Abstract
Adolescent humans and rodents have been shown to consume more alcohol than their adult counterparts. Given that corticosterone (CORT) has been shown to be related to intake of several drugs of abuse, this study assessed ontogenetic effects of low-moderate doses of ethanol on CORT increases and recovery. Despite no significant differences in baseline (home-cage) CORT levels, CORT responses to ethanol were greater in females than in males, and in adult females than adolescent females; males, however, showed less marked age differences in CORT levels following ethanol. Adolescent BECs were lower than those of adults, although these BEC differences appear insufficient to account for the ontogenetic differences in CORT levels. Collectively, these findings suggest that it is unlikely that age differences in CORT elevations provide a major contribution to the ontogenetic differences in alcohol intake seen between adolescents and adults.
Keywords: Corticosterone, adolescence, ethanol, ontogeny, rat
Introduction
During adolescence, many neurochemical, behavioral, and hormonal changes may contribute to the elevated ethanol consumption levels that are often seen in adolescents relative to adults in both humans and rodents (see Spear, 2000 for review). These age-related consumption differences may be related in part to ontogenetic alterations in sensitivity to many of ethanol’s effects. For example, adolescent rodents have been reported to be less sensitive to ethanol-induced sedation, motor impairment, locomotor sensitization, hypothermia, anxiolysis and dysphoria than adults (Anderson et al., 2010; Hefner and Holmes, 2007; Silveri and Spear, 1998; Stevenson et al., 2008; Swartzwelder et al., 1998; Varlinskaya and Spear, 2002; Vetter-O’Hagen et al., 2009). In contrast, adolescents have been found to be more sensitive than their adult counterparts to ethanol-induced social facilitation, spatial memory impairment, and locomotor activation (Markwiese et al., 1998; Quoilin et al., 2010; Stevenson et al., 2008; Spear and Varlinskaya, 2005; Swartzwelder et al., 1995; Varlinskaya and Spear, 2002, 2006, 2008), with reports of both increases (Pautassi et al., 2008; Ristuccia and Spear, 2008) and decreases (Dickinson et al., 2009) in ethanol-related reward sensitivity among adolescents relative to adults.
Administration of ethanol, like many drugs of abuse, activates the hypothalamus-pituitary- adrenal (HPA) axis (see Ellis, 1966; Thiagarajan et al., 1989), resulting in elevated corticosterone (CORT) release in adulthood (e.g. Ellis, 1966; Ogilvie and Rivier, 1996; 1997; Patterson-Buckendahl et al., 2005; Rivier, 1993; Silveri and Spear, 2004; Spencer and McEwan, 1997) following acute ethanol administration intraperitoneally, intragastrically, or via vapor inhalation (Lee and Rivier, 2003; Ogilvie et al., 1997). Such ethanol-induced increases in CORT may be important given that CORT has been shown to modulate voluntary ethanol intake (Fahlke et al., 1994a,b). For example, animals subjected to adrenalectomy demonstrated decreased ethanol intake that was reversed following administration of CORT (Fahlke et al., 1994a), effects that appeared more pronounced in ethanol-preferring strains and lines (Fahlke et al., 1994b). In adult rats, females have been shown not only to ingest significantly more ethanol than their male counterparts (Doremus et al., 2005; Lancaster et al., 1996; Vetter-O’Hagen et al, 2009), but also to have higher basal CORT levels (Critchlow et al., 1963) and to release more CORT than adult males in response to stressors (Duchesne et al., 2009) or an acute ethanol challenge (Ogilvie and Rivier, 1996; 1997; Rivier, 1993). These sex differences in CORT response to ethanol were not seen in weanling (postnatal day (P)21) or peripubertal (P41) rats (Ogilvie and Rivier, 1996).
Attenuation of CORT release in younger relative to more mature animals has been reported following administration of a variety of drugs of abuse, including cocaine, amphetamine, and morphine (Adriani and Laviola, 2000; Bailey and Kitchen, 1987; Laviola et al., 1995, 1999). Several studies have also examined age-related differences in ethanol-induced release of CORT. In an earlier study from our group, age-related increases in CORT levels following ethanol challenge were found to be particularly marked in females (Silveri and Spear, 2004), although no vehicle-injected or home cage controls were included, making it difficult to disentangle ethanol effects per se from CORT release associated with the process of manipulating and injecting the animals. In a study by Ogilvie and Rivier (1996) in which ethanol’s effects on CORT levels in weanling (P21), late adolescent (P41), and young adult (P61) rats were analyzed, females were found to have significantly higher CORT levels than males only in adult animals. These animals were shipped during development, however, and subjected to surgical implantation of intravenous and intraperitoneal cannulations prior to testing – potential stressors that may have influenced later psychopharmacological and behavioral sensitivity, perhaps more markedly during the juvenile and adolescent periods than in adulthood (Vetter-O’Hagen and Spear, in press; see McCormick et al., 2010 for review). Thus, the present study was conducted to add to the current knowledge of ethanol-induced increases in CORT in adolescents and adults by utilizing critical control groups (saline injected and home cage controls) as well as animals bred in-house that were not exposed to procedural manipulations prior to testing.
