2. Tim regulates resistance to infection by S. pneumoniae and S. marcescens.
Mutants lacking resistance, or bacterial killing, have shorter survival times and greater bacterial loads than wild-type flies. Tim null mutants lack resistance to (A) S. pneumoniae and (B) S. marcescens. Tim mutants have bacterial loads similar to wild-type flies when infected with (C) B. cepacia and (D) S. typhimurium. p-values were obtained by two-tailed, unpaired t-test; all experiments were performed with day-time injections.
These results demonstrate that Tim regulates resistance mechanisms for specific bacterial pathogens. Different physiologies will be important for defense against each type of pathogen; thus specific microbes can be used to probe different aspects of the immune system. Circadian proteins like Tim are known to regulate many different types of behavior and up to 5–10% of the transcriptome of different tissues. If specific physiologies that contribute to the defense against certain pathogens are circadian-regulated in Drosophila, then Tim mutants will present immune phenotypes when infected with those pathogens. Thus we can use our knowledge about the defenses required for different pathogens to identify those that are circadian-regulated.
Light-induced degradation of Tim protein in wild-type flies causes sensitivity to S. pneumoniae
We chose to focus on Tim mutants and their dramatic defect in resistance against S. pneumoniae. We hypothesized that Tim mutants might lack resistance because of a developmental defect (such as malformation of immune tissues) or because of a direct effect of circadian disruption on immunity in the adult fly. To distinguish between these two possibilities, we disrupted Tim protein in genetically wild-type adult flies using constant light (Figure 3A). Blue light (∼380 to 475 nm) causes complete and rapid ubiquitin-mediated degradation of Tim protein [20]; normally, this allows the internal molecular clock to be synchronized with external light cues [4]. Flies reared in constant light exhibit complete loss of circadian rhythm [21]. Here we use constant exposure to light to induce a Tim-null phenocopy in adult wild-type flies, circumventing any developmental requirements for Tim protein. Wild-type flies were infected with S. pneumoniae and incubated either in darkness or constant light. We found that flies incubated after infection in constant light died faster than those incubated in darkness (p<0.0001). As a negative control, we treated Tim null mutants and found that they were not further sensitized by constant light, suggesting that Tim is epistatic to and therefore downstream of light treatment. These results are consistent with the hypothesis that Tim protein regulates resistance to S. pneumoniae in the adult fly and also demonstrate that environmental and genetic disruption of circadian regulation alters immunity.
Figure 3. Resistance against S. pneumonia correlates with Tim activity.
Shown here are Kaplan-Meier survival curves comparing wild-type flies and Tim mutants infected with S. pneumoniae and subjected to different light conditions as detailed in the schematic diagrams. (A) Light-induced degradation of Tim protein in wild-type flies results in increased sensitivity to S. pneumoniae. Infected wild-type flies incubated in constant light were more sensitive than wild-type flies incubated in darkness (p<0.0001). Tim mutants incubated in constant light died as rapidly as did Tim mutants incubated in darkness (p = 0.3404). (B) Wild-type flies were more sensitive to S. pneumoniae when infected at a time of day when Tim protein is low. Wild-type flies were less sensitive when infected 6 hours after lights off (DARK) than 6 hours after lights on, when Tim protein is minimally and maximally expressed, respectively (p<0.0001). Tim mutant sensitivity was not rescued by infection 6 hours after lights off (DARK) (p = 0.4134). (C) Light-induced sleep-wake rhythms (a startle response called “masking”) do not rescue Tim mutant sensitivity to infection. Tim mutant sensitivity was not rescued by incubation in cycling light:dark conditions compared to incubation in the dark. In fact, both Tim and wild-type flies were more sensitive to infection in cycling light:dark conditions than in the dark (p = 0.0125 and p<0.0001 respectively).
Wild-type flies are more sensitive to S. pneumoniae when infected at a time of day when Tim protein expression is low
If Tim protein positively regulates resistance in the adult fly and if Tim protein levels oscillate over the circadian day, we predicted that the resistance of wild-type flies should also oscillate over the circadian day. To test this, we infected two sets of flies, light-entrained in anti-phase with each other, at times that correspond to minimal and maximal Tim protein expression. Tim protein levels are lowest approximately 7 hours after lights turn on (Zeitgeber 07 or ZT07/day) and highest at approximately 7 hours after lights off (ZT19/night) [22]. Consistent with minimal and maximal Tim expression, wild-type flies infected during the day (ZT07) died significantly faster than those infected at night (ZT19) (Figure 3B). Tim null mutants showed no difference in survival time based on time of infection. These results suggest that increased Tim expression at night positively regulates a mechanism(s) of resistance required to fight infection by S. pneumoniae.
