Abstract
Summary
Weekly bisphosphonates are the primary agents used to treat osteoporosis. Although these agents are generally well tolerated, serious gastrointestinal adverse events, including hospitalization for gastrointestinal bleed, may arise. We compared the gastrointestinal safety between weekly alendronate and weekly risedronate and found no important difference between new users of these agents.
Introduction
Weekly bisphosphonates are the primary agents prescribed for osteoporosis. We examined the comparative gastrointestinal safety between weekly bisphosphonates.
Methods
We studied new users of weekly alendronate and weekly risedronate from June 2002 to August 2005 among enrollees in a state-wide pharmaceutical benefit program for seniors. Our primary outcome was hospitalization for upper gastrointestinal bleed. Secondary outcomes included outpatient diagnoses for upper gastrointestinal disease, symptoms, endoscopic procedures, use of gastroprotective agents, and switching between therapies. We used Cox proportional hazard models to compare outcomes between agents within 120 days of treatment initiation, adjusting for propensity score quintiles. We also examined composite safety outcomes and stratified results by age and prior gastrointestinal history.
Results
A total of 10,420 new users were studied, mean age=79 years (SD, 6.9), and 95% women. We observed 31 hospitalizations for upper gastrointestinal bleed (0.91 per 100 person-years) within 120 days of treatment initiation. Adjusting for covariates, there was no difference in hospitalization for upper gastrointestinal bleed among those treated with risedronate compared with alendronate (HR, 1.12; 95%CI, 0.55 to 2.28). Risedronate switching rates were lower; otherwise, no differences were observed for secondary or composite outcomes.
Conclusions
We found no important difference in gastrointestinal safety between weekly oral bisphosphonates.
Keywords: Bisphosphonates, Drug evaluation, Drug safety, Osteoporosis, Population studies, Treatment
Introduction
Weekly oral bisphosphonates are currently the primary pharmaceutical agents used for managing osteoporosis [1–4]. These agents are generally well tolerated with adverse events largely related to upper gastrointestinal complaints, erosions, and ulcers [5]. Prior evidence suggests that risedronate may have a more favorable gastrointestinal profile. Randomized controlled endoscopic studies show fewer gastric ulcers among daily risedronate users within 14 days of treatment initiation compared with daily alendronate recipients [6, 7]. Observational studies also identify fewer gastrointestinal events within 4 months of treatment initiation among new recipients of risedronate versus alendronate [8, 9]. However, more recent data from a randomized controlled trial found no difference in gastrointestinal tolerability or adverse events causing discontinuation when comparing the efficacy and tolerability of weekly dosing of these agents [10, 11]. This finding has biological plausibility as a weekly dosing interval may provide time for early esophageal lesions to heal between exposures [12]. Nonetheless, due to exclusion criteria, results from this trial may not be widely applicable to the general population of patients treated with these agents [13]. Clinicians may therefore struggle with determining the best option for osteoporosis treatment from a safety standpoint. To examine the comparative gastrointestinal safety of weekly risedronate versus weekly alendronate, we performed an observational study of new recipients of these agents eligible for a state-wide pharmaceutical benefit program. We hypothesized that we would observe no clinically significant difference in gastrointestinal safety between agents.
Materials and methods
Study cohort
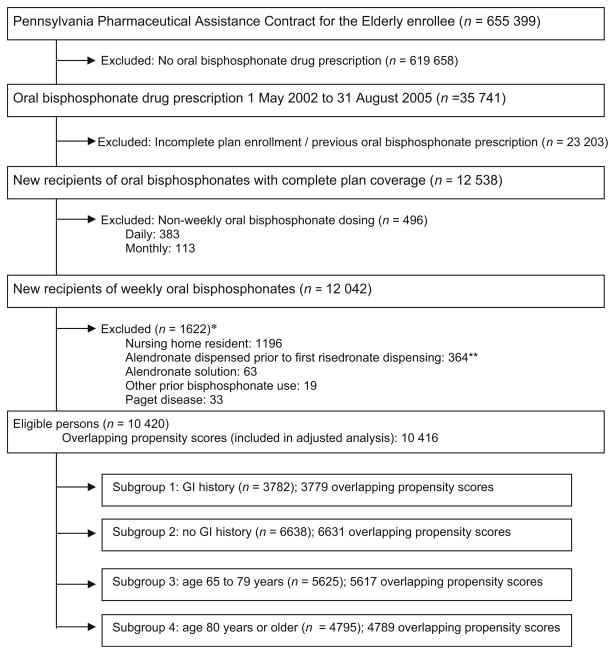
The study population was identified from health care utilization data for enrollees in the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE). This state-run program provides drug coverage without restriction for low-income residents aged 65 or more years with annual household income too high for Medicaid. Study eligibility was limited to patients with one or more claims in both Medicare and PACE in each of the three 6-month intervals preceding the index prescription. This was to ensure a minimum of 12 months of enrollment without any use of the study drugs prior to defining new use. We assembled a cohort of new recipients of weekly bisphosphonate therapy approved for osteoporosis treatment restricted to the period when both agents were available and dispensed in our cohort. Our cohort therefore included new recipients (no prescription filled for any oral bisphosphonate approved for osteoporosis management within the previous 365 days) of weekly alendronate (70 mg) and weekly risedronate (35 mg) between June 16, 2002 (date risedronate first dispensed in our cohort) and August 31, 2005 (Fig. 1). We excluded residents of nursing homes (where prescription data may not be complete), patients with a Medicare claim for Paget disease (International Classification of Diseases, Ninth Revision, Clinical Modification code 731.0), use of alendronate solution, and those with a history of any other bisphosphonate use (e.g., etidronate, which is not approved for osteoporosis management in the United States) within the year prior to index prescription. Our data included all PACE beneficiaries that met eligibility criteria. At the time of analysis, we had complete Medicare data through to December 31, 2005.
Fig. 1.
Flow diagram of cohort assembly and inclusion into the main and subgroup analyses. Single asterisk May meet more than one exclusion criterion. Double asterisk Study restricted to when both agents were used in our cohort, defined by the first date risedronate was dispensed in our cohort (June 16, 2002). Oral bisphosphonates were doses approved for the prevention or treatment of osteoporosis (alendronate [5, 10, 35, 70 mg], risedronate [5 or 35 mg], ibandronate [150 mg]). Weekly oral bisphosphonates studied were alendronate (70 mg) and risedronate (35 mg)
Outcomes
Our primary outcome was hospitalization for upper gastrointestinal bleed (hemorrhage or perforation) defined by primary discharge diagnosis in Medicare claims using previously validated criteria [14, 15]. Secondary outcomes included outpatient diagnosis for gastrointestinal diseases (peptic ulcer disease, gastrointestinal reflux disease, or gastritis), outpatient diagnosis for gastrointestinal symptoms (abdominal pain, dyspepsia, heartburn, nausea, or vomiting), upper gastrointestinal endoscopy, and use of gastroprotective agents (H2-receptor antagonists, proton pump inhibitors, misoprostol, or sucrulfate). Finally, we examined switching between agents as a general marker of side effects associated with initial therapy. Table 3 of the Appendix provides specific diagnostic and procedural codes used in our study.
