Abstract
Aims
More than 90% of cases of renovascular disease (RVD) are caused by atherosclerosis; thus patients with this condition are at high risk for vascular events. We examined the association of statins with prognosis in patients with RVD.
Methods and results
We performed a population-based cohort study in 4040 patients with RVD older than 65 years using province-wide health data in Ontario, Canada. The primary outcome was time to first cardiorenal event, specifically myocardial infarction, stroke, heart failure, acute renal failure, dialysis or death; the primary analysis used a time-dependent covariate for statin exposure. Despite having a greater burden of cardiovascular and renal comorbidity, the risk of the primary outcome was significantly lower in statin users than in non-users [unadjusted hazard ratio (HR) 0.51, 95% confidence interval (CI) 0.47–0.57; P < 0.0001]. This association was materially unchanged after adjusting for demographic characteristics, cardiovascular risk factors, other comorbidities, measures of health-care utilization, screening, and concomitant medications (adjusted HR 0.51, 95% CI 0.46–0.57). An analysis using the same endpoint in a propensity-matched cohort without time-dependent statin exposure revealed a lower risk of the primary outcome in statin-treated patients but with a substantially more conservative point estimate (HR 0.82, 95% CI 0.71–0.95).
Conclusion
These data suggest that statins are associated with improved prognosis in elderly patients with RVD.
Keywords: Statins, Renovascular disease, Cohort studies, Prognosis
Introduction
Atherosclerotic renovascular disease (RVD) is a highly prevalent vascular condition, particularly among the elderly, with nearly 7% of community dwelling persons 65 years or older demonstrating RVD on duplex sonography.1 In addition, patients with RVD incur high rates of cardiovascular and renal events. In the recent Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial, 37% of participants suffered a major cardiovascular event and 15% suffered a major renal event over a median follow-up of 34 months.2 Among elderly patients with RVD captured in US Medicare data, annual rates of stroke, acute coronary syndrome, heart failure, and death were 18, 30, 19, and 17%, respectively.3
Randomized trials in RVD have typically focused on the role of revascularization in the management of this condition; remarkably few trials have assessed the effects of medical therapy on prognosis. Because >90% of RVD is caused by atherosclerosis, most experts recommend statin therapy for affected patients, although no statin trial has been conducted in this setting. Patients with RVD are on an average sicker, older and more likely to have renal impairment than the typical participant recruited to a statin ‘mega-trial’; RVD might therefore complicate the risk-benefit ratio of statins. Alternatively, since RVD is often a marker of diffuse multisystem atherosclerosis, affected patients might have more to gain from adding a statin to their regimen.
We conducted a retrospective, population-based cohort study to evaluate the association between statins and cardiorenal outcomes in a defined sample of patients with RVD.4 Because non-adherence is common in statin users, we performed our analyses using time-dependent covariates to model statin exposure throughout follow-up. In a sensitivity analysis, we also matched statin users to controls using propensity-based matching, which accounts for the likelihood of being prescribed a statin according to measured baseline characteristics. Finally, because statins seem to exert beneficial effects across disparate vascular beds, we assessed a spectrum of major cardiac, cerebral, and renal events in the primary analysis, while also testing these outcomes separately in secondary analyses.
Methods
Setting and data sources
We conducted our study in Ontario, Canada using linked health-care databases in accordance with a fully prespecified research protocol. Ontario is Canada’s most populous and ethnically diverse province with a total population of >13 million, of whom 1.8 million are older than 65. Elderly patients in Ontario have universal access to health-care services, including outpatient medical visits, hospital care, home care, and prescription drugs. The large databases that record this care have been used extensively in past research, contain little missing information and have been validated for a diverse range of cardiovascular and renal events.5–8
We used six health databases: the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), which records all hospital admissions in the province, including detailed diagnostic and procedural information; the Ontario Health Insurance Plan (OHIP) Database, which records information on outpatient medical visits and testing; the National Ambulatory Care Reporting System Database, which records emergency department visits, dialysis, oncological care, and cardiac catheterization; the Canadian Institute for Health Information Same Day Surgery (CIHI-SDS) Database, which records information on ‘same day’ interventions and procedures; the Ontario Drug Benefit Database, which records all prescription medications dispensed to patients 65 years of age or older; and the Registered Persons Database, which collects vital statistics on all Ontario residents. The study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre and the Privacy Office of the Institute for Clinical Evaluative Sciences.
Sample and selection
We included consecutive patients older than 65 years with codes identifying ‘renal artery stenosis’ or RVD in the CIHI-DAD, CIHI-SDS, and OHIP databases from 1 July 1994 to 1 July 2007 (a span of 13 years). Using a similar code set, Murphy et al.9 calculated a specificity of 96% and sensitivity of 80% for angiographically verified RVD in a five-state validation study. We focused on patients older than 65 years because such individuals receive universal prescription coverage, and prescriptions dispensed are recorded in the Ontario Drug Benefit database. Furthermore, these subjects are highly likely to have atherosclerotic RVD. We excluded patients with fibro-muscular dysplasia (n = 21), death within 120 days of cohort entry (n = 672), invalid health card number (n = 215), missing age or sex (n = 1), non-residents of Ontario (n = 320), nursing home placement (n = 563), or end stage renal disease prior to cohort entry (n = 598). The rationale for each of these exclusions is detailed in Appendix 1.
Appendix 1.
Cohort exclusion criteria
| Exclusion | Rationale | Patients excluded |
|---|---|---|
| Fibromuscular dysplasia | FMD is a non-atherosclerotic cause of RVD typically treated with angioplasty rather than statins. It is most often present in patients under 50 | n = 21 |
| Death within 120 days of cohort entry | Our definition of statin exposure required an initial 120 day exposure assessment window following cohort entry. Patients who died within this window would tend to assort into the non-exposed control group, thereby introducing immortal time bias in the statin group | n = 672 |
| Invalid health card number | Cohort entry required a valid health card number to link patients across administrative databases | n = 215 |
| Missing age or sex | Important demographic variables for cohort characterization | n = 1 |
| Non-residents of Ontario | Non-residents may have a health-care episode in Ontario and then return home for follow-up care in their own jurisdiction, potentially leading to unreliable outcome and exposure status | n = 320 |
| Nursing home residents | These individuals receive medication from onsite formularies which are not tracked in the administrative databases | n = 563 |
| End stage renal disease | By definition, patients with end stage renal disease prior to cohort entry cannot incur new renal events, such as acute renal failure and new onset dialysis | n = 598 |
Exposure
We defined statin exposure as receipt of one or more prescriptions for a hydroxymethylglutaryl coA reductase inhibitor within 120 days following the first identifying code for RVD (hereafter labelled the ‘index date’). Since initially untreated patients might start a statin after this window, and initially treated patients might discontinue statin therapy, we modelled ongoing statin exposure as a time-dependent covariate to reduce dilution bias from these factors. On the basis of serial prescription refills, we deemed cessation to have occurred following the last drug claim (if any) in the treatment group, and deemed initiation to have occurred following the first drug claim (if any) in the control group. As described below, we replicated this analysis using the more traditional ‘intention-to-treat’ approach, in which ongoing statin treatment was not reclassified following the initial exposure assessment.