Loss of resistance seen in Tim mutants cannot be rescued by a sleep/wake cycle induced by environmental light cues
“Masking” is a phenomenon in which flies lacking endogenous circadian rhythm exhibit circadian-like, light-stimulated locomotor activity and sleep-wake patterns due to external light cues (startle response) [23]. Thus Tim mutants in masking light/dark conditions exhibit a locomotor response to light stimulus even in the absence of Tim protein. We set out to ask if masking would restore the immune function of Tim mutants back to that of wild-type flies. To test this, we infected wild-type flies and Tim mutants with S. pneumoniae and incubated them either in the dark (relying on endogenous circadian rhythm) or in circadian light-dark conditions (masking) (Figure 3C). Masking conditions did not rescue the immune phenotype of Tim mutants infected with S. pneumoniae, suggesting that this phenotype is not rescued by light-stimulated locomotor activity but requires the normal expression of Tim protein.
The melanization response is not circadian-regulated
We next examined the three main Drosophila immune responses for circadian regulation: melanization (generation of reactive oxygen species, or ROS), anti-microbial peptide (AMP) synthesis, and phagocytosis by hemocytes. We tested each immune response for time-of-day differences in wild type and Tim mutants and found evidence for circadian regulation of phagocytosis but not melanization or AMP gene induction. This is consistent with our survival data for S. pneumonia, as phagocytic hemocytes are crucial to control the growth of S. pneumonia in Drosophila [24].
We first tested if the melanization response is circadian-regulated. Insects react to injury and some bacterial infections with an enzymatic cascade that generates toxic reactive oxygen species (ROS) and results in the deposition of melanin, visible through the cuticle as dark black spots [19]. If melanization is circadian-regulated, we would expect to see a difference in melanized spot formation between wild-type flies and Tim mutants as well as between wild-type flies at different times of day, but not between Tim mutants at different times of day. We assayed melanization in wild type and Tim mutants infected with S. pneumoniae, but did not observe a systemic melanization response, consistent with published reports [24], [25]. We then assayed the melanization response of flies infected with L. monocytogenes. Flies exhibit a strong melanization response after L. monocytogenes infection and require melanization enzymes for resistance against this pathogen [19]. We found no significant difference in melanized spot formation between wild-type flies and Tim mutants or between wild-type flies at different times of day (Figure 4A). These data suggest that melanization is not regulated by Tim.
Figure 4. Tim protein does not regulate melanization or AMP gene expression.
(A) Wild type and Tim mutants injected with L. monocytogenes at ZT07 (DAY) or ZT19 (NIGHT) have similar levels of melanization. p-values for pair-wise comparisons were not significant: WT (DAY) vs. Tim (DAY), p = 0.3551; WT (NIGHT) vs. Tim (NIGHT), p = 1.000; WT (DAY) vs. WT (NIGHT), p = 0.4031; Tim (DAY) vs. Tim (NIGHT), p = 0.8717. p-values were obtained by unpaired, two-tailed t-test; n = 18–20 flies per genotype per condition. (B) Basal (uninduced) levels of Drosomycin expression were increased at ZT11 relative to ZT05, ZT17, ZT23 in both wild type and Tim mutants, suggesting that this change in expression is not mediated by Tim. (C) Infection-induced levels of Drosomycin expression were not significantly different after injection of wild type and Tim mutants with M. luteus at ZT05 (DAY) or ZT 17 (NIGHT). Every pair-wise comparison resulted in p-values greater than 0.05 (not significant) by Mann-Whitney test. (D) Infection-induced levels of Diptericin expression were not significantly different after injection of wild type and Tim mutants with E. coli at ZT05 (DAY) or ZT17 (NIGHT). Every pair-wise comparison resulted in p-values greater than 0.05 (not significant) by Mann-Whitney test.
AMP gene expression induced by septic injury is not circadian-regulated
We next tested if antimicrobial peptide (AMP) gene expression is circadian-regulated. Expression of AMP genes in response to infection is the best characterized immune response of Drosophila; these small peptides secreted by the fat body are thought to control microbial growth by mechanisms such as disrupting bacterial membranes. Previously, others had found that expression of several immunity signaling genes controlling AMP gene expression such as Rel and Imd is circadian-regulated [8], [10], [11], [18]. Here we measured the basal (uninduced) and pathogen-induced expression of two representative AMP genes, Drosomycin (Drs) and Diptericin (Dpt), which are widely used as reporters for induction of the Toll and imd signaling pathways, respectively [26].