Covariates
Patient demographics were determined at the time of treatment initiation and other variables by medical and pharmacy claims within the year prior to treatment initiation. Covariates included factors plausibly related to upper gastrointestinal morbidity [16–25] such as demographics (age, sex, race), upper gastrointestinal-related (e.g., in- and outpatient gastrointestinal disease, varices, Mallory–Weiss syndrome), osteoporosis-related diagnoses (e.g., kyphosis, osteoporosis, vertebral fracture), comorbidities (e.g., alcohol abuse, coagulation defects, chronic liver disease, Crohn’s disease, gastroenteritis, depression, overweight/obesity), drug use (e.g., antiplatelet/antithrombotic, gastroprotective agents, glucocorticoids, selective Cox-2 inhibitors, other nonsteroidal antiinflammatory, number of generics), and prior hospitalization. We also included calendar time (month and year) of the index prescription to adjust for potential secular trends in prescribing or coding. Table 4 of the Appendix lists all variables, definitions, and coding. If a record of a specific diagnosis, procedure, or prescription was lacking, patients were coded as not having these characteristics. As a result of this coding rule, there were no participants for whom exposure, confounder, or outcome information was missing.
Statistical analysis
Descriptive characteristics and covariates were summarized by user group. We used Cox proportional hazard models to compare the rates of occurrence of each outcome between weekly risedronate and weekly alendronate, censoring only on the date of death or end of follow-up (120 days following treatment initiation). We therefore followed surviving patients throughout the 120-day period of follow-up, regardless of whether or not prescription refills suggested continued exposure. We tested proportional hazards assumptions by including an interaction term between exposure and the log of time, finding no violations over the 120-day period of follow-up.
Alendronate was selected as the reference category in all Cox proportional hazard models. We developed exposure propensity scores for risedronate prescribing using logistic regression to adjust for confounding. Propensity score methods of adjustment are comparable to conventional multiple variable adjustment [26]. However, propensity score methods of adjustment reduce the number of covariates included in the outcome model and are thus advantageous when studying rare outcomes [27, 28]. We also restricted the adjusted analyses to recipients with overlapping propensity scores; minimum propensity scores equal to the lowest value observed among risedronate recipients (“exposed”) and maximum value equal to that observed among alendronate recipients (“unexposed”). Risedronate propensity score quintiles (four dummy variables) were used to adjust for confounding in Cox proportional hazard models.
In sensitivity analyses, we examined two composite outcomes: (1) any upper gastrointestinal diagnosis or procedure and (2) any gastrointestinal outcome considered, as well as modified our outcome coding for upper gastrointestinal procedure by restricting it to the most commonly identified codes in our cohort. In addition, we completed analyses stratified by age, defined by the median age of our cohort (less than or equal to median age versus greater than median age) and history of gastrointestinal events (gastrointestinal neoplasm or any upper gastrointestinal diagnosis, symptom, or procedure; versus none) within the year prior to treatment initiation. Analyses were completed using SAS 9.1 (SAS, Cary, NC, USA).
In a secondary analysis, we additionally censored patients on the first day of switching agents, losing drug plan eligibility, entering a nursing home, or discontinuing drug therapy (last date covered by drug plus 15 days, allowing for 30-day gaps between prescriptions) [13]. Finally, we examined the extent of residual confounding necessary to account for a null finding if in fact differences in hospitalization for gastrointestinal bleed exist between agents [29]. In applying the “array approach” (Microsoft Excel file available at www.drugepi.org) [29], we allowed the relative risk between the possible unmeasured confounder and gastrointestinal bleed to vary from 1 to 5.5 and the prevalence of this unmeasured confounder among risedronate recipients (exposed) to vary from 0% to 50%. We produced separate figures to compare results assuming that the prevalence among alendronate recipients (unexposed) was 10%, 20%, or 40%. We were specifically interested in examining the extent of confounding if the true relative risk was 0.70 (based on a previously reported estimate for a broad gastrointestinal outcome where the hazard ratio was 1.4 for alendronate compared to risedronate) [9].
The Partners HealthCare Institutional Review Board approved this project. Data Use Agreements are in place from PACE and the Centers for Medicare and Medicaid Services.
Results
Study cohort
Of 12,042 new recipients of weekly bisphosphonates identified, 1,622 were excluded (1,196 nursing home residents, 364 treated before the first dispensing of risedronate in our cohort, 63 dispensed alendronate solution, 19 had other previous bisphosphonate use, and 33 Paget disease). We studied the remaining 10,420 new recipients (Table 1). The mean age of our cohort was 78.7 years (SD=6.9, range 65–101, median=79), 95% women and 95% Caucasian. There was little evidence of potential confounding between alendronate and risedronate recipients, with each group being of similar age and having similar disease risk profiles (prevalence of comorbidities). However, slightly more risedronate recipients had a background history of gastrointestinal events (38% versus 35%) and had been treated with gastroprotective agents (38% versus 34%). In contrast, more alendronate recipients (23% versus 20%) had been hospitalized in the year prior to their index prescription. These differences disappeared within risedronate propensity score quintiles, with a mean difference between quintiles of 0.1% for gastrointestinal event history, 0.3% for gastroprotective agents, and 0.2% for previous hospitalization (Table 5 of the Appendix).
Table 1.
Characteristics of new recipients of weekly alendronate and weekly risedronate, June 2002–August 2005
| Alendronate recipients (n=5,818) | Risedronate recipients (n=4,602) | |
|---|---|---|
| Mean age (SD), years | 78.7 (6.8) | 78.7 (6.9) |
| Mean generic drugs (SD), n | 9.4 (5.4) | 9.5 (5.5) |
| Median comorbidity score (25th,75th percentile), n | 1 (0–3) | 1 (0–3) |
| Median physician visits (25th,75th percentile), n | 7 (3–12) | 7 (4–12) |
| Male, % | 5.2 | 4.0 |
| Caucasian, % | 94.7 | 95.9 |
| Hospitalization within previous year, % | 23.3 | 20.3 |
| Gastrointestinal-related comorbidities, % | ||
| Inpatient gastrointestinal diagnosis (ulcer or bleed) | 0.7 | 0.7 |
| Outpatient gastrointestinal diagnosis | 19.0 | 21.9 |
| Outpatient gastrointestinal disorder or discomfort | 23.2 | 23.3 |
| Upper gastrointestinal endoscopy | 4.9 | 4.8 |
| Helicobacter pylori infection | 0.3 | 0.2 |
| Varices or Mallory–Weiss syndrome | 0.1 | 0.1 |
| Gastrointestinal neoplasm | 6.1 | 6.9 |
| Any of the above (gastrointestinal event history) | 35.3 | 37.5 |
| Osteoporosis-related diagnoses, % | ||
| Kyphosis | 2.9 | 3.0 |
| Osteoporosis | 50.1 | 53.9 |
| Prior fracture—vertebral | 8.3 | 7.5 |
| Prior hip or arm fracture | 6.1 | 5.0 |
| Other comorbidities, % | ||
| Alcohol abuse | 0.3 | 0.1 |
| Chronic liver disease | 2.1 | 2.9 |
| Coagulation defects | 2.8 | 2.6 |
| Crohn’s disease or gastroenteritis | 4.0 | 3.5 |
| Depression | 10.0 | 10.4 |
| Diabetes mellitus | 13.9 | 14.6 |
| Heart failure | 14.3 | 14.2 |
| Hypertension | 68.3 | 68.8 |
| Nongastrointestinal, nonskin neoplasm | 18.5 | 19.0 |
| Osteoarthritis | 38.9 | 39.4 |
| Overweight or obese | 2.4 | 2.2 |
| Rheumatoid arthritis | 4.5 | 4.4 |
| Medication use, % | ||
| Antiplatelet/antithrombotic | 11.1 | 10.8 |
| Cardiovascular | 78.5 | 78.8 |
| Gastroprotective | 33.6 | 38.0 |
| Glucocorticoids | 13.5 | 13.5 |
| Selective Cox-2 inhibitor | 21.1 | 19.8 |
| Other nonsteroidal antiinflammatory | 14.2 | 13.3 |
| SSRI | 16.8 | 17.1 |
| Non-SSRI antipsychotic | 8.9 | 10.5 |
Comparative gastrointestinal safety
We observed 31 hospitalizations for upper gastrointestinal bleed (0.91 per 100 person-years) within 120 days of treatment initiation. We found no difference between the rates of hospitalization for upper gastrointestinal bleed (HR, 1.12; 95%CI, 0.55 to 2.28) or any other outcome considered in our primary analyses (Table 2). Although the number of events was too small to examine differences in hospitalization by gastrointestinal history or among those aged younger than 80 years, we found no difference between agents among those aged 80 or more years (2.58 bleeds per 100 person-years of follow-up; HR, 0.97; 95%CI, 0.45 to 2.08).