Characteristics and comorbidities
For each patient in the cohort, we assessed demographic characteristics, cardiovascular risk factors, major comorbidities, measures of health-care utilization, screening, and concomitant medications by searching the health-care databases for the 3-year interval preceding the index date. Variables were selected from a literature review of prognostic factors in RVD supplemented by additional characteristics likely to affect patient outcome.3,10–14 At baseline, we assessed inpatient and outpatient claims for diagnostic modalities that are typically used to test for RVD such as renal angiography and renal Doppler ultrasonography as well as diagnostic tests for related cardiovascular conditions since such manoeuvres may lead to statin prescribing. We adjusted for treatment with 15 distinct classes of medications at baseline, specifically, statins, calcium channel blockers, thiazide diuretics, alpha-blockers, beta-blockers, vasodilators, non-statin lipid drugs, anticoagulants, loop diuretics, antiplatelet agents, anti-arrhythmic agents, potassium-sparing diuretics, non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers. All variables were entered as covariates in all statistical models.
Outcomes
The primary outcome was time to a major cardiorenal event comprising myocardial infarction, stroke, heart failure, acute renal failure, dialysis, and death. Myocardial infarction, stroke, heart failure, and acute renal failure required admission to hospital with a most responsible diagnosis of the condition in question; death and dialysis were defined using both outpatient and inpatient databases (Appendix 2 for specific coding). We examined the six components of the primary outcome separately in secondary analyses. Follow-up for each patient began on the index date and continued until the event in question, death (for the secondary analyses) or 31 March 2008 (whichever came first). The primary analysis used time-dependent statin exposure assessment.
Appendix 2.
Diagnosis coding for study outcomes
| Outcome | Database | Definition |
|---|---|---|
| Myocardial infarction | CIHI-DAD NACRS | Admission to hospital or the emergency room with a most responsible diagnosis of acute myocardial infarction, as coded by ICD9 410; ICD10 I21, I22 |
| Stroke | CIHI-DAD NACRS | Admission to hospital or the emergency room with a most responsible diagnosis of stroke, as coded by ICD9 431, 434, 436; ICD10 I61, I63, I64, G46 |
| Heart failure | CIHI-DAD | Admission to hospital with a most responsible diagnosis of heart failure, systemic volume overload, or pulmonary oedema, as coded by ICD9 276.6, 425, 428, 514, 518.4; ICD10 E87.7, I09.9, I11.0, I13, I25.5, I42, I43, I50, J81 |
| Dialysis | CIHI-DAD CIHI-SDS NACRS OHIP | In-hospital or in-community performance of peritoneal dialysis, haemodialysis, or ultrafiltration (including continuous dialysis) as coded by CCP 51.95, 66.98; CCI 1PZ21; OHIP G082 G083, G085, G090, G091, G092, G093, G094, G095, G096, G294, G295, G325, G326, G330, G331, G332, G333, G860, G861, G862, G863, G864, G865, G866, H540, H740, R849 |
| Acute renal failure | CIHI-DAD | Admission to hospital with a most responsible diagnosis of acute renal failure, pre-renal azotaemia, or anuria, as coded by ICD9 584; ICD10 N17, R39.2 |
| Malignant hypertension | CIHI-DAD | Admission to hospital with a most responsible diagnosis of malignant hypertension or hypertensive encephalopathy, as coded by ICD9 401.0, 402.0, 403.0, 404.0, 405.0, 437.2; ICD10 I10.1, I15.01, I15.11, I15.21, I15.81, I15.91, I67.4 |
| Renal hospitalization | CIHI-DAD | Admission to hospital with a most responsible renal diagnosis, as coded by ICD9 276.2, 276.6, 276.7, 403, 404, 580–589, 593.9, 788.5, 782.3, 791.0, 791.7, V42.0, V45.1, V56.0, V56.8; ICD10 E10.2x, E11.2x, E13.2x, E14.2x, I12, I13, I15.10, I15.11, M10.3, N00-08, N10-12, N14.1, N14.2, N16.5, N17-19, N25.0, N25.8, N25.9, N26, N27, N28.9, N29.8, R34, R39.2, R80, T86.1, T86.100, T86.101, T86102, Z94.0 |
| Revascularization | CIHI-DAD CIHI-SDS NACRS OHIP | In-hospital or in-community performance of any arterial revascularization procedure, as coded by CCP 48.1, 48.11–48.19, 48.02, 48.03, 48.09, 48.2, 48.3, 50.1, 50.11–50.19, 51.28, 51.25, 51.29, 51.26, 51.22, 51.24, 50.28, 50.38; CCI 1ID76MU, 1IJ26, 1IJ27, 1IJ50, 1IJ54, 1IJ57, 1IJ76, 1IJ80, 1IL35, 1JE50, 1JE57, 1JE87, 1JM58, 1JW35, 1JW50, 1JW57, 1JW76, 1JX50, 1JX57, 1JX76, 1KA50, 1KA57, 1KA76, 1KE26, 1KE35, 1KE50, 1KE57, 1KE76, 1KG26, 1KG35, 1KG50, 1KG57, 1KG76MI, 1KG87, 1KR38; OHIP E645, E645C, E645B, E646, E649, E651, E652, E654, E672, E679, G262, G298, J025, J050, J058, J066, N220, N223, R741, R742, R743, R780, R783, R784, R785, R787, R791, R792, R794, R797, R804, R806, R809, R814, R815, R830, R831, R832, R855, R856, R860, R861, R867, R933, R934, R936, R937, S421, Z434 |
CCI, Canadian Classification of Health Interventions; CCP, Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures; CIHI-DAD, Canadian Institute for Health Information Discharge Abstract Database; CIHI-SDS, Canadian Institute for Health Information Same Day Surgery Database; ICD9, International Classification of Diseases, Ninth Revision; ICD10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (Canadian enhancement); NACRS, National Ambulatory Care Reporting System; OHIP, Ontario Health Insurance Plan.
Sensitivity analyses
We conducted several additional analyses to test the robustness of our findings. First, we repeated our primary analysis using an intention-to-treat framework, which did not categorize statin exposure as a time-dependent covariate. The purpose of this analysis was to reduce healthy adherer bias whereby patients who are more adherent to statins may be innately healthier than patients who are less adherent to statins. Second, we replicated our primary analysis in specific settings which might influence the effect of statins, specifically dividing the cohort into subgroups by age, sex, history of diabetes mellitus, chronic kidney disease, coronary artery disease, and previous renal artery revascularization. Third, to capture the full extent of incident cardiorenal events in RVD, we prespecified three additional outcomes: hospitalizations with a most responsible diagnosis of malignant hypertension, hospitalizations with a most responsible diagnosis of renal disease (i.e. ‘any renal hospitalization’), and revascularization procedures (comprising cerebrovascular, coronary, and peripheral arterial interventions). The intent of this analysis was to test the association of statins with morbid events which might not be captured in the primary outcome and its components. Fourth, we repeated our primary analysis using a traditional propensity score matching algorithm, pairing statin-exposed patients and untreated controls by propensity score (± 0.2 standard deviations), age (± 1 year), and sex. Of note this analysis does not use a time-dependent statin exposure assessment covariate but rather characterizes exposure according to treatment at baseline. It is therefore inherently more conservative since it does not account for treatment uptake among controls or treatment cessation and erratic adherence among statin users. Finally, we replicated our primary analysis in patients who did not change treatment over time, specifically excluding treated patients who discontinued statin therapy even temporarily together with controls who initiated statin therapy during follow-up.