We first compared the basal (uninduced) expression of Drs and Dpt in wild type and Tim mutants at four time points around the circadian cycle using qRT-PCR: ZT05, ZT11, ZT17, and ZT23. We found that both wild type and Tim mutants exhibited higher basal expression of the Toll-regulated AMP Drs at ZT11 than other time points (Figure 4B). This result suggests that basal expression of Drs varies with time of day but that this difference in expression is not mediated by Tim protein. This time-of-day difference in basal expression is statistically significant but very small relative to infection-induced expression levels (Figure 4 C, D). Basal expression of Dpt was not significantly different at any time of day in either wild type or Tim mutants (Figure S1A).
We next measured Drs and Dpt expression after S. pneumoniae infection in wild type and Tim mutants at ZT05 and ZT17, approximately when wild-type flies exhibit differences in survival and Tim mutants do not (see Figure 3). If AMP induction were circadian-regulated, we would expect to see a difference in AMP expression between wild-type flies at ZT05 and ZT17 and between wild type and Tim mutants at ZT17 but not between Tim mutants at ZT05 and ZT17. We found that S. pneumoniae-induced expression of Drs and Dpt was not significantly different at either time of day in either wild type or Tim mutants (Figure S1B, C).
S. pneumoniae is not typically used to induce AMP expression and did not cause the dramatic induction of AMP gene expression seen with other bacteria relative to media alone [27]. Thus we next examined circadian-regulation of AMP expression in response to two bacteria, M. luteus and E. coli, that have been most extensively used to probe AMP induction and more generally, to quantify signaling through the Toll pathway (activated by M. luteus) or the Imd pathway (activated by E. coli) [26]. We examined Drs and Dpt induction in wild-type flies and Tim mutants infected at either ZT07 or ZT19 with either M. luteus or E. coli and collected six hours later for qRT-PCR analysis. Again, we found that infection-induced expression levels of Drs and Dpt were not significantly different at these time points in wild type and Tim mutants (Figure 4C,D). Taken together, these data suggest that, for both Drs and Dpt, basal levels of expression and short-term expression induced by these bacteria (S. pneumonia, M. luteus, E. coli) are not circadian-regulated.
Tim regulates phagocytosis of pathogens by immune cells
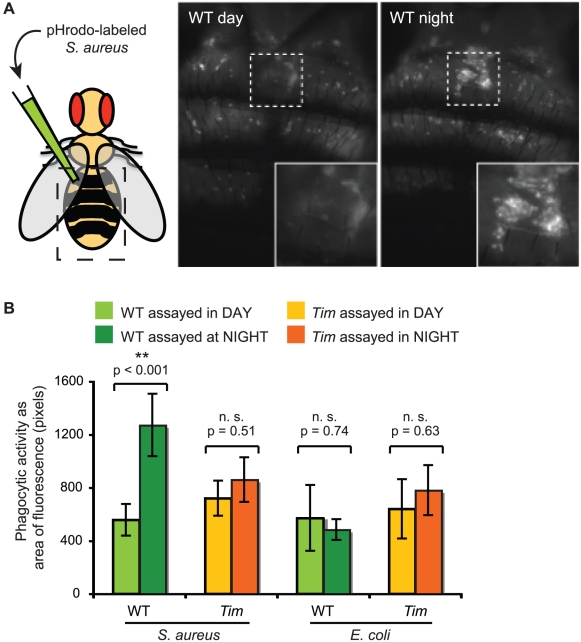
Finally, we tested for circadian regulation of the Drosophila immune response of phagocytosis—the physical engulfment and destruction of bacteria by specialized immune cells. Phagocytosis is typically assayed by measuring the internalization of fluorescently-labeled bacteria such as S. aureus or E. coli. Here, we measured phagocytic activity at different times of day in both wild-type flies and Tim mutants. If phagocytosis is regulated by Tim protein, we predict that phagocytic activity will be high at night and low during the day in wild-type flies but will not vary with time of day in Tim mutants. We assayed phagocytic activity in adult flies by injecting dead S. aureus labeled with pHrodo, a pH-sensitive rhodamine dye that fluoresces in acidic environments. When pHrodo-labeled bacteria are phagocytosed and processed into acidic lysosomes, the phagocytic cell emits red fluorescence and can be imaged through the dorsal surface of live, intact flies. We found that phagocytic activity was significantly higher at ZT19 (night) than ZT07 (day) in wild-type flies. Phagocytic activity did not vary with the circadian cycle in Tim mutants (Figure 5A, B). Thus, phagocytic activity oscillates with circadian rhythm in vivo in wild-type flies but not in Tim mutants, consistent with the hypothesis that Tim protein up-regulates phagocytosis of S. aureus at night. If phagocytosis is required to clear S. aureus, these data are consistent with previous studies demonstrating that wild-type flies exhibit higher rates of survival when infected with S. aureus at night than when infected during the day [18].