Table 2.
Upper gastrointestinal event rates by agent and relative safety of weekly risedronate compared with weekly alendronate
| Outcome of Interest | Alendronate
|
Risedronate
|
Hazard ratio (95%CI)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Events, n | Ratea | Events, n | Ratea | Unadjusted | P value | Adjustedb | P value | |
| Hospitalization for upper gastrointestinal bleed | 16 | 0.84 | 15 | 1.00 | 1.19 (0.59–2.40) | 0.63 | 1.12 (0.55–2.28) | 0.76 |
| Upper gastrointestinal disease | 612 | 34.26 | 508 | 36.10 | 1.05 (0.94–1.19) | 0.39 | 0.95 (0.84–1.07) | 0.41 |
| Upper gastrointestinal symptom | 662 | 37.26 | 516 | 36.70 | 0.99 (0.88–1.11) | 0.80 | 0.96 (0.85–1.07) | 0.45 |
| Upper gastrointestinal endoscopy | 134 | 7.13 | 90 | 6.05 | 0.85 (0.65–1.11) | 0.23 | 0.82 (0.62–1.07) | 0.142 |
| Gastroprotective treatment | 1,843 | 130.45 | 1,588 | 146.60 | 1.11 (1.04–1.19) | 0.002 | 0.98 (0.92–1.05) | 0.65 |
| Switched between therapies | 111 | 5.89 | 60 | 4.02 | 0.68 (0.50–0.93) | 0.02 | 0.67 (0.49–0.92) | 0.01 |
| Any upper gastrointestinal diagnosis or procedure | 1,058 | 62.31 | 867 | 64.87 | 1.04 (0.95–1.14) | 0.39 | 0.98 (0.89–1.07) | 0.61 |
| Any upper gastrointestinal diagnosis or procedure, or gastroprotective treatment | 2,263 | 170.71 | 1,920 | 189.90 | 1.10 (1.03–1.17) | 0.003 | 0.99 (0.93–1.05) | 0.75 |
Patients were censored on the date of death or 120 days after treatment initiation
CI confidence interval, HR hazard ratio
Rate per 100 person-years of follow-up
Adjusted for risedronate propensity score quintiles as four dummy variables
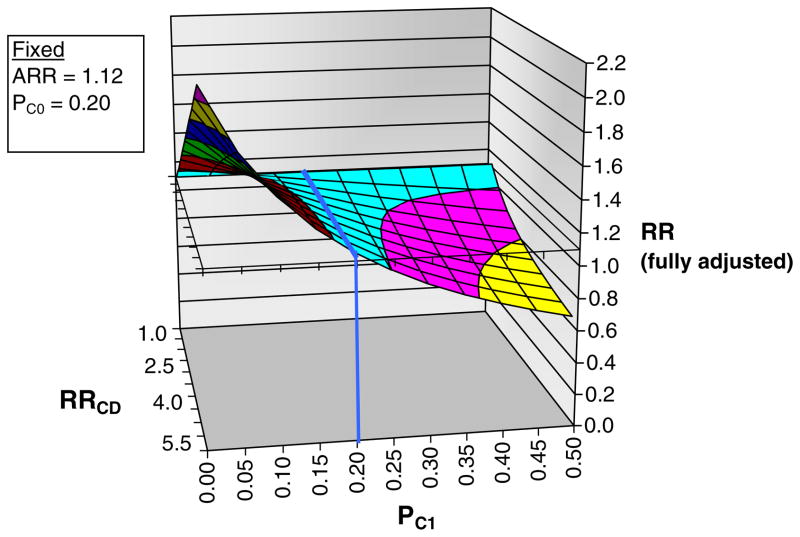
Using the array approach, it is unlikely that our null finding is due to residual confounding. For example, if an unmeasured confounder has a strong relationship with our outcome (relative risk of 5.0) and its prevalence is 20% among alendronate recipients, then its prevalence would need to be twofold (over 40%) higher among risedronate recipients to bring the “fully adjusted” estimate from the observed value of 1.12 to 0.70 (Fig. 3 of the Appendix). Similarly, if the prevalence of the confounder was only 10% (instead of 20% as described in the figure) among alendronate recipients, the prevalence of the confounder would need to be more than twofold higher (22% or more) to bring an observed estimate of 0.95 (e.g., observed for outpatient visits for gastrointestinal disease in our study) to a fully adjusted estimate of 0.7 (figure not shown).
Overall, we document no large differences between agents for secondary or composite outcomes, except that risedronate users had lower switching rates (Fig. 2). Lower switching rates among risedronate versus alendronate users was also observed in the secondary (“on-treatment”) analysis. In the main on-treatment analysis (hospitalization for gastrointestinal bleed), 59% were censored at the end of the 120 days of observation, and 39% were censored due to stopping treatment. Patients persisted with therapy for a mean of 87.6 days (SD=41.2), and there was no difference in persistence with therapy between agents (P=0.17). Among those with known history of gastrointestinal events, we found that rates for upper gastrointestinal endoscopic procedures were lower among risedronate (HR, 0.70; 95% CI, 0.49 to 0.99) compared with alendronate recipients. However, when endoscopies and switching between therapies were combined with upper gastrointestinal diagnoses and symptoms within 120 days after treatment initiation as the outcome, we found no large difference between agents (HR, 0.98; 95%CI, 0.92 to 1.04). Similar results were found when we examined endoscopies using the more restrictive coding (data not shown).