Statistical analysis
Sample size calculations suggested that 2627 patients were required to detect as statistically significant a relative risk reduction of 20% or more, based on equal numbers of treated patients and controls, and an overall primary event risk of 30% in the control group (α = 0.05, β = 0.10).3 We assumed a median follow-up of 2.5 years and attrition of 10%; this is conservative as emigration is <0.5% per year among older individuals in our region. Given that in actual fact controls were somewhat more numerous than treated patients, 2709 patients were actually required to exclude the same risk reduction. We used Cox proportional hazards regression to test the association of statins with outcomes and to compute hazard ratios (HR) with 95% confidence intervals (CI). Multivariable analyses were adjusted for demographic characteristics, cardiovascular risk factors, comorbidities, measures of health-care utilization, screening, and medications. A two-tailed P-value of <0.05 was considered statistically significant. We performed all statistical analyses using SAS version 9.1 (SAS Institute, Carey, NC, USA).
Results
Over the 13-year accrual interval, we studied 4040 patients with RVD (Table 1). Comorbidities were highly prevalent, including hypertension (89%), coronary artery disease (54%), peripheral artery disease (46%), heart failure (46%), and cerebrovascular disease (30%). Fewer than half of all patients received a statin at baseline (n = 1682; 42%). Not surprisingly, cardiovascular risk factors and comorbidities were more common in the statin group than among controls including diabetes (33 vs. 25%), hypertension (91 vs. 87%), chronic kidney disease (63 vs. 57%), coronary artery disease (62 vs. 48%), and cerebrovascular disease (32 vs. 29%). Diagnostic methods in this cohort included renal artery ultrasound (41%), catheter renal angiography (25%), computed tomographic angiography (23%), captopril nephrography (14%), and magnetic resonance angiography (7%).
Table 1.
Selected baseline characteristics of the study cohort by initial statin exposure (n = 4040)
| Characteristic | Statin group (n = 1682) | Control group (n = 2358) | P-value |
|---|---|---|---|
| Demographic factors (%) | |||
| Age, y | 74.1 ± 5.3 | 74.9 ± 5.9 | <0.001 |
| Sex (female) | 769 (46) | 1080 (46) | 0.96 |
| Rural residence | 238 (14) | 411 (17) | 0.005 |
| Socioeconomic status (high) | 943 (56) | 1287 (55) | 0.53 |
|
| |||
| Cardiovascular risk factors (%) | |||
| Diabetes mellitus | 557 (33) | 583 (25) | <0.001 |
| Hypertension | 1526 (91) | 2055 (87) | <0.001 |
| Cerebrovascular disease | 540 (32) | 672 (29) | 0.014 |
| Peripheral artery disease | 750 (45) | 1109 (47) | 0.13 |
| Coronary artery disease | 1037 (62) | 1129 (48) | <0.001 |
| Heart failure | 787 (47) | 1078 (46) | 0.50 |
| Aortic aneurysm | 606 (36) | 847 (36) | 0.94 |
| Chronic kidney disease | 1065 (63) | 1347 (57) | <0.001 |
| Atrial fibrillation/flutter | 245 (15) | 387 (16) | 0.11 |
| Other dysrhythmia | 522 (31) | 535 (23) | <0.001 |
|
| |||
| Other comorbidities (%) | |||
| Chronic liver disease | 43 (3) | 90 (4) | 0.03 |
| Chronic lung disease | 670 (40) | 998 (42) | 0.11 |
| Dementia | 83 (5) | 181 (8) | <0.001 |
| Peptic ulcer disease | 259 (15) | 435 (18) | 0.011 |
| Systemic malignancy | 62 (4) | 145 (6) | <0.001 |
| Prior venous thrombosis | 188 (11) | 273 (12) | 0.69 |
|
| |||
| Health-care utilization and testing, n in past year | |||
| Family medicine visits | 13 ± 9 | 13 ± 10 | 0.51 |
| Cardiology visits | 2 ± 3 | 2 ± 2 | <0.001 |
| Endocrinology visits | 0.3 ± 1 | 0.2 ± 1 | 0.07 |
| Nephrology visits | 1 ± 2 | 1 ± 2 | <0.001 |
| Internist visits | 1 ± 3 | 1 ± 2 | 0.30 |
| Vascular surgeon visits | 2 ± 2 | 2 ± 2 | 0.02 |
| Cholesterol tests | 1 ± 2 | 1 ± 1 | <0.001 |
| Creatinine tests | 4 ± 4 | 3 ± 4 | <0.001 |
| Creatinine kinase tests | 0.5 ± 1 | 0.1 ± 1 | <0.001 |
| Transaminase tests | 2 ± 2 | 1 ± 1 | <0.001 |
| Hospitalizations (past 3 years) | 2 ± 2 | 2 ± 2 | <0.001 |
| Number of drugs (past 6 months) | 12 ± 6 | 10 ± 6 | <0.001 |
| Echocardiography (%) | 667 (40) | 788 (33) | <0.001 |
| Carotid ultrasound (%) | 435 (26) | 452 (19) | <0.001 |
| Coronary angiography (%) | 294 (18) | 162 (7) | <0.001 |
| Holter monitoring (%) | 167 (10) | 211 (9) | 0.29 |
| Stress testing (%) | 576 (34) | 560 (24) | <0.001 |
| Bone density testing (%) | 104 (6) | 85 (4) | <0.001 |
| Renal artery ultrasound (%) | 698 (42) | 969 (41) | 0.80 |
| Renal MRA (%) | 190 (11) | 79 (3) | <0.001 |
| Renal CTA (%) | 411 (24) | 533 (23) | 0.18 |
| Plain renal angiography (%) | 514 (31) | 500 (21) | <0.001 |
| Captopril nephrography (%) | 234 (14) | 337 (14) | 0.73 |
Data are mean ± SD or n (%). High socioeconomic status was defined by quintiles 3, 4, and 5. Numeric health-care data were rounded to the nearest integer. CTA, computed tomographic angiography; MRA, magnetic resonance angiography.
The sample provided a total of 12 489 patient-years of follow-up with a median of 3.3 years (interquartile range 1.4–5.0 years). In the primary analysis, statins were associated with a substantially lower risk of cardiorenal events (unadjusted HR 0.51, 95% CI 0.47–0.57; P < 0.0001). The primary outcome occurred at a rate of 63 and 103 events per 100 patient years at risk in statin users and non-users, respectively. After adjusting for demographic characteristics, cardiovascular risk factors, comorbidities, measures of health-care utilization, screening, and concomitant medications, this protective association was materially unchanged (adjusted HR 0.51, 95% CI 0.46–0.57; Table 2). In fully adjusted models, statins were associated with reduced rates of stroke (HR 0.72, 95% CI 0.54–0.96), heart failure (HR 0.83, 95% CI 0.69–0.99), dialysis (HR 0.66, 95% CI 0.52–0.86), and death (HR 0.27, 95% CI 0.24–0.31), with a trend towards fewer myocardial infarctions (HR 0.82, 95% CI 0.65–1.04, P = 0.099). Statins were not associated with any protective association for acute renal failure (HR 0.89, 95% CI 0.63–1.27); however, the latter was the least frequent component of the primary endpoint and this analysis may have been underpowered. As with the primary analysis, results were largely consistent in unadjusted and adjusted models (Table 3). Propensity score analysis in 1061 matched statin user-control pairs (total n = 2122) still yielded significant (albeit more conservative) results (HR 0.82, 95% CI 0.71–0.95).