Figure 5. Tim protein regulates an early stage of phagocytosis of bacteria by hemocytes.
(A) Wild-type flies in the night phase of the circadian cycle have more phagocytic activity than wild-type flies in the day phase. Shown here are images of the dorsal surface of representative flies after injection of dead S. aureus labeled with the fluorophore, pHrodo, which emits fluorescence in acidic environments such as the lysosomes. Hemocytes that have phagocytosed these bacteria become fluorescent. Dashed lines indicate the region enlarged in the inset. (B) Phagocytic activity for wild type and Tim mutant flies was quantified by measuring areas of fluorescence. Wild-type flies exhibited significant circadian differences in phagocytosis of S. aureus (p<0.001) but not E. coli (p = 0.74). This circadian difference is not present in Tim mutant flies with either S. aureus (p = 0.51) or E. coli (p = 0.63). p-values were obtained by unpaired, two-tailed t-test.
To test if circadian-regulated phagocytosis is bacteria-specific, we then assayed phagocytic activity with a different type of bacteria. We injected wild type and Tim mutants with pHrodo-labeled E. coli at ZT07 (day) and ZT19 (night) (Figure 5B). In contrast to injections of S. aureus, wild-type flies did not exhibit a circadian difference in phagocytosis of E. coli. Tim mutants also exhibited no circadian difference in phagocytosis of E. coli. These results suggest that Tim regulates bacteria-specific phagocytosis by immune cells.
Phagocytosis can be generally described as three steps: receptor-mediated substrate recognition and binding, particle engulfment, and phagosome maturation. Fluorescently-labeled S. aureus and E. coli have often been used in phagocytosis assays to determine whether specific phagocytic components discriminate between different types of bacteria. Many cellular steps of phagocytosis subsequent to substrate recognition do not appear to be bacteria-specific. For example, inhibition of β-COP, thought to be involved in phagosome maturation, leads to decreased phagocytosis of both S. aureus and E. coli [28]. In contrast, some molecular components responsible for phagocyte recognition of bacteria are bacteria-specific. For example, inhibition of the phagocytic receptor PGRP-LC leads to decreased binding and phagocytosis of E. coli but not S. aureus [28]. Another phagocytic receptor, PGRP-Sc1a, mediates phagocytosis of S. aureus but not E. coli [29]. Thus our finding that Tim up-regulates phagocytosis of S. aureus but not E. coli suggests that Tim regulates a bacteria-specific step of this process such as substrate recognition or binding.
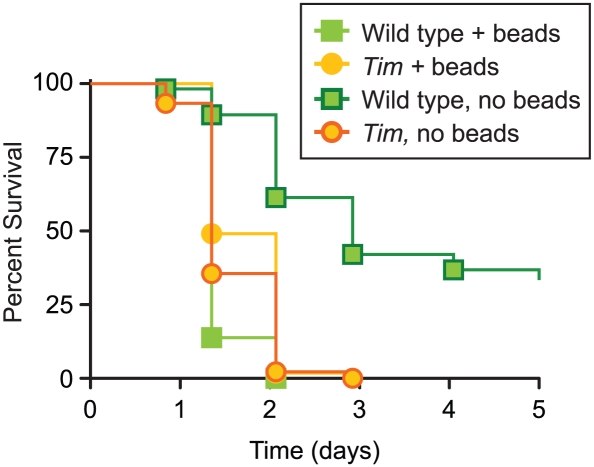
Bead inhibition of phagocytosis eliminates the survival difference between wild type and Tim mutants after infection with S. pneumoniae
Because phagocytosis is crucial in defense against S. pneumoniae [24], these results suggest that differences in phagocytic activity might contribute to the difference in survival after S. pneumoniae infection between wild type and Tim mutants. Thus we tested if inhibition of phagocytic activity would decrease these survival differences. Phagocytosis can be inhibited in vivo by injection of polystyrene beads; phagocytes engulf these beads and are unable to phagocytose subsequent injections of fluorescently-labeled bacteria [30]. Thus we compared the survival kinetics of wild type and Tim mutants infected with S. pneumoniae with or without bead pre-injection (Figure 6). As described above, Tim mutants are highly sensitive to S. pneumoniae infection relative to wild type flies. Consistent with published results, we found that bead pre-injection increased sensitivity of wild type flies. Bead pre-injection of wild type flies decreased survival rate to those similar to Tim mutants, suggesting that inhibition of phagocytosis is sufficient to recapitulate Tim mutant sensitivity. Consistent with this, we also found that bead pre-injection did not increase the sensitivity of Tim mutants to S. pneumoniae, suggesting that phagocytosis is already impaired in these flies. Interestingly, in some experiments, bead-inhibited wild type flies appear to be more sensitive than bead-inhibited Tim mutants, suggesting that the presence of Tim protein in the absence of phagocytosis may have a negative effect on survival. Taken together, these results suggest that Tim-mediated phagocytosis plays an important role in survival of S. pneumoniae and that loss of Tim is equivalent to total inhibition of phagocytosis, though Tim mutants are still able to phagocytose. Inhibition of phagocytosis is thought to inhibit phagocytes' ability to signal the presence of infection; perhaps Tim also plays a role in these downstream signaling events. Importantly, our results do not rule out the possibility of multiple roles for Tim protein in immunity against bacterial infection, including S. pneumoniae infection.