Fig. 2.
Adjusted hazard ratio (HR) estimates and 95% confidence intervals within 120 days of treatment initiation among new recipients of weekly risedronate versus weekly alendronate (alendronate is the reference group). a Primary analysis stratified by age. b Primary analysis stratified by gastrointestinal history. c Secondary (on-treatment) analysis, censoring patients on the first day of switching agents, losing drug plan eligibility, entering a nursing home, or discontinuing drug therapy (last date covered by drug plus 15 days, allowing for 30-day gaps between prescriptions), or 120 days after treatment initiation. GIDX gastrointestinal disease (outpatient diagnosis for peptic ulcer disease, gastrointestinal reflux disease, or gastritis). GISX gastrointestinal symptoms (outpatient diagnosis for abdominal pain, dyspepsia, heartburn, nausea, or vomiting). GIPX upper gastrointestinal endoscopy. DXSXPX any gastrointestinal diagnostic outcome (including hospitalization for upper gastrointestinal bleed) or procedure. GITX gastroprotective agent. SWITCH switched between therapies. ANY any gastrointestinal diagnostic outcome or procedure, or gastroprotective treatment, or switched between therapies
Discussion
A recent systematic review concluded that there is no difference in the rates of upper gastrointestinal events between alendronate or risedronate and placebo [30]. Our findings further suggest that there is no important difference in the gastrointestinal safety between weekly risedronate and weekly alendronate tablets. We did an English language MEDLINE search through April 2008 to identify any large comparative observational studies and relevant head-to-head trials examining gastrointestinal safety between weekly oral bisphosphonates. Prior observational studies comparing gastrointestinal safety of these agents document fewer gastrointestinal events with daily risedronate compared with daily or weekly alendronate [8, 9]. Although we found no difference in gastrointestinal safety, identified by upper gastrointestinal bleed, diagnosis, or symptom, we did identify that fewer risedronate users compared to alendronate users switched between therapies. The clinical significance of this finding, however, is unclear because we found no difference in persistence with therapy or in gastrointestinal diagnoses or symptoms documented between agents. The decision to switch between therapies indicated for the same condition is made based on the prescriber’s perceptions about the general adverse events caused by each agent. Risedronate was heavily marketed as having a safer gastrointestinal risk profile compared to alendronate. Caution in interpretation regarding the clinical significance of switching between agents in this observational study is thus warranted.
Our findings are consistent with results from the Fosamax Actonel Comparison Trial (FACT) that found comparable gastrointestinal safety between weekly alendronate and risedronate among 1,053 postmenopausal women (mean age= 64.5 years) with low bone mineral density [10]. Randomized controlled trials such as the FACT establish drug efficacy within defined patient populations, often not representative of those who may benefit from pharmacotherapy or how the agents, are used in practice [31]. We studied a much larger and older cohort of new bisphosphonate recipients, and our findings complement and corroborate FACT results.
Although our study design may allow for wider generalizability compared to prior studies [8–10], we are limited because our study relies entirely on claims data. Data not captured within administrative claims may be associated with gastrointestinal outcomes. For example, we lacked data on cigarette smoking [23], alcohol consumption, body mass index [18], and the use of over-the-counter products such as nonsteroidal antiinflammatory agents or gastroprotective medications. More risedronate recipients in our study had a background history of gastrointestinal disease and had been prescribed a gastro-protective agent. Channeling of patients with gastrointestinal disease to risedronate versus alendronate has also been observed in a cohort of chronic glucocorticoid users [32]. If risedronate recipients in our study also had more risk factors for upper gastrointestinal disease not measured by our data, our null findings may be attributed to residual confounding. However, using the “array approach” for sensitivity analysis [29], a strong confounder (e.g., relative risk of 5.0) and large imbalances between exposure groups (e.g., 20% alendronate versus 40% risedronate) would be required to account for our null findings. Instead, we document very small differences in background history of gastrointestinal disease (about 3%) using data measured in healthcare utilization data. We therefore do not believe that the differences between unmeasured factors such as alcohol consumption, cigarette smoking, or body mass index would fully explain our findings of comparable gastrointestinal safety.
We found a consistent lack of difference in risk between agents among those with no gastrointestinal history. However, among the smaller cohort of patients with history of gastrointestinal events, risedronate recipients had lower rates of endoscopy. Although the definition of our primary outcome (hospitalization for upper gastrointestinal bleed) has been validated, we cannot rule out the possibility that outpatient diagnoses and symptoms identified in claims data reflect care to “rule-out” upper gastrointestinal disease or follow-up visits for preexisting conditions. It is thus difficult to make strong conclusions based on this secondary endpoint alone. It could be that endoscopy represents more severe gastrointestinal side effects, or it could represent a differential in practice style between physicians who prescribe alendronate versus risedronate. Indeed, after combining endoscopy with outpatient diagnoses for upper gastrointestinal disease or symptoms, we observed no difference between agents. Therefore, the clinical importance of differences in the rates of upper gastrointestinal endoscopy identified by our study is unclear and caution in interpretation is advised.
In addition to those already mentioned, four limitations are worth noting. First, by studying a cohort of frail older adults from Pennsylvania, our results may not be general-izable to all users of these agents, such as younger patients with chronic glucocorticoid treatment. However, given that oral bisphosphonates are largely prescribed to treat primary osteoporosis, we believe our study population to be a significant strength. We were able to study a large cohort of older adults with complete drug coverage without restrictions (alendronate and risedronate are fully covered with the same co-payment), and older frail adults are at the highest risk for gastrointestinal disease [19].
Second, our study database only contained claims data to determine gastrointestinal outcomes and may thus have misclassified some events. Prior studies have found that gastrointestinal adverse events are one of the main reasons for discontinuing bisphosphonate treatment for osteoporosis [33–36]. In our secondary (on-treatment) analysis, we document that 39% were censored due to lack of persistence with treatment and another 1% switched between agents. Although there were no differences in persistence or reasons for censoring between agents, we may have underestimated the extent of gastrointestinal side effects resulting from bisphosphonate treatment. For these reasons, we completed our primary analyses using an “intention-to-treat scenario” and followed surviving patients for 120 days. This analytic approach allows for a time lag between when a patient’s symptoms appear (may stop taking medication) and presentation in a physician’s office with an upper gastrointestinal complaint (requires time to book an appointment with a physician or to be referred for endoscopy). We also focused on hospitalization for upper gastrointestinal bleed using previously validated criteria [14, 15] as our primary outcome, and there is no reason to believe that there would be differential misclassification in hospitalizations between bisphosphonate therapies.
Third, our study is limited by statistical power due to few events observed for our primary outcome (hospitalization for gastrointestinal bleed). One method to increase the number of events under study would be to lengthen the period of observation beyond 120 days. However, prior research has documented that upper gastrointestinal problems from oral bisphosphonate therapy occur early in the time course of treatment [6, 7], and thus, 120 days is an appropriate risk window of observation. Extending the length of observation could also introduce information bias such as depletion of susceptibles [37]. Similarly, we were concerned about the potential for informative censoring using an on-treatment analysis, whereby patients experiencing early gastrointestinal discomfort stop taking their bisphosphonate and would thus be censored from analysis before a signal is detected in claims data.
