Abstract
Objective
To assess maternal cardiovascular function using echocardiography in normal and preeclamptic women in the third trimester of pregnancy.
Methods
40 subjects, 20 with preeclampsia and 20 normotensive controls with >34 weeks gestation and singleton pregnancy were recruited. Baseline characteristics, maternal and fetal outcome were studied with systolic and diastolic parameters on echocardiography.
Results
The following parameters were higher in preeclamptic subjects as compared to normotensive controls–mean cardiac output (66.85 ± 4.56 ml/min vs. 56.1 ± 1.77 ml/min); mean LV diastolic mass (131.15 ± 16.85 vs. 104.90 ± 23.17 g); systolic mass (88.5 ± 7.34 vs. 83.33 ± 23.84 g); total vascular resistance (1396.85 ± 150.2 vs. 1204.5 ± 71.182 dyne, s cm5). Women with preeclampsia delivered smaller babies (2410 ± 426.16 g) as compared to normotensive controls (2895 ± 276.20 g). Student ‘t’ test was used as a test of significance.
Conclusion
Women with preeclampsia have significant systolic and diastolic dysfunction compared to normotensive controls. Blood pressure monitoring alone is insufficient to identify effectively, risk of cardiovascular complications in these subjects.
Keywords: Echocardiography, Maternal, Systolic, Diastolic, Normal, Preeclampsia
Introduction
Preeclampsia is a disease unique to pregnancy and it is characterized by progressive hypertension, pathological edema and proteinuria. It affects 5% of pregnancies [1]. The hemodynamics of preeclampsia is a subject of controversies. Cross-sectional studies of women with preeclampsia have revealed diverse hemodynamic findings such as elevated cardiac output [2], high vascular resistance and reduced cardiac output [3] and reduced myocardial contractility [4]. The data on changes in left ventricular diastolic function is scarce. In addition, there is conflicting information about left ventricular performance both during normotensive and hypertensive pregnancy. Normal, increased and depressed function have all been reported at various stages of gestation [5–8].
This exploratory study was undertaken to assess the cardiovascular hemodynamic alterations in preeclampsia on echocardiography and its impact on maternal and fetal outcome.
Subjects and Methods
This case control study was carried out in the department of Obstetrics and Gynecology, SSG Hospital, Government Medical College Baroda. Forty subjects were enrolled, of which 20 had preeclampsia and 20 were normotensive. Subjects were classified as preeclamptic if blood pressure (BP) was ≥140/90 mmHg with proteinuria greater than +1 on urine dipstick examination. Blood pressure was measured using standard auscultatory method with help of pneumatically operated mercurial type random zero sphygmomanometer. Blood pressure was measured in left arm in sitting position with arm at the level of heart. Appearance of sound (Phase I Korotkoff) and disappearance of sound (Phase V) was recorded as systolic and diastolic BP respectively. Subjects with gestational hypertension have not been included in this study. Women were included in the study at gestational age >34 weeks and singleton pregnancy. The criteria for exclusion were: gestational age <34 weeks or unsure of dates, Chronic hypertension (excluded by history and presentation), heart disease, medical problems (e.g. diabetes mellitus, renal disease), moderate or severe anemia, twin pregnancy and alcohol use, tobacco use.
Subjects were categorized as having mild and severe preeclampsia based on the criteria of Easterling et al. [1].
Echocardiography
Echocardiographic examination was performed using Hewlett Packard SONOS 2500 with a 2.5 MHz transducer. Two-dimensional echocardiography facilitated M-mode recordings and color flow mapping facilitated Doppler measurements were performed. An initial 2-dimensional study in standard parasternal long axis and short axis planes followed by the apical 4-chamber plane view to evaluate cardiac structure and obtain visual assessment of left ventricle (LV) contractile function was performed. M–mode studies were performed at the level of aorta, left atrium and LV at midposition between the tips of the mitral value and papillary muscles. Pulsed Doppler flow across the mitral value was recorded to obtain the LV diastolic filling pattern. Systolic parameters studied were left ventricle end systolic volume (LV ESV), stroke volume (SV), cardiac output (CO), aortic root diameter (ARD), left ventricular outflow tract (LVOT) and left ventricle mass (LVM). Diastolic parameters studied were E wave, A wave, E/A ratio, isovolumetric relaxation time (IVRT), E deceleration time (DtE), E wave velocity time integral (E VTI), and A wave velocity time integral (A VTI).