Figure 6. Bead inhibition of phagocytosis in wild type flies eliminates the difference in survival kinetics with Tim mutants after S. pneumoniae infection.
Shown here are Kaplan-Meier survival curves comparing wild-type flies and Tim mutants infected with S. pneumoniae with and without pre-injection of beads to inhibit phagocytosis. Infected wild-type flies pre-injected with beads were more sensitive than wild-type flies without pre-injection (p<0.0001) and had similar survival kinetics as Tim mutants (p = 0.0502). Infected Tim mutants had identical survival kinetics with or without bead pre-injection (p = 0.1327). In this experiment infected, bead-injected wild-type flies were more sensitive than infected, bead-injected Tim mutants (p<0.0001); however, this result varied from experiment to experiment.
Circadian regulation of immunity has long been reported anecdotally in humans and other animals. Here we show that Tim protein has complex, pathogen-specific effects on immunity, regulating resistance to infection in Drosophila. Resistance against S. pneumoniae is upregulated by Tim in the adult fly and is not simply a diurnal response (i.e., activated by light/dark cycles even in the absence of circadian regulatory protein function). We also identified phagocytosis as a specific circadian-regulated immune mechanism. This is the first demonstration of a link between Tim protein and the cellular immune response in vivo. Our data suggests that circadian regulation affects phagocytic immune cells at an early stage of specific pathogen recognition and makes a significant contribution to survival of infection.
This work has implications for people whose occupations disrupt their circadian regulation such as night-shift workers, flight attendants, and hospital staff. Recently, it was shown that chronic jet lag (shifts in circadian rhythm) in mice did not cause loss of sleep or increased stress but did cause circadian dysregulation of the innate immune system and dramatic vulnerability to LPS-induced endotoxemic shock [31]. Furthermore, mice infected with S. pneumoniae during their rest phase died significantly less quickly than mice infected during their active phase [32]. In modern society, circadian dysregulation and exposure to bacterial infection at night are likely not uncommon. This work demonstrates that disruption of circadian oscillations in phagocytosis can have significant adverse effects on resistance against infection.
Materials and Methods
Fly and bacterial strains
The Canton S (CS) wild-type and CS tim01 lines have been previously described [7]. Infections were performed with the following bacteria as described [33]: Streptococcus pneumoniae strain SP1, a streptomycin-resistant variant of D39 and gift from Elizabeth Joyce in Stan Falkow's laboratory at Stanford University [34]; Listeria monocytogenes strain 10403S, gift from Julie Theriot at Stanford University [35]; Serratia marcescens strain DB1140, gift from Man Wah Tan at Stanford University [36]; Salmonella typhimurium strain SL1344, gift from Falkow lab at Stanford University [37]; Burkholderia cepacia ATCC strain 2541; Escheria coli strain DH5-alpha; and Micrococcus luteus ATCC strain 4698. S. pneumoniae was frozen at OD600 0.15 in 1 ml aliquots with 10% glycerol, pelleted upon thawing, rediluted three-fold in fresh BHI (Brain Heart Infusion media, Difco), and incubated (standing) for 1.5–2 hour at 37°C with 5% CO2 to OD600 0.15 for injection into flies, which were incubated at 29°C. L. monocytogenes was grown in standing BHI, overnight at 37°C, and diluted to OD600 of 0.1 for injection into flies, which were incubated at 25°C. S. marcescens was grown in shaking BHI, overnight at 37°C, and diluted to OD600 ranging from 0.1 to 0.6 for injection into flies, which were incubated at 29°C. S. typhimurium was grown in standing LB, overnight at 37°C, and diluted to OD600 of 0.1 for injection into flies, which were incubated at 29°C. B. cepacia was grown in standing BHI, overnight at 29°C, and diluted to OD600 of 0.01 to 0.001 for injection into flies, which were incubated at 18° or 25°C. E. coli was grown in shaking LB, overnight at 37°C, and diluted to OD600 0.1 for injection into flies, which were incubated at 25°C. M. luteus was grown in standing LB, overnight at 30°C, and diluted to OD600 0.1 for injection into flies, which were incubated at 25°C.