Finally, we were unable to examine the comparative gastrointestinal safety of newer oral bisphosphonate formulations. Ibandronate received FDA approval for osteoporosis treatment using monthly dosing in March 2005. The prolonged dose-free interval may result in fewer gastrointestinal events [12], but this remains to be documented by comparison with weekly bisphosphonates in prospective studies. Risedronate also received FDA approval in April 2007 for monthly dosing (75 mg tablet on two consecutive days) to treat postmenopausal osteoporosis. Future studies that examine the comparative gastrointestinal safety of oral bisphosphonates are of interest.
In conclusion, we found no important difference in upper gastrointestinal safety between weekly alendronate and weekly risedronate. Future studies may help to further identify if differences in gastrointestinal safety exist between oral bisphosphonates, particularly by dosing interval (e.g., weekly versus monthly) and among patients with different risk profiles (e.g., younger age and gastrointestinal risk).
Acknowledgments
The authors thank Dr. Sebastian Schneeweiss, MD, ScD, Director for Drug Evaluation and Outcomes Research, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, for helpful comments and discussions.
Grant support Dr Brookhart is supported by the National Institute on Aging (K25 AG027400), Dr Cadarette was supported by the Canadian Institutes of Health Research (Post-Doctoral Fellowship), Dr Katz is supported by the National Institute of Arthritis and Muskuloskeletal and Skin Diseases (K24 AR02123 and P60 AR47782), Dr Solomon is supported by the Arthritis Foundation and the National Institutes of Health (K24 AR055989, R21 AG027066, and P60 AR47782), and Dr. Stürmer is supported by the National Institute on Aging (RO1 AG023178) and the UNC-GSK Center for Excellence in Pharmacoepidemiology and Public Health.
Appendix
Table 3.
Study outcome coding (diagnoses and procedures)
| Variable | Definition |
|---|---|
| Primary outcomea | |
| Hospitalization for upper gastrointestinal bleed (hemorrhage or perforation) | Primary discharge diagnosis of ICD-9-CM codes 531.0x, 531.1x, 531.2x, 531.4x, 531.5x, 531.6x, 532.0x, 532.1x, 532.2x, 532.4x, 532.5x, 532.6x, 533.0x, 533.1x, 533.2x, 533.4x, 533.5x, 533.6x, 578.0x, 578.1x, 578.9x |
| Secondary outcomesb | |
| 1 Outpatient diagnosis for gastrointestinal disease (peptic ulcer disease, gastrointestinal reflux disease, or gastritis) | ICD-9-CM codes 530.1x, 530.2x, 530.3x, 530.4x, 530.8, 530.81, 530.82, 530.89, 530.9x, 531.xx, 532. xx, 533.xx, 535.0x, 535.4x, 535.5x, 535.6x |
| 2 Outpatient diagnosis for gastrointestinal symptom (abdominal pain, dyspepsia, heartburn, nausea, or vomiting) | ICD-9-CM codes 536.2x, 536.8x, 536.9x, 537.89, 537.9x, 578.xx, 787, 787.0x, 787.1x, 787.2x, 787.3x, 789, 789.0x |
| 3 Upper gastrointestinal endoscopy | ICD-9-CM codes 45.13, 45.14, 45.16, 44.43 and CPT-4 codes 43234–43259 |
| 4 Use gastroprotective agent | Any pharmacy claim for H2- receptor antagonists, proton pump inhibitors, misoprostol, or sucrulfate |
| 5 Switched between therapies | First date filled prescription for the other agent |
| 6 Any upper gastrointestinal diagnosis or procedure | Primary outcome and/or secondary outcome nos. 1–3 |
| 7 Any gastrointestinal diagnostic outcome or procedure or gastroprotective treatment | Any of the above |
CPT-4 Current Procedural Terminology, 4th edition, ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification
Validation studies that compared claims data to hospital medical charts in Canada [14] and Spain [15] have found that over 90% of patients with one of these codes as their primary hospital discharge diagnosis were indeed hospitalized for upper gastrointestinal bleed
Although we are unaware of validation data for outpatient diagnostic codes, the positive predictive value of using ICD-9-CM procedural codes for upper gastrointestinal endoscopy has been reported to be over 97% [38]. We supplemented these ICD-9-CM procedural codes with CPT-4 codes to improve the sensitivity of our estimates [8]. Of those identified as having had an upper gastrointestinal endoscopy in our study (N=224), 5% had ICD-9-CM codes only, 41% had CPT-4 codes only, and 54% had both types of codes. In a sensitivity analysis, we restricted coding to the most commonly identified codes (CPT-4 codes 43235, 43239; N=180)
Table 4.
Definition and coding of variables included in logistic regression model used to create propensity scores
| Variable | Definition | Coding |
|---|---|---|
| Medicare enrolment information at time of index prescription | ||
| Age | Age in years | Categorical, one category for each age, except grouping ages 96+ into a single reference group (31 dummies) |
| Male sex | Male sex | Dichotomous (yes/no) |