Table 2.
Multivariable predictors for the primary outcome
| Characteristic | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Statins | 0.51 (0.46–0.57) | <0.0001 |
| Age (per year) | 1.03 (1.02–1.04) | <0.0001 |
| Diabetes mellitus | 1.39 (1.25–1.54) | <0.0001 |
| Cerebrovascular disease | 1.17 (1.06–1.30) | 0.003 |
| Peripheral arterial disease | 1.12 (1.01–1.24) | 0.03 |
| Coronary artery disease | 1.16 (1.04–1.30) | 0.007 |
| Heart failure | 1.37 (1.21–1.55) | <0.0001 |
| Aortic aneurysm (including surgery for) | 0.85 (0.76–0.96) | 0.009 |
| Chronic kidney disease | 1.22 (1.10–1.36) | 0.0002 |
| Chronic liver disease | 1.30 (1.03–1.64) | 0.03 |
| Chronic lung disease | 1.12 (1.01–1.24) | 0.03 |
| Peptic ulcer disease | 0.83 (0.73–0.94) | 0.003 |
| Systemic malignancy | 1.51 (1.26–1.81) | <0.0001 |
| CADG3 (‘likely to recur’) | 0.80 (0.68–0.95) | 0.01 |
| CADG7 (‘chronic specialty stable’) | 0.82 (0.72–0.94) | 0.004 |
| Hospitalizations (n, past 3 years) | 1.07 (1.05–1.10) | <0.0001 |
| Drugs (n, past 6 months) | 1.02 (1.01–1.03) | 0.003 |
| Calcium channel blockers | 1.13 (1.02–1.25) | 0.02 |
| Loop diuretics | 1.23 (1.09–1.40) | 0.0008 |
| Non-steroidal anti-inflammatory drugs | 0.84 (0.73–0.98) | 0.03 |
CADG, chronic ambulatory disease grouping.
Table 3.
Primary and secondary outcomes
| Outcome | Event rate per 1000 patient-years at risk
|
Unadjusted analysis hazard ratio (95% CI) | Adjusted analysisa hazard ratio (95% CI) | |
|---|---|---|---|---|
| Statin | Control | |||
| Primaryb | 62.6 | 102.7 | 0.51 (0.47–0.57) | 0.51 (0.46–0.57) |
| Myocardial infarction | 10.5 | 12.6 | 0.82 (0.67–1.01) | 0.82 (0.65–1.04) |
| Stroke | 4.7 | 6.4 | 0.68 (0.52–0.89) | 0.72 (0.54–0.96) |
| Heart failure | 24.7 | 29.3 | 0.82 (0.69–0.97) | 0.83 (0.69–0.99) |
| Acute renal failure | 5.5 | 6.1 | 0.84 (0.61–1.17) | 0.89 (0.63–1.27) |
| Dialysis | 6.7 | 9.9 | 0.81 (0.65–1.02) | 0.66 (0.52–0.86) |
| Death | 31.6 | 89.0 | 0.28 (0.24–0.31) | 0.27 (0.24–0.31) |
| Renal hospitalization | 14.5 | 20.3 | 0.73 (0.59–0.90) | 0.69 (0.55–0.87) |
| Malignant hypertension | 2.0 | 10.7 | 0.37 (0.14–1.03) | 0.18 (0.05–0.60) |
| Revascularizationc | 68.9 | 64.9 | 1.26 (1.09–1.46) | 1.08 (0.91–1.27) |
Adjusted for demographic characteristics, cardiovascular risk factors, comorbidities, measures of health-care utilization, screening, and medications.
Defined as first occurrence of myocardial infarction, stroke, heart failure, acute renal failure, dialysis, or death.
Coronary, cerebrovascular, and peripheral revascularization procedures.
We conducted an intention-to-treat analysis to test the robustness of these findings, as well as to further evaluate absolute risk differences. In the unadjusted analysis, statins continued to exert a positive effect on the primary outcome although the strength of the association was approximately halved (HR 0.75, 95% CI 0.69–0.83). These results were consistent with the adjusted analysis (HR 0.77, 95% CI 0.69–0.85). Replicating our results in patients who did not cross over between treated and exposed groups over time yielded similar findings (unadjusted HR 0.85, 95% CI 0.75–0.97, P = 0.014; adjusted HR 0.85, 95% CI 0.73–0.99, P = 0.031). Specifically, of the initially statin-treated patients, 1077 patients temporarily or permanently discontinued therapy; of the initially untreated controls, 711 patients later initiated statins during follow-up.
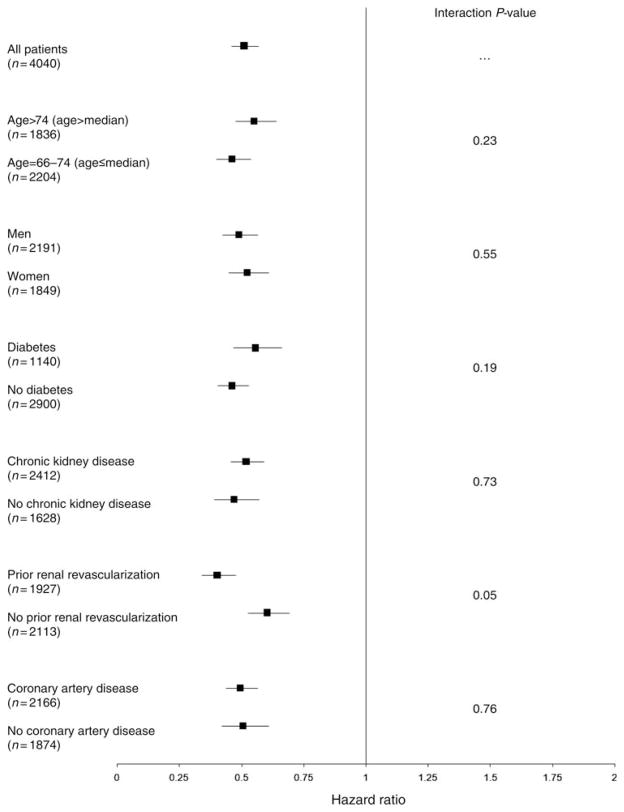
We also found a consistently positive association between statins and prognosis across each of the predefined subgroups, in both unadjusted and adjusted analyses (Figure 1). A borderline statistical interaction was present for patients with a history of renal artery revascularization (P = 0.053), with a stronger effect in those who received revascularization (HR 0.40, 95% CI 0.34–0.48) than in those who did not (HR 0.60, 95% CI 0.53–0.69). Of the cohort, 1927 patients had a history of renal revascularization and incurred a total of 767 primary outcome events, whereas 2113 had no previous history of renal revascularization (in whom 1202 events transpired). Finally, statin treatment was associated with a lower risk of all renal hospitalizations (HR 0.69, 95% CI 0.55–0.87) and malignant hypertension hospitalizations (HR 0.18, 95% CI 0.05–0.60) but not vascular interventions (HR 1.08; 95% CI 0.91–1.27; Table 3). Time-to-event curves showed gradual and continual separation for the primary outcome (Figure 2) and mortality (Figure 3) throughout the entirety of follow-up.