Injection
All experiments, including injections, were performed with male flies, 5–7 days post-eclosion. Flies were raised at 25°C, 55–65% humidity on yeasted molasses food in a 12h light/dark cycle. For injections, flies were anesthetized with CO2. Injections were carried out with a pulled glass capillary needle. A Picospritzer III (Parker-Hannifan) or custom-made Tritech microinjector (Tritech) was used to inject 50 nL of liquid into each fly. Volume was calibrated by measuring the diameter of the expelled drop under oil. Injections comparing ZT07 (day) and ZT19 (night) were conducted in a dark room using red safety lights as light sources.
Survival assays
60–85 flies per genotype per condition were assayed for each survival curve and placed in 3 vials of dextrose food with approximately 20 flies each. In each experiment, 20–40 flies of each line were also injected with media as a wounding control. Death was recorded daily. Data was converted to Kaplan-Meier format using custom Excel-based software called Count the Dead. Survival curves are plotted as Kaplan-Meier plots and statistical significance is tested using log-rank analysis using GraphPad Prism software. All experiments were performed at least three times and yielded similar results.
CFU determination
Following challenge with microbes, six individual flies were collected at each time point. These flies were homogenized, diluted serially and plated on appropriate media (tryptic soy blood agar for S.pneumoniae, LB for all others). Statistical significance was determined using non-parametric two-tailed t-tests. All experiments were performed at least three times and yielded similar results.
Melanization assays
Flies were injected with S. pneumoniae (OD600 0.1) or L. monocytogenes (OD600 0.1) and incubated at 29°C. On day 2 of the S. pneumoniae infection or day 3 of the L. monocytogenes infection, flies were visually inspected for deposits of melanin resulting from the proteolytic cascade that generates reactive oxygen species. Injection with media typically causes a wound-induced melanization response, a small deposit of melanin at the site of injection within 24 hours. Infection with certain pathogenic bacteria such as L. monocytogenes or S. typhimurium also causes a disseminated melanization response, with large spots of melanin deposition observed elsewhere than the site of injection: under the cuticle or in deeper tissue on both dorsal and ventral sides of the abdomen, thorax, and head. Flies were quantified for both number and size of spots.
Antimicrobial peptide gene expression analysis
Flies were injected with 50 nL of bacterial culture at OD600 0.10 (S. pneumoniae, M. luteus, or E. coli), or media alone (BHI, Difco). Following injection, flies were placed in vials containing molasses food and incubated at 25°C for five hours at the same light: dark cycle to which they had been entrained. Three groups of six flies were homogenized in Trizol and stored at −80°C until processed. RNA was isolated using a standard Trizol preparation and the samples treated with DNAse (Invitrogen). Fermentas RevertAid First Strand cDNA synthesis kit was used to produce the cDNA following the manufacturer's protocol. Quantitative RT-PCR was performed using a Stratagene Mx3000 qPCR machine, with Roche FastStart Universal SYBR Green Master (Rox) mix and the following primer sets: Drosomycin, Diptericin, Rpl1 [38]. Total mRNA concentration was normalized using Rpl1 expression. Differences in the infection-induced gene expression were calculated by normalizing to basal gene expression in uninfected CS (day sample) taken at the same time point; p-values were obtained by Mann-Whitney test. The data shown in Figures 4 and S1 pool the results of three independent trials, each having three biological replicates of six flies in each sample for each condition.
Phagocytosis assays
5–7 day old male flies were injected with 50 nL of 20 mg/ml pHrodo-labeled S. aureus or E. coli in water (Molecular Probes, cat# A10010 and P35361). The flies were allowed to phagocytose the particles for 30–60 min. The wings of the flies were removed and flies were pinned with a minutien pin onto a silicon pad. Fluorescence images were taken of the dorsal surface using epifluorescent illumination with a Leica MZ3 microscope fitted with an ORCA camera (Hamamatsu). Images were captured with Openlab (Improvision) software. The exposure was set such that the brightest images had a very small number of saturated pixels. The experiment was repeated three times with 6–10 flies for each treatment.