Statistical Analysis
The study was designed to test two hypotheses (1) that CO in preeclamptic women is less than CO of normotensive women (CO pe < CO nt) and (2) total vascular resistance (TVR) of preeclamptic women is greater than mean of normotensive women. Mean arterial pressure was calculated using the formula
 |
TVR was calculated using the formula—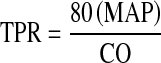 in which CO = cardiac output. Cardiac output was not corrected for body surface area as it has been demonstrated as poor correlating factor [1].
in which CO = cardiac output. Cardiac output was not corrected for body surface area as it has been demonstrated as poor correlating factor [1].
Student ‘t’ test was used as a test of significance. A P value of <0.05 was considered to be statistically significant.
Results
Table 1 shows the baseline features of the study population. Age and body mass index (BMI) were similar in the two groups. Differences in systolic BP, diastolic BP, mean arterial pressure (MAP) and birth weight were statistically significant between two groups. MAP of the subjects with preeclampsia was 115.88 ± 9.87, higher than that of controls (83.85 ± 5.71). Women with preeclampsia delivered smaller babies than women with normotensive pregnancy. Twelve subjects had mild and eight subjects had severe preeclampsia. No maternal or fetal complications were reported.
Table 1.
Baseline characteristics of two study groups
| Parameter | Normotensive (n = 20) | Hypertensive (n = 20) | P Value | Valensise et al. [12] |
|---|---|---|---|---|
| Age,(year) | 25.1 ± 2 | 25.75 ± 3.74 | 0.41 | NS |
| Body mass index | 22.7 ± 3.77 | 27.36 ± 7.02 | 0.11 | NS |
| Systolic BP (SBP) | 109.9 ± 23.48 | 155 ± 12.77 | 0.01 | <0.0001 |
| Diastolic BP (DBP) | 69.5 ± 6.04 | 96.5 ± 9.21 | 0.07 | <0.0001 |
| Mean arterial pressure (MAP) | 83.85 ± 5.71 | 115.88 ± 9.87 | 0.001 | <0.0001 |
| Birth weight (g) | 2895 ± 276.20 | 2410 ± 426.16 | 0.00001 | NS |
Table 2 shows comparison of systolic parameters between two study groups. Cardiac output in the preeclamptic group was 66.85 ± 4.56 ml/min as compared to 56.1 + 1.77 ml/min in the normotensive group. This observation was statistically significant at P < 0.004. Thus the first null hypothesis of our study was rejected. TVR in preeclampsia group was higher at 1396.85 ± 156.2 dyne, sec cm5 as compared to 1204.5 ± 71.18 dyne, sec cm5 in the normotensive group. This was statistically significant. Thus in this case the null hypothesis of our study was accepted.
Table 2.
Comparison of systolic parameters between two study groups
| Parameter | Normotensive (n = 20) | Hypertensive (n = 20) | P Value |
|---|---|---|---|
| LV ESV (ml) | 27.2 ± 3.5 | 36.04 ± 13.32 | 0.1 |
| LV EDV (ml) | 107.73 ± 5.66 | 108.23 ± 27.95 | 0.78 |
| SV (ml) | 70.8 ± 3.22 | 73.3 ± 14.19 | 0.39 |
| CO (ml/min) | 56.1 ± 1.77 | 66.85 ± 4.56 | 0.004 |
| LVM- | |||
| Diastolic (g) | 104.90 ± 23.17 | 131.15 ± 16.85 | 0.0001 |
| Systolic (g) | 83.33 ± 23.84 | 88.5 ± 7.34 | 0.04 |
| ARD (cm) | 2.02 ± 0.121 | 2.48 ± 0.286 | 0.00002 |
| LVOT (cm) | 1.56 ± 0.16 | 1.9 ± 0.2 | 0.14 |
| TVR (dyne, Sec cm5) | 1204.5 ± 71.18 | 1396.85 ± 156.2 | 0.03 |
Left ventricular diastolic mass and ARD was significantly higher in preeclamptic subjects. LV ESV, LV EDV and LVOT were not significantly different between two study groups.