Figure 1.
Subgroup analyses for the primary outcome of major cardiorenal events. All analyses were adjusted for demographic characteristics, cardiovascular risk factors, comorbidities, measures of health-care utilization, screening, and medications. For each subgroup, the square represents the hazard ratio with horizontal lines representing the 95% confidence interval.
Figure 2.
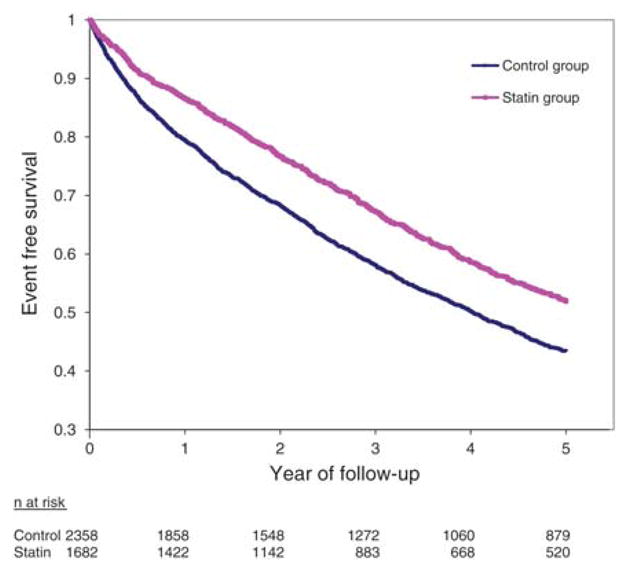
Time to primary outcome stratified by treatment with statins. Log-rank P < 0.0001 for the comparison of curves.
Figure 3.
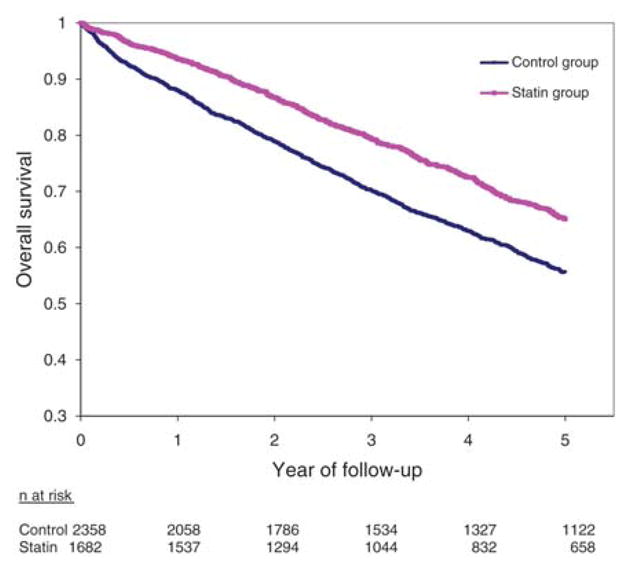
Overall survival stratified by treatment with statins. Log-rank P < 0.0001 for the comparison of curves.
Discussion
We found that statins were associated with a significantly lower rate of cardiorenal events in older patients with RVD. This finding was consistent with reductions in secondary endpoints, was present whether renal artery revascularization was performed or not, and was observed in the intention-to-treat analysis and propensity-based algorithm. The size of the apparent benefit is in keeping with the Heart Protection Study, the largest randomized trial of statins performed to date (n = 20 536), which reported a number needed to treat of 19 for major vascular events in patients with occlusive vascular disease or diabetes. Reductions in renal events in the present study are also in keeping with several large randomized trials.15–19
Relation to the previous literature
Several animal and human studies have reported on the effects of statins in RVD (Table 4). In randomized animal models of RVD, statins reduced renal fibrosis, improved kidney function and blood flow, prevented left ventricular hypertrophy, and increased myocardial perfusion.20–24 Four previous cohort studies demonstrated findings similar to ours, although the largest sample size in these reports was only a fifth of the present study.11,25–27 The most likely explanation is decreased progression, and possible regression, of renal artery stenosis with improved blood pressure under treatment with statins (as has been observed in one renal angiography cohort and several case reports28,29). In addition, anti-inflammatory, antithrombotic, and antioxidant effects of statins may account for these findings. Because our analytical models do not contain on-treatment cholesterol levels, we are unable to determine the precise mechanism of this relationship.
Table 4.
Literature on statins and renovascular disease
| Study | Design | Findings related to statin exposure |
|---|---|---|
| Animal studies | ||
| Chade et al.20,21 | Porcine unilateral RVD model | Renoprotective effects: reduced renal fibrosis and remodelling; increased renal blood flow and glomerular filtration rate |
| Zhu et al.22 | Porcine unilateral RVD model | Cardioprotective effects: left ventricular hypertrophy prevented; myocardial perfusion increased; reduced microvascular remodelling |
| Laina et al.23 | Rodent bilateral RVD model | Renoprotective effects: improved glomerular filtration rate, free water clearance, and fractional sodium excretion |
| Lavi et al.24 | Porcine unilateral RVD model | Renoprotective effects: improved endothelial function, reduced renal oxidative stress, inflammation and fibrosis; attenuated endothelial progenitor cell apoptosis |
|
| ||
| Human studies | ||
| Khong et al.29 | Case report (n = 1) | Marked regression of renal artery stenosis over 3 years following institution of statin therapy (and despite continued heavy smoking) |
| Basta et al.28 | Case report (n = 1) | Marked regression of renal artery stenosis, including spontaneous remission of hypertension and renin levels |
| Bates et al.11 | Cohort study (n = 748) | Reduced mortality over 11 year follow-up with statins (HR 0.71; 95% CI 0.53–0.95) |
| Davies et al.25 | Cohort study (n = 447) | Reduced restenosis after renal artery stenting as well as greater freedom from recurrent symptoms (hypertension and worsening renal failure) with statins; risk ratios not provided |
| Silva et al.26 | Cohort study (n = 104) | Reduced all-cause mortality (HR 0.13; 95% CI 0.04–0.44) and renal mortality (HR 0.21; 95% CI 0.07–0.64) |
| Cheung et al.27 | Cohort study (n = 79) | Reduced angiographic progression of RVD with statins (HR 0.28; 95% CI 0.10–0.77); increased likelihood of regression with statins (HR 4.88; 95% CI 1.32–19.4) |
Multivariable-adjusted results were provided in the table above (wherever possible).
Limitations
The major limitation of our study is the non-randomized comparison of statin use with untreated controls. Indeed the overall observational design raises possibilities of selection bias and residual confounding. We adjusted for 74 variables in our analyses and, if anything, statin users appeared to have greater cardiorenal comorbidity at baseline than controls. This imbalance would be expected to bias the results against treatment, yet this was not observed in any of the analyses. Adjusting for year of diagnosis did not materially change the results, suggesting that the present findings are not due solely to secular change. However, several variables could not be adjusted for such as laboratory measures of renal function, cholesterol, and anatomic grade of stenosis. Our data also lack lifestyle measures, such as obesity, smoking, and exercise. Outcomes in the current study were not blindly adjudicated and were based on administrative data. The coding of certain outcomes—such as malignant hypertension—have been validated in other jurisdictions but not in Ontario.