Bead inhibition of phagocytosis
Fluorescent 0.2 um polystyrene beads (Molecular Probes, cat# F13080) were injected into hemolymph to inhibit phagocytosis as previously described [30]. Briefly, 200 ul of beads in solution were washed three times in sterile water and resuspended in 20 ul final volume. 5–7 day old male flies were injected with 50 nL of bead solution. To confirm that phagocytosis was inhibited with this protocol, the in vivo phagocytosis assay was performed as described above. Phagocytosis was completely inhibited within 2 days of bead injection. Flies were then injected with S. pneumoniae at OD600 ranging from 0.05–0.15 (see Survival Assays, above).
Accession numbers
Accession numbers are listed below for the following genes, which were examined in this study. Accession numbers were obtained from www.uniprot.org: Timeless (P49021); Drosomycin (P41964); Diptericin (P24492)
Supporting Information
Tim protein does not regulate AMP gene expression. (A) Basal (uninduced) levels of Diptericin expression were very low and not significantly different at different times of day in wild type and Tim mutants. Every pair-wise combination resulted in p-value greater than 0.05 (not significant) by Mann-Whitney test. (B) Drosomycin expression levels induced by injection of S. pneumoniae or media (BHI) are not circadian-regulated. Wild type and Tim mutants were injected at ZT05 (DAY) or ZT 17 (NIGHT). Pair-wise comparisons of flies injected with S. pneumoniae resulted in p-values greater than 0.05 (not significant) by Mann-Whitney test. Tim mutants injected with media during the day exhibited significantly less Drosomycin expression than wild-type flies injected with media during the day or night or Tim mutants injected at night; all other pair-wise comparisons of flies injected with media did not show significant differences by Mann-Whitney test. S. pneumoniae-infected flies exhibited higher levels of Drosomycin expression than BHI-injected controls; p<0.01 by Mann-Whitney test. (C) Infection-induced levels of Diptericin expression were not significantly different after injection of wild type and Tim mutants with E. coli at ZT05 (DAY) or ZT17 (NIGHT). Pair-wise comparisons within the set of flies injected with S. pneumoniae resulted in p-values greater than 0.05 (not significant) by Mann-Whitney test. S. pneumoniae-infected and BHI-injected flies did not exhibit significant differences in Diptericin expression; p>0.05 by Mann-Whitney test.
(EPS)
Acknowledgments
We thank Elizabeth Joyce, Stan Falkow, Man Wah Tan, and Julie Theriot for providing bacteria; we thank Gerard Karsenty and the Karsenty lab for use of their qRT-PCR machine, Gary Struhl for his fluorescence microscope, and Laura Johnston and Andrew Tomlinson for microscopy equipment; and we thank Joel Shirasu-Hiza for help with computer programming. David Schneider, Joe Takahashi, Jen Zallen, Tarun Kapoor, and Julie Canman provided insightful comments on this manuscript. Marc Dionne, Paul Taghert, and Amita Sehgal provided invaluable discussion, as did members of the Schneider lab and other members of the Shirasu-Hiza lab (Victoria Allen, James Cheong, Albert Kim, Paul Kim, Yang “Sunny” Li, Reed O'Connor, Adam Ryan). We thank the reviewers for useful comments and experimental suggestions.
Footnotes
The authors have declared that no competing interests exist.