Table 3 shows comparison of diastolic parameters between normotensive and preeclamptic subjects. E wave velocity, peak A wave velocity, IVRT, E wave deceleration time and A VTI were higher in preeclampsia group.
Table 3.
Comparison of diastolic parameters between two study group
| Parameter | Normotensive (n = 20) | Hypertensive (n = 20) | P value |
|---|---|---|---|
| E wave, m/s | 0.675 ± 0.137 | 1.023 ± 0.1926 | 0.0000 |
| A Wave, m/s | 0.500 ± 0.130 | 0.775 ± 0.278 | 0.0000 |
| E/A ratio | 1.35 ± 0.224 | 1.497 ± 0.492 | 0.3 |
| E Dec time, ms | 126 ± 8.07 | 189.4 ± 49.73 | 0.02 |
| IVRT, ms | 83.3 ± 5.9 | 96.13 ± 9.13 | 0.03 |
| E VTI, ms | 12.34 ± 1.9 | 13.44 ± 4.13 | 0.55 |
| A VTI, ms | 2.72 ± 0.63 | 6.43 ± 1.04 | 0.03 |
When systolic and diastolic parameters were compared in subjects with mild and severe preeclampsia (table not shown here), TVR was significantly higher (P < 0.0000) in severe preeclamptic subjects compared to mild preeclamptic subjects, whereas other parameters were unchanged.
Discussion
In this small and preliminary study we have assessed the role of echocardiography and found it to be a useful technique for evaluation of maternal cardiac function in preeclamptic women. Preeclampsia in women is characterized by a high CO and high vascular resistance state. There are physiological changes in left ventricular structure and function during normal pregnancy but exaggerated physiological changes are seen in preeclamptic subjects. The study suggests that women with severe preeclampsia had a uniform pattern of high resistance and high CO.
High TVR in preeclampsia suggests elevated afterload which is linked with reduced emptying of left ventricle. Elevated end systolic volume suggests that elevated end systolic pressure is generated by increased afterload. Although SV is dependent on CO, this finding was not found to be statistically significant. The high E wave velocity in preeclamptic subjects, suggests that transmittal pressure gradient during early passive filling is greater and reflects changes in passive myocardial compliance in the hypertrophic ventricle. The higher peak A wave velocity in preeclamptic subjects suggests more important role of atrial systole in filling of the hypertrophied ventricle in these women. The prolonged IVRT in preeclamptic subjects suggests that longer time is required for left ventricular pressure to fall below atrial pressure. The prolongation of E wave deceleration time in preeclamptic subjects is consistent with increased passive filling of left ventricle during early diastole. TVR of subjects with severe preeclampsia was significantly higher than that of subjects with mild preeclampsia. A possible explanation for this is that MAP of severe preeclamptic subjects was 126.3 mmHg while that of subjects with mild preeclampsia was 108.9 mmHg.
Valensise et al. [9] found that CO, LVM and TVR in preeclamptic group was higher than that of normotensive group.
B. Vasapollo [10] and Bosio et al. [11] reported that the preclinical phase of preeclampsia is characterized by low TVR and high CO. Established preeclampsia is characterized by high TVR and low CO. Poppas et al. [7] suggest that TVR should be considered as the predominant parameter to characterize the systemic arterial load i.e. vascular after load. Valensise et al. [12] found that women with early mild gestational hypertension, who have high TVR (TVR > 1340 dyne, s/cm5) and concentric geometry of the left ventricle have a higher risk of developing maternal and fetal complications.