For these reasons, only a large randomized trial can conclusively demonstrate that statins improve prognosis in this setting; however, such a trial is unlikely to be performed given the high prevalence of compelling indications for statins in patients with RVD. In addition, our results might not apply to excluded patients, such as individuals with end stage renal disease, patients younger than 65, or those who died within 4 months of RVD diagnosis.
We cannot know for certain why patients were screened for RVD, but we did ascertain very high frequencies of hypertension (89%), chronic kidney disease (60%), and heart failure (46%) in this cohort. The fact that RVD is being detected in health-care databases, in concert with sizeable event rates, would suggest that patients with symptomatic RVD were likely enroled.
There are several reasons why some patients with RVD in our cohort may not have received statins. Patients who did not receive statins were less likely to have coronary artery disease, diabetes mellitus, and cerebrovascular disease at baseline. Such patients had equivalent rates of ambulatory care visits to general practitioners (P = 0.51) and internists (P = .30). We believe that adherence to treatment was unlikely to be a bar to statin therapy because controls still received an average of ten different drugs in the 6 months prior to cohort accrual. In addition, time-dependent exposure adjusts for medication adherence and drug uptake in both groups. Therefore, initially untreated controls still had a chance to receive statins after ‘time zero’ of follow-up, and indeed we found a general increase in statin treatment over the course of the study.
Notwithstanding the above remarks, we found some evidence of under-treatment in our study, with many patients with concomitant extra-renal atherosclerotic vascular disease not receiving statin therapy. Results in patients with or without coronary artery disease were virtually identical as shown in our predefined subgroup analyses. The beginning of our accrual period predated the results of the major statin trials in coronary disease, cerebro-vascular disease, and peripheral arterial disease, during which time lipoprotein targets and thresholds became ever more rigorous. By the end of our accrual interval, statin users were outnumbering controls by a ratio of 2 to 1.
Conclusions
These findings suggest an association between statins and cardiorenal prognosis in patients with RVD (in keeping with data from previous studies).11–27,29 Our study also underscores the high rates of morbidity and mortality in this condition; overall, 49% of patients suffered a primary event and 37% of patients died during a median follow-up of 3.3 years. Given these rates, which are consistent with the previous literature, patients with RVD require careful surveillance and diligent risk factor modification to prevent cardiorenal complications.2,3 While randomized data do not exist to guide the nature of medical therapy for this condition, our findings suggest that patients with RVD should potentially be considered for statin therapy.
Appendix 3.
Adjusted covariates in multivariable models
| Demographic factors | Other investigations |
|---|---|
| Age | Echocardiography |
| Income | Carotid ultrasound |
| Locale | Coronary angiography |
| Sex | Holter monitoring |
| Stress testing | |
| Cardiovascular risk factors and comorbidities | Bone density testing |
| Diabetes mellitus | Renal artery ultrasound |
| Hypertension | Renal magnetic resonance angiography |
| Cerebrovascular disease | Renal computed tomographic angiography |
| Peripheral arterial disease | Renal angiography (catheter-based) |
| Coronary artery disease | Captopril nephrography |
| Heart failure | Renin immunoassay |
| Aortic aneurysm | Aldosterone immunoassay |
| Chronic kidney disease | |
| Atrial fibrillation/flutter | Medications |
| Other dysrhythmias | Statins |
| Calcium channel blockers | |
| Other comorbidities | Thiazide diuretics |
| Chronic liver disease | Alpha-blockers |
| Chronic lung disease | Beta-blockers |
| Dementia | Vasodilators |
| Peptic ulcer disease | Non-statin lipid drugs |
| Systemic malignancy | Anticoagulants |
| Venous thromboembolism | Loop diuretics |
| Antiplatelets | |
| Chronic ambulatory disease groupings | Anti-arrhythmics |
| Acute major | Potassium-sparing diuretics |
| Acute minor | Non-steroidal anti-inflammatories |
| Likely to recur | Angiotensin-converting enzyme inhibitors |
| Asthma | Angiotensin receptor blockers |
| Chronic medical unstable | |
| Chronic medical stable | |
| Chronic specialty stable | |
| Eye/dental | |
| Chronic specialty unstable | |
| Psychosocial | |
| Prevention, administration | |
| Health-care access and screening (n, past year) | |
| Family doctor visits | |
| Cardiologist visits | |
| Nephrologist visits | |
| General internist visits | |
| Vascular surgeon visits | |
| Cholesterol tests | |
| Creatinine tests | |
| Hospitalizations (past 3 years) | |
| Distinct drugs (past 6 months) | |
| Creatinine kinase tests | |
| Liver transaminase tests |
Appendix 4.
Baseline characteristics for the propensity-matched cohort
| Variable | Value | Control (n = 1061) | Statin (n = 1061) | Overall (n = 2122) |
|---|---|---|---|---|
| Income quintile 1 (%) | 231 (21.8) | 242 (22.8) | 473 (22.3) | |
| Income quintile 2 (%) | 227 (21.4) | 225 (21.2) | 452 (21.3) | |
| Income quintile 3 (%) | 218 (20.5) | 222 (20.9) | 440 (20.7) | |
| Income quintile 4 (%) | 197 (18.6) | 186 (17.5) | 383 (18.0) | |
| Income quintile 5 (%) | 188 (17.7) | 186 (17.5) | 374 (17.6) | |
| Female (%) | 494 (46.6) | 494 (46.6) | 988 (46.6) | |
| Rural location (%) | 158 (14.9) | 173 (16.3) | 331 (15.6) | |
| Age | Mean ± SD | 74.07 ± 5.36 | 74.09 ± 5.36 | 74.08 ± 5.36 |
| Median (IQR) | 73 (70–78) | 73 (70–78) | 73 (70–78) | |