This work was funded by R01AI069164 and T32AI007328. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 3.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714–722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Beaver LM, Giebultowicz JM. Regulation of copulation duration by period and timeless in Drosophila melanogaster. Curr Biol. 2004;14:1492–1497. doi: 10.1016/j.cub.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan N, Davis AJ, Giebultowicz JM. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem Biophys Res Commun. 2008;374:299–303. doi: 10.1016/j.bbrc.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirasu-Hiza MM, Dionne MS, Pham LN, Ayres JS, Schneider DS. Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol. 2007;17:R353–355. doi: 10.1016/j.cub.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 11.Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, et al. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y, Cahyaningsih U, Abe A, Tanabe A. Presence of the diurnal rhythms of monocyte count and macrophage activities in chicks. Poult Sci. 1992;71:296–301. doi: 10.3382/ps.0710296. [DOI] [PubMed] [Google Scholar]
- 14.Kurepa Z, Rabatic S, Dekaris D. Influence of circadian light-dark alternations on macrophages and lymphocytes of CBA mouse. Chronobiol Int. 1992;9:327–340. doi: 10.3109/07420529209064544. [DOI] [PubMed] [Google Scholar]
- 15.Hriscu ML. Modulatory factors of circadian phagocytic activity. Ann N Y Acad Sci. 2005;1057:403–430. doi: 10.1196/annals.1356.032. [DOI] [PubMed] [Google Scholar]
- 16.Roy B, Singh R, Kumar S, Rai U. Diurnal variation in phagocytic activity of splenic phagocytes in freshwater teleost Channa punctatus: melatonin and its signaling mechanism. J Endocrinol. 2008;199:471–480. doi: 10.1677/JOE-08-0270. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen-Legros J, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol. 2000;196:245–313. doi: 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee J-E, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2008;18:195–199. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:2764–2773. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidoo N, Song W, Hunter-Ensor M, Sehgal A. A role for the proteasome in the light response of the timeless clock protein. Science. 1999;285:1737–1741. doi: 10.1126/science.285.5434.1737. [DOI] [PubMed] [Google Scholar]
- 21.Konopka RJ, Pittendrigh C, Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet. 1989;6:1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieger D, Stanewsky R, Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 24.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayres J, Schneider D. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramet M, Pearson A, Manfruelli P, Li X, Koziel H, et al. Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity. 2001;15:1027–1038. doi: 10.1016/s1074-7613(01)00249-7. [DOI] [PubMed] [Google Scholar]
- 29.Garver LS, Wu J, Wu LP. The peptidoglycan recognition protein PGRP-SC1a is essential for Toll signaling and phagocytosis of Staphylococcus aureus in Drosophila. Proc Natl Acad Sci U S A. 2006;103:660–665. doi: 10.1073/pnas.0506182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol. 2000;10:781–784. doi: 10.1016/s0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- 31.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feigin RD, San Joaquin VH, Haymond MW, Wyatt RG. Daily periodicity of susceptibility of mice to pneumococcal infection. Nature. 1969;224:379–380. doi: 10.1038/224379a0. [DOI] [PubMed] [Google Scholar]
- 33.Schneider DS, Ayres JS, Brandt SM, Costa A, Dionne MS, et al. Drosophila eiger mutants are sensitive to extracellular pathogens. PLoS Pathog. 2007;3:e41. doi: 10.1371/journal.ppat.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce EA, Kawale A, Censini S, Kim CC, Covacci A, et al. LuxS is required for persistent pneumococcal carriage and expression of virulence and biosynthesis genes. Infect Immun. 2004;72:2964–2975. doi: 10.1128/IAI.72.5.2964-2975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansfield BE, Dionne MS, Schneider DS, Freitag NE. Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol. 2003;5:901–911. doi: 10.1046/j.1462-5822.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 36.Flyg C, Xanthopoulos K. Insect Pathogenic Properties of Serratia marcescens. Passive and Active Resistance to Insect Immunity Studied with Protease-deficient and Phage-resistant Mutants. J Gen Microbiol. 1983;129:453–464. [Google Scholar]
- 37.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 38.Clark RI, Woodcock KJ, Geissmann F, Trouillet C, Dionne MS. Multiple TGF-beta superfamily signals modulate the adult Drosophila immune response. Curr Biol. 2011;21:1672–1677. doi: 10.1016/j.cub.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tim protein does not regulate AMP gene expression. (A) Basal (uninduced) levels of Diptericin expression were very low and not significantly different at different times of day in wild type and Tim mutants. Every pair-wise combination resulted in p-value greater than 0.05 (not significant) by Mann-Whitney test. (B) Drosomycin expression levels induced by injection of S. pneumoniae or media (BHI) are not circadian-regulated. Wild type and Tim mutants were injected at ZT05 (DAY) or ZT 17 (NIGHT). Pair-wise comparisons of flies injected with S. pneumoniae resulted in p-values greater than 0.05 (not significant) by Mann-Whitney test. Tim mutants injected with media during the day exhibited significantly less Drosomycin expression than wild-type flies injected with media during the day or night or Tim mutants injected at night; all other pair-wise comparisons of flies injected with media did not show significant differences by Mann-Whitney test. S. pneumoniae-infected flies exhibited higher levels of Drosomycin expression than BHI-injected controls; p<0.01 by Mann-Whitney test. (C) Infection-induced levels of Diptericin expression were not significantly different after injection of wild type and Tim mutants with E. coli at ZT05 (DAY) or ZT17 (NIGHT). Pair-wise comparisons within the set of flies injected with S. pneumoniae resulted in p-values greater than 0.05 (not significant) by Mann-Whitney test. S. pneumoniae-infected and BHI-injected flies did not exhibit significant differences in Diptericin expression; p>0.05 by Mann-Whitney test.
(EPS)