One limitation of this study is that it was not possible to follow up subjects in the postpartum period to examine whether the altered cardiovascular hemodynamic state reverts to normal after pregnancy. Also the sample size was small as subjects had to be taken to a private heart care facility for the echocardiography assessment.
This study shows that there are significant structural and functional changes in the cardiovascular dynamics in subjects with preeclampsia. It appears that BP monitoring alone is insufficient to identify effectively, risk of cardiovascular complications in these subjects. Maternal echocardiography if introduced into the routine management protocol, could help to identify women who are at high risk to develop complications.
References
- 1.Easterling TR, Benedetti TJ, Schmucker BC, et al. Maternal hemodynamics in normal and pre-eclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990;76(6):1061–1069. [PubMed] [Google Scholar]
- 2.Escudero EM, Favaloro LE, Moreira C, et al. Study of left ventricular function in pregnancy induced hypertension. Clin Cardiol. 1988;11:329–333. doi: 10.1002/clc.4960110511. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli L, Ferro G, Nappi C, et al. Early diastolic time intervals during hypertensive pregnancy. Clin Cardiol. 1987;10:567–572. doi: 10.1002/clc.4960101011. [DOI] [PubMed] [Google Scholar]
- 4.Moran AM, Colan SD, Mauer MB, et al. Adaptive mechanisms of left ventricular diastolic function to the physiologic load of pregnancy. Clin Cardiol. 2002;25:124–131. doi: 10.1002/clc.4960250308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai DK, Moodley J, Naidoo DP, et al. Cardiac abnormalities in pulmonary edema associated with hypertensive crises in pregnancy. Br J Obstet Gynaecol. 1996;103:523–528. doi: 10.1111/j.1471-0528.1996.tb09800.x. [DOI] [PubMed] [Google Scholar]
- 6.Kametas NA, McAuliffe F, Cook B, et al. Maternal left ventricular transverse and long axis systolic function during pregnancy. Ultrasound Obstet Gynecol. 2001;18:467–474. doi: 10.1046/j.0960-7692.2001.00574.x. [DOI] [PubMed] [Google Scholar]
- 7.Poppas A, Shroff SG, Korcarz CE, et al. Serial assessment of the cardiovascular system in normal pregnancy. Circulation. 1997;95:2407–2415. doi: 10.1161/01.cir.95.10.2407. [DOI] [PubMed] [Google Scholar]
- 8.Simmons LA, Gillin AG, Jeremy RW. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol. 2001;283:1627–1633. doi: 10.1152/ajpheart.00966.2001. [DOI] [PubMed] [Google Scholar]
- 9.Valensise H, Novelli GP, Vasapollo B, et al. Maternal diastolic dysfunction and left ventricular geometry in gestational hypertension. Hypertension. 2001;37:1209–1215. doi: 10.1161/01.hyp.37.5.1209. [DOI] [PubMed] [Google Scholar]
- 10.Vasapollo B, Valensise H, Novelli GP, et al. Abnormal maternal cardiac function precedes the clinical manifestation of fetal growth restriction. Ultrasound Obstet Gynecol. 2004;24:23–29. doi: 10.1002/uog.1095. [DOI] [PubMed] [Google Scholar]
- 11.Bosio PM, Mckenna PJ, Conroy R, et al. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:978–984. doi: 10.1016/S0029-7844(99)00430-5. [DOI] [PubMed] [Google Scholar]
- 12.Valensise H, Vasapollo B, Novella GP, et al. Maternal total vascular resistance and concentric geometry. Br J Obstet Gynecol. 2006;113:1044–1052. doi: 10.1111/j.1471-0528.2006.01013.x. [DOI] [PubMed] [Google Scholar]