| Family doctor ambulatory visits in the past year, n | Mean ± SD | 12.61 ± 8.74 | 12.88 ± 8.94 | 12.74 ± 8.84 |
| Median (IQR) | 11 (7–17) | 11 (7–17) | 11 (7–17) | |
| Cardiologist ambulatory visits in the past year, n | Mean ± SD | 2.03 ± 2.70 | 2.03 ± 2.31 | 2.03 ± 2.51 |
| Median (IQR) | 1 (0–3) | 1 (0–3) | 1 (0–3) | |
| Endocrinologist ambulatory visits in the past year, n | Mean ± SD | 0.27 ± 1.03 | 0.25 ± 0.96 | 0.26 ± 1.00 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| Nephrologist ambulatory visits in the past year, n | Mean ± SD | 1.03 ± 2.20 | 0.94 ± 1.94 | 0.99 ± 2.08 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | |
| General internist ambulatory visits in the past year, n | Mean ± SD | 1.55 ± 2.41 | 1.44 ± 3.09 | 1.49 ± 2.77 |
| Median (IQR) | 1 (0–2) | 0 (0–2) | 1 (0–2) | |
| Vascular surgeon ambulatory visits in the past year, n | Mean ± SD | 1.70 ± 2.26 | 1.69 ± 2.06 | 1.70 ± 2.16 |
| Median (IQR) | 1 (0–3) | 1 (0–3) | 1 (0–3) | |
| Number of cholesterol laboratory tests in the past year | Mean ± SD | 0.95 ± 1.07 | 0.96 ± 1.00 | 0.96 ± 1.04 |
| Median (IQR) | 1 (0–1) | 1 (0–1) | 1 (0–1) | |
| Number of creatinine laboratory tests in the past year | Mean ± SD | 3.08 ± 3.24 | 3.04 ± 3.54 | 3.06 ± 3.39 |
| Median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | |
| Number of hospitalizations in past 3 years | Mean ± SD | 2.34 ± 1.98 | 2.24 ± 2.02 | 2.29 ± 2.00 |
| Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | |
| Number of distinct drugs in the past 6 months | Mean ± SD | 11.66 ± 6.20 | 11.70 ± 5.45 | 11.68 ± 5.83 |
| Median (IQR) | 11 (7–15) | 11 (8–15) | 11 (8–15) | |
| Number of CK tests in the past year | Mean ± SD | 0.21 ± 0.63 | 0.25 ± 0.56 | 0.23 ± 0.60 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| Number of ALT/AST tests in the past year | Mean ± SD | 1.06 ± 1.45 | 1.10 ± 1.41 | 1.08 ± 1.43 |
| Median (IQR) | 1 (0–1) | 1 (0–2) | 1 (0–2) | |
| Diabetes mellitus (%) | 288 (27.1) | 306 (28.8) | 594 (28.0) | |
| Hypertension (%) | 955 (90.0) | 951 (89.6) | 1906 (89.8) | |
| Cerebrovascular disease (%) | 340 (32.0) | 331 (31.2) | 671 (31.6) | |
| Peripheral arterial disease (%) | 487 (45.9) | 487 (45.9) | 974 (45.9) | |
| Coronary artery disease (%) | 613 (57.8) | 618 (58.2) | 1231 (58.0) | |
| Heart failure (%) | 488 (46.0) | 495 (46.7) | 983 (46.3) | |
| Aortic aneurysm (%) | 407 (38.4) | 392 (36.9) | 799 (37.7) | |
| Chronic kidney disease (%) | 635 (59.8) | 635 (59.8) | 1270 (59.8) | |
| Atrial fibrillation/flutter (%) | 160 (15.1) | 169 (15.9) | 329 (15.5) | |
| Other arrhythmias (%) | 292 (27.5) | 280 (26.4) | 572 (27.0) | |
| Chronic liver disease (%) | 22 (2.1) | 30 (2.8) | 52 (2.5) | |
| Chronic lung disease (%) | 441 (41.6) | 433 (40.8) | 874 (41.2) | |
| Dementia (%) | 47 (4.4) | 57 (5.4) | 104 (4.9) | |
| Peptic ulcer disease (%) | 177 (16.7) | 181 (17.1) | 358 (16.9) | |
| Systemic malignancy (%) | 40 (3.8) | 44 (4.1) | 84 (4.0) | |
| Prior venous thrombosis/thromboembolism (%) | 111 (10.5) | 121 (11.4) | 232 (10.9) | |
| CADG—‘acute minor’ (%) | 999 (94.2) | 1007 (94.9) | 2006 (94.5) | |
| CADG—‘acute major’ (%) | 1045 (98.5) | 1044 (98.4) | 2089 (98.4) | |
| CADG—‘likely to recur’ (%) | 968 (91.2) | 966 (91.0) | 1934 (91.1) | |
| CADG—‘asthma’ (%) | 115 (10.8) | 118 (11.1) | 233 (11.0) | |
| CADG—‘chronic medical unstable’ (%) | 1060 (99.9) | 1060 (99.9) | 2120 (99.9) | |
| CADG—‘chronic medical stable’ (%) | 1042 (98.2) | 1046 (98.6) | 2088 (98.4) | |
| CADG—‘chronic specialty stable’ (%) | 144 (13.6) | 129 (12.2) | 273 (12.9) | |
| CADG—‘eye dental’ (%) | 395 (37.2) | 401 (37.8) | 796 (37.5) | |
| CADG—‘chronic specialty unstable’ (%) | 296 (27.9) | 305 (28.7) | 601 (28.3) | |
| CADG—‘psychosocial’ (%) | 547 (51.6) | 557 (52.5) | 1104 (52.0) | |
| CADG—‘prevention, administration’ (%) | 718 (67.7) | 726 (68.4) | 1444 (68.0) | |
| Echocardiography (%) | 384 (36.2) | 409 (38.5) | 793 (37.4) | |
| Carotid ultrasound (%) | 269 (25.4) | 251 (23.7) | 520 (24.5) | |
| Coronary angiography (%) | 130 (12.3) | 135 (12.7) | 265 (12.5) | |
| Holter monitoring (%) | 107 (10.1) | 104 (9.8) | 211 (9.9) | |
| Stress testing (%) | 320 (30.2) | 312 (29.4) | 632 (29.8) | |
| Bone density testing (%) | 45 (4.2) | 52 (4.9) | 97 (4.6) | |
| Renal artery ultrasound (%) | 440 (41.5) | 441 (41.6) | 881 (41.5) | |
| Renal MRA (%) | 69 (6.5) | 73 (6.9) | 142 (6.7) | |
| Renal CTA (%) | 270 (25.4) | 260 (24.5) | 530 (25.0) | |
| Plain renal angiogram (%) | 276 (26.0) | 278 (26.2) | 554 (26.1) | |
| Captopril nephrography (%) | 178 (16.8) | 161 (15.2) | 339 (16.0) | |
| Renin immunoassay (%) | 7 (0.7) | 6 (0.6) | 13 (0.6) | |
| Aldosterone immunoassay (%) | 10 (0.9) | 8 (0.8) | 18 (0.8) | |
| Calcium channel blocker (%) | 616 (58.1) | 618 (58.2) | 1234 (58.2) | |
| Thiazide diuretic (%) | 333 (31.4) | 336 (31.7) | 669 (31.5) | |
| Alpha-blocker (%) | 107 (10.1) | 116 (10.9) | 223 (10.5) | |
| Beta-blocker (%) | 576 (54.3) | 558 (52.6) | 1134 (53.4) | |
| Vasodilator (%) | 94 (8.9) | 82 (7.7) | 176 (8.3) | |
| Oral anticoagulant (%) | 155 (14.6) | 148 (13.9) | 303 (14.3) | |
| Loop diuretic (%) | 520 (49.0) | 537 (50.6) | 1057 (49.8) | |
| Antiplatelet (%) | 383 (36.1) | 389 (36.7) | 772 (36.4) | |
| Anti-arrhythmic (%) | 169 (15.9) | 174 (16.4) | 343 (16.2) | |
| Potassium-sparing diuretic (%) | 105 (9.9) | 104 (9.8) | 209 (9.8) | |
| NSAID (%) | 119 (11.2) | 120 (11.3) | 239 (11.3) | |
| ACE-inhibitor (%) | 499 (47.0) | 515 (48.5) | 1014 (47.8) | |
| ARB (%) | 123 (11.6) | 114 (10.7) | 237 (11.2) |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CADG, chronic ambulatory disease group; CK, creatine kinase; CTA, computed tomographic angiography; IQR, interquartile range; MRA, magnetic resonance angiography; NSAID, non-steroidal anti-inflammatory drug.