| Race | Caucasian race | Dichotomous (yes/no) |
| Date | Month/year of index prescription | Categorical (month/year), one category for each month/year (38 dummies) |
| Medicare claims within 365 days prior to index drug prescription | ||
| Hospitalization | Any | Dichotomous (yes/no) |
| Comorbidity score | Charlson comorbidity score [39, 40] | Ordinal (quartiles, 3 dummies) |
| Upper gastrointestinal (GI) | ||
| Inpatient GI diagnosis (ulcer or bleed) | Any hospital discharge diagnosis of ICD-9-CM codes 531.xx-535.xx, 578.xx, 537.83 | Dichotomous (yes/no) |
| Outpatient GI diagnosis | ICD-9-CM codes 530.xx-535.xx | Dichotomous (yes/no) |
| Outpatient GI disorder or abdominal discomfort | ICD-9-CM codes 536.xx-538.xx, 787.xx, 789.xx, 793.4 | Dichotomous (yes/no) |
| Upper GI endoscopy | ICD-9-CM codes 45.13, 45.14, 45.16, 44.43 and CPT-4 codes 43234-43259 | Dichotomous (yes/no) |
| Helicobacter pylori infection | ICD-9-CM codes 041.86 | Dichotomous (yes/no) |
| Varices or Mallory–Weiss syndrome | ICD-9-CM codes 456.0x, 456.20,456.1x, 456.21, | Dichotomous (yes/no) |
| Gastrointestinal neoplasm | ICD-9-CM codes 140.xx-159, 210.xx, 211.xx, 230.xx, 235.1x-235.5x, | Dichotomous (yes/no) |
| Osteoporosis-related | ||
| Osteoporosis | ICD-9-CM codes 733.0x | dichotomous (yes/no) |
| Kyphosis | ICD-9-CM codes 737.1x, 737.41, 737.3x | dichotomous (yes/no) |
| Prior vertebral fracture | Vertebral (ICD-9-CM codes 733.13, 805.xx) | dichotomous (yes/no) |
| Prior hip or arm fracture | ICD-9-CM codes 820.xx, 733.14, 812.xx, 733.11, 813.xx, 733.12 | dichotomous (yes/no) |
| Other relevant comorbidities | ||
| Alcohol abuse | ICD-9-CM codes 303.xx, V11.3, 291.xx, 571.0x, 571.1x, 571.2x, 571.3x, 357.5x, 535.3x, 425.5x, 265.2x, E860.0x | Dichotomous (yes/no) |
| Chronic liver disease | ICD-9-CM codes 571.4x, 571.6x, 571.8x, 571.9x, 573.xx, 070.xx | Dichotomous (yes/no) |
| Coagulation defects | ICD-9-CM codes 286.xx | Dichotomous (yes/no) |
| Crohn’s disease or gastroenteritis | ICD-9-CM codes 555.xx, 556.xx, 558.xx | Dichotomous (yes/no) |
| Depression | ICD-9-CM codes 293.83, 296.2x. 296.3x, 298.0x, 300.4x, 309.0x, 309.1x, 309.28, 311.xx | Dichotomous (yes/no) |
| Diabetes mellitus | ICD-9-CM codes 250.xx and recipient of diabetic drug | Dichotomous (yes/no) |
| Heart failure | ICD-9-CM codes 428.xx | |
| Hypertension | ICD-9-CM codes 416.0x, 401.xx, 402.xx | Dichotomous (yes/no) |
| Liver disease | ICD-9-CM codes 571.4x, 571.6x, 571.8x, 571.9x, 573.xx, 070.xx | Dichotomous (yes/no) |
| Non-GI, non- skin neoplasm | ICD-9-CM codes 160.xx- 171.xx, 174.xx-208.xx, 212.xx-229.xx, 231.xx- 234.xx, 235.6x-239.xx | Dichotomous (yes/no) |
| Osteoarthritis | ICD-9-CM codes 715.xx | Dichotomous (yes/no) |
| Overweight or obese | ICD-9-CM codes 278, 278.0x | Dichotomous (yes/no) |
| Rheumatoid arthritis | ICD-9-CM codes 714.xx | Dichotomous (yes/no) |
| Pharmacy claims within 365 days prior to index drug prescription | ||
| Number of generics | Count | Ordinal (quintiles, 4 dummies) |
| Antiplatelet/antithrombotic | Any pharmacy claim | Dichotomous (yes/no) |
| Gastroprotective | Any pharmacy claim | Dichotomous (yes/no) |
| Glucocorticoids | Any pharmacy claim | Dichotomous (yes/no) |
| Selective Cox-2 inhibitor | Any pharmacy claim | Dichotomous (yes/no) |
| Other NSAID | Any pharmacy claim | Dichotomous (yes/no) |
| SSRI | Any pharmacy claim | Dichotomous (yes/no) |
| Non-SSRI antipsychotic | Any pharmacy claim | Dichotomous (yes/no) |
COX-2 cyclooxygenase-2, GI gastrointestinal, ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification, NSAID nonsteroidal antiinflammatory drug, SSRI selective serotonin reuptake inhibitors
Table 5.
Cohort characteristics within propensity score quintiles for risedronate prescribing by drug received, overlapping propensity scores (N=10,416)
| Covariate | Quintile 1
|
Quintile 2
|
Quintile 3
|
Quintile 4
|
Quintile 5
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALD | RSD | ALD | RSD | ALD | RSD | ALD | RSD | ALD | RSD | |
| No. | 1,400 | 683 | 1,244 | 839 | 1,152 | 932 | 1,091 | 992 | 929 | 1,154 |
| Mean age, years | 79.4 | 79.5 | 78.3 | 77.8 | 78.4 | 78.1 | 78.4 | 78.6 | 79.0 | 79.3 |
| Men, % | 11.9 | 10.5 | 5.5 | 5.1 | 3.1 | 3.2 | 1.9 | 2.2 | 0.8 | 1.5 |
| White, % | 88.6 | 88.3 | 94.1 | 93.8 | 95.9 | 97.0 | 99.0 | 98.2 | 98.2 | 99.0 |
| Year of index prescription, % | ||||||||||
| 2002 | 39.5 | 38.2 | 21.9 | 22.6 | 15.2 | 14.7 | 9.6 | 9.7 | 3.7 | 3.1 |
| 2003 | 21.0 | 20.6 | 27.7 | 28.5 | 30.0 | 30.8 | 34.6 | 32.2 | 35.4 | 36.9 |
| 2004 | 11.0 | 12.3 | 21.7 | 18.8 | 29.5 | 28.5 | 36.2 | 39.0 | 50.2 | 51.0 |
| 2005 | 28.5 | 28.8 | 28.8 | 30.0 | 25.3 | 26.0 | 19.5 | 19.2 | 10.8 | 8.9 |
| Hospitalizations in previous year, % | 37.9 | 40.6 | 24.6 | 22.5 | 21.4 | 18.8 | 13.9 | 15.5 | 12.6 | 11.9 |
| Gastrointestinal-related comorbidities, % | ||||||||||
| Hospitalization for peptic ulcer disease or bleed | 0.9 | 0.7 | 0.5 | 0.4 | 0.8 | 0.6 | 0.7 | 0.5 | 0.4 | 1.0 |
| Outpatient GI diagnosis | 10.9 | 12.3 | 13.3 | 12.8 | 17.5 | 16.1 | 22.6 | 22.9 | 36.2 | 38.0 |
| Outpatient disease or discomfort | 24.6 | 24.3 | 23.6 | 21.9 | 21.4 | 20.6 | 20.9 | 22.2 | 25.5 | 26.9 |
| Upper GI endoscopy | 6.0 | 5.0 | 4.7 | 3.7 | 4.2 | 4.7 | 4.2 | 5.4 | 5.1 | 5.1 |
| Helicobacter pylori infection | 0.5 | 0.6 | 0.5 | 0.0 | 0.2 | 0.4 | 0.1 | 0.1 | 0.0 | 0.1 |
| Varices or Mallory–Weiss syndrome | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.2 | 0.3 |
| GI neoplasm | 3.8 | 3.1 | 5.9 | 3.3 | 5.1 | 6.4 | 7.0 | 7.6 | 10.1 | 11.7 |
| Any GI event (any of above) | 30.5 | 31.2 | 30.9 | 29.2 | 32.3 | 30.7 | 36.8 | 37.2 | 50.4 | 53.1 |
| Osteoporosis-related, % | ||||||||||
| Kyphosis | 2.6 | 2.6 | 2.7 | 1.9 | 2.9 | 2.8 | 3.8 | 3.0 | 2.5 | 4.1 |
| Osteoporosis | 36.6 | 41.0 | 41.7 | 43.5 | 51.6 | 46.7 | 57.8 | 59.1 | 70.9 | 70.5 |
| Prior vertebral fracture | 11.9 | 13.6 | 8.5 | 8.0 | 7.6 | 6.5 | 7.0 | 6.4 | 5.3 | 5.5 |
| Prior hip or arm fracture | 12.4 | 13.2 | 5.6 | 6.0 | 5.1 | 3.8 | 3.1 | 2.5 | 1.8 | 2.4 |
| Other relevant comorbidities, % | ||||||||||
| Alcohol abuse | 0.9 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Chronic liver disease | 0.5 | 0.7 | 1.4 | 0.7 | 1.5 | 2.1 | 2.1 | 1.6 | 6.5 | 7.4 |
| Coagulation defects | 4.6 | 4.4 | 2.9 | 2.9 | 1.7 | 2.1 | 2.2 | 2.1 | 2.2 | 2.0 |
| Crohn’s disease or gastroenteritis | 5.9 | 8.1 | 4.7 | 3.9 | 3.2 | 3.0 | 2.4 | 2.1 | 3.0 | 2.0 |
| Depression | 9.9 | 10.7 | 8.0 | 7.9 | 8.5 | 8.3 | 11.5 | 10.0 | 12.8 | 14.0 |
| Diabetes mellitus | 11.9 | 11.6 | 11.8 | 14.1 | 14.7 | 13.8 | 14.5 | 15.1 | 18.3 | 16.7 |
| Heart failure | 16.8 | 19.2 | 12.1 | 10.8 | 12.8 | 12.3 | 14.6 | 12.0 | 15.1 | 17.1 |
| Hypertension | 68.8 | 70.0 | 62.8 | 65.9 | 66.9 | 66.3 | 69.7 | 71.5 | 75.2 | 69.9 |
| Non-GI, nonskin neoplasm | 17.6 | 17.9 | 16.8 | 17.5 | 18.7 | 14.7 | 18.7 | 18.9 | 21.4 | 24.3 |
| Osteoarthritis | 39.8 | 38.9 | 36.6 | 36.8 | 39.0 | 36.8 | 38.1 | 37.7 | 41.4 | 44.9 |
| Overweight or obese | 3.1 | 3.2 | 2.5 | 2.0 | 3.0 | 3.2 | 1.9 | 1.5 | 1.3 | 1.6 |
| Rheumatoid arthritis | 5.2 | 4.8 | 3.9 | 3.7 | 5.2 | 4.7 | 4.2 | 4.5 | 3.7 | 4.3 |
| Mean comorbidity score, No. | 1.8 | 1.8 | 1.6 | 1.5 | 1.6 | 1.5 | 1.6 | 1.6 | 1.8 | 1.8 |
| Mean generics, No. | 9.3 | 9.8 | 9.0 | 9.1 | 9.1 | 9.1 | 9.6 | 9.3 | 10.3 | 10.2 |
| Pharmacy claims within 365 days prior to index osteoporosis drug prescription, % | ||||||||||
| Antiplatelet/antithrombotic | 12.9 | 11.9 | 10.1 | 11.2 | 11.3 | 11.7 | 10.1 | 9.4 | 10.7 | 10.5 |
| Cardiovascular | 77.6 | 77.5 | 77.3 | 78.1 | 77.6 | 77.9 | 79.9 | 80.9 | 80.9 | 78.9 |
| Gastroprotective | 20.0 | 21.2 | 23.2 | 23.5 | 33.6 | 32.6 | 40.7 | 40.5 | 59.4 | 60.5 |
| Glucocorticoids | 14.1 | 13.3 | 12.9 | 12.6 | 13.5 | 14.1 | 11.8 | 13.6 | 15.4 | 13.7 |
| Selective Cox-2 inhibitor | 25.2 | 27.2 | 24.0 | 23.2 | 21.8 | 19.2 | 16.3 | 17.9 | 15.6 | 15.1 |
| Other NSAID | 17.7 | 17.9 | 15.4 | 15.9 | 14.9 | 13.9 | 11.0 | 11.3 | 10.4 | 10.1 |
| SSRI | 17.7 | 17.4 | 14.5 | 13.8 | 16.2 | 14.8 | 18.7 | 19.0 | 17.2 | 19.6 |
| Non-SSRI antipsychotic | 4.0 | 6.4 | 5.5 | 6.4 | 8.8 | 6.1 | 9.8 | 10.6 | 19.8 | 19.1 |
COX-2 cyclooxygenase-2, GI gastrointestinal, NSAID nonsteroidal antiinflammatory drug, SSRI selective serotonin reuptake inhibitors, ALD alendronate, RSD risedronate
Fig. 3.
Sensitivity analysis of residual confounding using the array approach for our primary outcome (hospitalization for upper gastrointestinal bleed). To facilitate interpretation by the reader, we have maintained the notation used in the explanatory article and Excel program that we modified (available at www.drugepi.org) to produce this figure [29]. ARR apparent exposure relative risk, i.e., the relative risk (hazard ratio) observed in the current study (risedronate versus alendronate), fixed at 1.12. PC0 prevalence of counfounder among unexposed (alendronate), fixed at 20% in this example. PC1 prevalence of confounder among exposed (risedronate) group, varied from 0% to 50% in the figure. RRCD association between confounder and disease outcome, varied from 1.0 to 5.5 in figure. RR fully adjusted or “true” exposure relative risk. Blue line no confounding present, prevalence of confounder same among alendronate and risedronate recipients
Footnotes
Conflicts of interest Co-authors have received salary support in the last 5 years from research grants to the Brigham and Women’s Hospital for unrelated work from: Amgen (Dr Brookhart), GlaxoSmithKline (Dr Stürmer), Merck (Drs. Solomon and Stürmer), Novartis (Dr Katz), Pfizer, Procter & Gamble, and Savient (Dr Solomon). There was no pharmaceutical industry support for this study.