Acknowledgments
We thank Nick Daneman, Nadia Khan, Marko Mrkobrada, Donald Redelmeier, and Matthew Weir for comments on the manuscript.
Funding
The study was supported by a peer-reviewed grant-in-aid from the Physician Services Incorporated Foundation. D.G.H. was supported by a new investigator award from the Canadian Institutes for Health Research. P.C.A. was supported by a career investigator award from the Heart and Stroke Foundation of Ontario. A.X.G. was supported by a clinician scientist award from the Canadian Institutes for Health Research. The Institute for Clinical Evaluative Sciences is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by the Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. D.G.H. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of interest: None for D.G.H., A.X.G., F.W., P.L. or P.C.A. M.M.M. has received honoraria from Pfizer and AstraZeneca and was employed at Pfizer Global Pharmaceuticals from January 2006 to April 2007. S.W.T. has received honoraria from Merck, BMS-Sanofi-aventis, and Servier, and has received research funding from AstraZeneca, Ortho Biotech, Novartis, and Amgen.
References
- 1.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 2.The ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 3.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Hackam DG, Duong-Hua M, Mamdani M, Li P, Tobe SW, Spence JD, Garg AX. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008;156:549–555. doi: 10.1016/j.ahj.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 6.Juurlink D, Preyra C, Croxford R, Chong A, Austin P, Tu J, Laupacis A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 7.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10:67–71. [PubMed] [Google Scholar]
- 9.Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by medicare beneficiaries, 1996–2000. Am J Roentgenol. 2004;183:561–568. doi: 10.2214/ajr.183.3.1830561. [DOI] [PubMed] [Google Scholar]
- 10.de SR, Loh H, Rigby AS, Nikitin NP, Witte KK, Goode K, Bhandari S, Nicholson A, Clark AL, Cleland JG. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol. 2007;100:273–279. doi: 10.1016/j.amjcard.2007.02.098. [DOI] [PubMed] [Google Scholar]
- 11.Bates MC, Campbell JE, Stone PA, Jaff MR, Broce M, Lavigne PS. Factors affecting long-term survival following renal artery stenting. Catheter Cardiovasc Interv. 2007;69:1037–1043. doi: 10.1002/ccd.21121. [DOI] [PubMed] [Google Scholar]
- 12.Mui KW, Sleeswijk M, van den Hout H, van BJ, Navis G, Woittiez AJ. Incidental renal artery stenosis is an independent predictor of mortality in patients with peripheral vascular disease. J Am Soc Nephrol. 2006;17:2069–2074. doi: 10.1681/ASN.2005080827. [DOI] [PubMed] [Google Scholar]
- 13.Losito A, Errico R, Santirosi P, Lupattelli T, Scalera GB, Lupattelli L. Long-term follow-up of atherosclerotic renovascular disease. Beneficial effect of ACE inhibition. Nephrol Dial Transplant. 2005;20:1604–1609. doi: 10.1093/ndt/gfh865. [DOI] [PubMed] [Google Scholar]
- 14.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med. 2005;165:207–213. doi: 10.1001/archinte.165.2.207. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H, Mizuno K, Ohashi Y, Yoshida T, Hirao K, Uchida Y. Pravastatin and cardiovascular risk in moderate chronic kidney disease. Atherosclerosis. 2009;206:512–517. doi: 10.1016/j.atherosclerosis.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Huskey J, Lindenfeld J, Cook T, Targher G, Kendrick J, Kjekshus J, Pedersen T, Chonchol M. Effect of simvastatin on kidney function loss in patients with coronary heart disease: Findings from the Scandinavian Simvastatin Survival Study (4S) Atherosclerosis. 2009;205:202–206. doi: 10.1016/j.atherosclerosis.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Athyros VG, Mikhailidis DP, Liberopoulos EN, Kakafika AI, Karagiannis A, Papageorgiou AA, Tziomalos K, Ganotakis ES, Elisaf M. Effect of statin treatment on renal function and serum uric acid levels and their relation to vascular events in patients with coronary heart disease and metabolic syndrome: A subgroup analysis of the GREek Atorvastatin and Coronary heart disease Evaluation (GREACE) Study. Nephrol Dial Transplant. 2007;22:118–127. doi: 10.1093/ndt/gfl538. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14:1605–1613. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Kastelein JJP, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK for the Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) Study. Clin J Am Soc Nephrol. 2007;2:1131–1139. doi: 10.2215/CJN.04371206. [DOI] [PubMed] [Google Scholar]
- 20.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 21.Chade AR, Zhu XY, Grande JP, Krier JD, Lerman A, Lerman LO. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26 doi: 10.1097/HJH.0b013e328302833a. [DOI] [PubMed] [Google Scholar]
- 22.Zhu XY, Daghini E, Chade AR, Napoli C, Ritman EL, Lerman A, Lerman LO. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol. 2007;18:1209–1217. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]
- 23.Laina A, Benyamin G, Levtov O, Getter R, Serban I, Wollman Y, Rubinstein A, Cabili S, Peer G, Blum M. Effect of chronic cholesterol loading in the development of acute ischemic renal failure in rats. Ren Fail. 1994;16:117–123. doi: 10.3109/08860229409044853. [DOI] [PubMed] [Google Scholar]
- 24.Lavi R, Zhu XY, Chade AR, Lin J, Lerman A, Lerman LO. Simvastatin decreases endothelial progenitor cell apoptosis in the kidney of hypertensive hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2010;30:976–983. doi: 10.1161/ATVBAHA.109.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies MG, Saad WA, Bismuth JX, Peden EK, Naoum JJ, Lumsden AB. Outcomes of endoluminal reintervention for restenosis after percutaneous renal angioplasty and stenting. J Vasc Surg. 2009;49:946–952. doi: 10.1016/j.jvs.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Silva VS, Martin LC, Franco RJS, Carvalho FC, Bregagnollo EA, Castro JH, Gavras I, Gavras H. Pleiotropic effects of statins may improve outcomes in atherosclerotic renovascular disease. Am J Hypertens. 2008;21:1163–1168. doi: 10.1038/ajh.2008.249. [DOI] [PubMed] [Google Scholar]
- 27.Cheung CM, Patel A, Shaheen N, Cain S, Eddington H, Hegarty J, Middleton RJ, Cowie A, Mamtora H, Kalra PA. The effects of statins on the progression of atherosclerotic renovascular disease. Nephron Clin Pract. 2007;107:c35–c42. doi: 10.1159/000107552. [DOI] [PubMed] [Google Scholar]
- 28.Basta LL, Williams C, Kioschos JM, Spector AA. Regression of atherosclerotic stenosing lesions of the renal arteries and spontaneous cure of systemic hypertension through control of hyperlipidemia. Am J Med. 1976;61:420–423. doi: 10.1016/0002-9343(76)90381-8. [DOI] [PubMed] [Google Scholar]
- 29.Khong TK, Missouris CG, Belli AM, MacGregor GA. Regression of atherosclerotic renal artery stenosis with aggressive lipid lowering therapy. J Hum Hypertens. 2001;15:431–433. doi: 10.1038/sj.jhh.1001196. [DOI] [PubMed] [Google Scholar]