Contributor Information
S. M. Cadarette, Email: s.cadarette@utoronto.ca, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Leslie Dan Faculty of Pharmacy, University of Toronto, 144 College Street, Toronto, Ontario M5S 3M2, Canada
J. N. Katz, Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Department of Orthopaedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
M. A. Brookhart, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
T. Stürmer, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
M. R. Stedman, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
R. Levin, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
D. H. Solomon, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
References
- 1.Stafford RS, Drieling RL, Hersh AL. National trends in osteoporosis visits and osteoporosis treatment, 1988–2003. Arch Intern Med. 2004;164:1525–1530. doi: 10.1001/archinte.164.14.1525. [DOI] [PubMed] [Google Scholar]
- 2.Lee E, Wutoh AK, Xue Z, Hillman JJ, Zuckerman IH. Osteoporosis management in a Medicaid population after the Women’s Health Initiative study. J Womens Health. 2006;15:155–161. doi: 10.1089/jwh.2006.15.155. [DOI] [PubMed] [Google Scholar]
- 3.Udell JA, Fischer MA, Brookhart MA, Solomon DH, Choudhry NK. Effect of the Women’s Health Initiative on osteoporosis therapy and expenditure in Medicaid. J Bone Miner Res. 2006;21:765–771. doi: 10.1359/jbmr.060119. [DOI] [PubMed] [Google Scholar]
- 4.Cadarette SM, Katz JN, Brookhart MA, Levin R, Stedman MR, Choudhry NK, Solomon DH. Trends in drug prescribing for osteoporosis after hip fracture, 1995–2004. J Rheumatol. 2008;35:319–326. [PMC free article] [PubMed] [Google Scholar]
- 5.Bobba RS, Beattie K, Parkinson B, Kumbhare D, Adachi JD. Tolerability of different dosing regimens of bisphosphonates for the treatment of osteoporosis and malignant bone disease. Drug Safety. 2006;29:1133–1152. doi: 10.2165/00002018-200629120-00005. [DOI] [PubMed] [Google Scholar]
- 6.Lanza FL, Hunt RH, Thomson ABR, Provenza JM, Blank MA Risedronate Endoscopy Study Group. Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterol. 2000;119:631–638. doi: 10.1053/gast.2000.16517. [DOI] [PubMed] [Google Scholar]
- 7.Thomson ABR, Marshall JK, Hunt RH, Provenza JM, Lanza FL, Royer MG, Li Z, Blank MA Risedronate Endoscopy Study Group. 14 day endoscopy study comparing risedronate and alendronate in postmenopausal women stratified by Helicobacter pylori status. J Rheumatol. 2002;29:1965–1974. [PubMed] [Google Scholar]
- 8.Kane S, Borisov NN, Brixner D. Pharmacoeconomic evaluation of gastrointestinal tract events during treatment with risedronate or alendronate: a retrospective cohort study. Am J Manag Care. 2004;10:S216–S226. [Google Scholar]
- 9.Miller RG, Bologness M, Worley K, Solis A, Sheer R. Incidence of gastrointestinal events among bisphosphonate patients in an observational setting. Am J Manag Care. 2004;10:S207–S215. [Google Scholar]
- 10.Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE Fosamax Actonel Comparison Trial Investigators. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- 11.Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SM, Sebba A, Kagan R, Chen E, Thompson DE, de Papp AE Fosamax Actonel Comparison Trial (FACT) Investigators. Comparison of weekly treatment of post-menopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab. 2006;91:2631–2637. doi: 10.1210/jc.2005-2602. [DOI] [PubMed] [Google Scholar]
- 12.Epstein S, Delmas PD, Emkey R, Wilson KM, Hiltbrunner V, Schimmer RC. Oral ibandronate in the management of postmenopausal osteoporosis: review of upper gastrointestinal safety. Maturitas. 2006;54:1–10. doi: 10.1016/j.maturitas.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Rainford DS, Gutthann SP, Rodriguez LAG. Positive predictive value of ICD-9 codes in the identification of cases of complicated peptic ulcer disease in the Saskatchewan Hospital Automated Database. Epidemiol. 1996;7:101–104. doi: 10.1097/00001648-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Cattaruzzi C, Troncon MG, Agostinis L, Rodriguez LAG. Positive predictive value of ICD-9th codes for upper gastrointestinal bleeding and perforation in the Sistema Informativo Sanitario Regionale Database. J Clin Epidemiol. 1999;52:499–502. doi: 10.1016/s0895-4356(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 16.Dalton SO, Johansen C, Mellemkjær L, Nørgård B, Sørensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Donahue JG, Chan KA, Andrade SE, Beck A, Boles M, Buist DSM, Carey VJ, Chandler JM, Chase GA, Ettinger B, Fishman P, Goodman M, Guess HA, Gurwitz JH, LaCroix AZ, Levin TR, Platt R. Gastric and duodenal safety of daily alendronate. Arch Intern Med. 2002;162:936–942. doi: 10.1001/archinte.162.8.936. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Body-mass index and symptoms of gastroesophageal reflux in women. New Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zullo A, Hassan C, Campo SMA, Morini S. Bleeding peptic ulcer in the elderly: risk factors and prevention strategies. Drugs Aging. 2007;24:815–828. doi: 10.2165/00002512-200724100-00003. [DOI] [PubMed] [Google Scholar]
- 20.Tata LJ, Fortun PJ, Hubbard RB, Smeeth L, Hawkey CJ, Smith CJP, Whitaker HJ, Farrington CP, Card TR, West J. Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding? Aliment Pharmacol Ther. 2005;22:175–181. doi: 10.1111/j.1365-2036.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-Diaz S, Rodriguez LAG. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990 s. Arch Intern Med. 2000;160:2093–2099. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi T, Sugimoto T, Yamauchi M, Matsumori Y, Tsutsumi M, Chihara K. Multiple vertebral fractures are associated with refractory reflux esophagitis in postmenopausal women. J Bone Miner Metab. 2005;23:36–40. doi: 10.1007/s00774-004-0538-7. [DOI] [PubMed] [Google Scholar]
- 23.Weil J, Langman MJS, Wainwright P, Lawson DH, Rawlins M, Logan RFA, Brown TP, Vessey MP, Murphy M, Colin-Jones DG. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000;46:27–31. doi: 10.1136/gut.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007;76:1005–1012. [PubMed] [Google Scholar]
- 25.Udd M, Miettinen P, Palmu A, Heikkinen M, Janathuinen E, Pasanen P, Tarvainen R, Mustonen H, Julkunen R. Analysis of the risk factors and their combinations in acute gastroduodenal ulcer bleeding: a case-control study. Scand J Gastroenterol. 2007;42:1395–1403. doi: 10.1080/00365520701478758. [DOI] [PubMed] [Google Scholar]
- 26.Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161:891–898. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–447. [Google Scholar]
- 28.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 30.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay R. Beyond clinical trials: the importance of large databases in evaluating differences in the effectiveness of bisphosphonate therapy in postmenopausal osteoporosis. Bone. 2007;40:S32–S35. [Google Scholar]
- 32.Curtis JR, Westfall AO, Allison JJ, Freeman A, Saag KG. Channeling and adherence with alendronate and risedronate among chronic glucocorticoid users. Osteoporos Int. 2006;17:1268–1274. doi: 10.1007/s00198-006-0136-8. [DOI] [PubMed] [Google Scholar]
- 33.Tosteson ANA, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, Pressman AR, Ettinger B. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115:209–216. doi: 10.1016/s0002-9343(03)00362-0. [DOI] [PubMed] [Google Scholar]
- 34.Carr AJ, Thompson PW, Cooper C. Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int. 2006;17:1638–1644. doi: 10.1007/s00198-006-0166-2. [DOI] [PubMed] [Google Scholar]
- 35.Ideguchi H, Ohno S, Hattori H, Ishigatsubo Y. Persistence with bisphophonate therapy including treatment courses with multiple sequential bisphosphonates in the real world. Osteoporos Int. 2007;18:1421–1427. doi: 10.1007/s00198-007-0406-0. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259–262. doi: 10.1007/s00198-002-1370-3. [DOI] [PubMed] [Google Scholar]
- 37.Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47:731–737. doi: 10.1016/0895-4356(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 38.Cooper GS, Chak A, Lloyd LE, Yurchick PJ, Harper DL, Rosenthal GE. The accuracy of diagnosis and procedural codes for patients with upper GI hemorrhage. Gastrointest Endosc. 2000;51:423–426. doi: 10.1016/s0016-5107(00)70442-1. [DOI] [PubMed] [Google Scholar]
- 39.Schneeweiss S, Wang PS, Avorn J, Maclure M, Levin R, Glynn RJ. Consistency of performance ranking of comorbidity adjustment scores in Canadian and U.S. utilization data. J Gen Intern Med. 2004;19:444–450. doi: 10.1111/j.1525-1497.2004.30109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]