Methods
Subjects
A total of 514 male and female Sprague-Dawley rats derived from 160 litters from our breeding colony at Binghamton University were used in this study. On the day after birth, P1, all litters were culled to 8–10 pups (6 males and 4 females kept whenever possible) and housed with their mother and father in standard breeding cages until weaning on P21 at which point they were pair-housed with a same-sex littermate. All animals were housed in a temperature-controlled (20–22°C) vivarium on a 14-/10-hr light/dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and tap water. All animals were treated in accordance with guidelines established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental Design
The design of the study was a 2 (age: adolescent, adult) × 2 (sex: male, female) × 3 (ethanol dose: 0, 0.5, 1.5 g/kg) × 5 (time point: 15, 30, 60, 90, 180 min) factorial with a home-cage control group for each age and sex. Seven to nine experimental animals were randomly assigned to each group, with the constraint that no more than one animal from a given litter was placed in any particular condition to avoid confounding litter with treatment effects (Holson and Pearce, 1992).
Experimental Protocol
All testing was conducted between 0900 and 1300 hours on either P34–36 or on P75–85. The earlier age range was chosen to represent early-to-mid adolescence, whereas the P75–85 range was chosen to characterize young adults, with animals at this age from both sexes past the traditional zone between adolescence and adulthood during which some adolescent typical characteristics may persist (see Spear, 2000 for review). On test day animals were removed from their home cage, injected with either saline or ethanol, and then placed alone in a holding cage until sacrificed for trunk blood collection. Subjects were placed individually in holding cages post-injection (instead of being returned to the home cage) to prevent changes in CORT levels due to interactions with their cage-mate. Home cage controls were taken from the home cage and immediately sacrificed for trunk blood collection. Sacrifice and blood collection took place in a room adjacent to the colony room. No animals were present in the room while blood collection was taking place, with the door between this room and the colony area remaining closed during the collection process.
Ethanol Administration
Ethanol was administered intraperitoneally (i.p.) as a 12.6% (v/v) solution in physiological saline, a relatively low concentration known to induce little (if any) tissue irritation at the site of injection. Dose of ethanol (0.5 and 1.5 g/kg) was varied by altering volume rather than concentration to avoid concentration-induced differences in ethanol absorption rate (Linakis and Cunningham, 1979). Control subjects were injected with physiological saline isovolumetric to the highest dose of ethanol administered (1.5% body weight). All solutions were administered at room temperature. The 0.5 g/kg dose was chosen as a dose at which age-related behavioral dissociations are seen, with this dose inducing social facilitation in adolescents, but not adults (Varlinskaya and Spear, 2006; Varlinskaya et al., 2010; Willey et al., 2009). A 1.5g/kg challenge dose was also utilized as it has been found to be aversive to both adolescents and adults (Anderson et al., 2010).
Blood Ethanol Determination
Trunk blood samples were collected in heparinized tubes and frozen at −80°C until analysis of blood ethanol concentration (BEC). Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min, and then extracted and injected a 1.0 ml sample of the gas headspace into the HP 5890 series Gas Chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions. The range of standards used was 5–600 mg/dl.
Corticosterone Determination
Trunk blood samples were centrifuged at 2°C for 20 min at 3,000 rpm. Plasma samples were then collected and stored at −80°C until the time of assay. Plasma CORT levels were determined by radioimmunoassay using RIA kits obtained from ICN Biomedicals, Inc. (Orangeburg, NY) with 100% specificity for rat CORT. The sensitivity of the competitive binding assay is 80 pg/tube, with inter- and intra-assay coefficients of variation less than 10% and 5% respectively. Standards and samples were assayed in duplicate, with disintegrations per minute averaged against a standard curve performed with each assay and input into GraphPad Prism 2.0 software for calculation of ng/ml in each sample.
Data Analyses
Outlier tests were run within each test group for CORT levels and BECs. Any subject with a score exceeding 2 standard deviations above or below the group mean for either measure was removed from further analysis. A total of 33 subjects (6%) were removed from analyses (13 based on BECs, 16 based on CORT, and 4 based on both measures), with no more than 2 animals removed from any group, thereby resulting in final sample sizes of 6–8 for each sex/dose/time point group. Between-subjects analyses of variance (ANOVAs) were used to analyze BECs and CORT levels. Significant effects and interactions were further investigated using Fisher’s LSD post-hoc contrasts.
Results
CORT levels
The ANOVA of baseline CORT levels revealed no significant age or sex differences among the home-cage control animals (means (ng/ml) ± SEM’s: adolescent females 6.43 ± 2.92; adolescent males 2.29 ± 2.29; adult females 19.33 ± 5.85; and adult males 10.88 ± 7.26).
A 2 age × 2 sex × 3 dose × 5 time point ANOVA of CORT release following ethanol administration revealed significant main effects of age [F(1,393) = 46.49, p < 0.001], sex [F(1,393) = 181.61, p < 0.001], dose [F(2,393) = 68.21, p < 0.001], and time point [F(4,393) = 67.63, p < 0.001], as well as numerous interactions involving these factors, including interactions of age and time point with sex [F(4,393) = 3.37, p < 0.01], and with dose [F(8, 393) = 4.81, p < 0.001]. Adult females generally had significantly higher CORT levels than adult males, with adolescent females also having significantly higher levels of CORT than their male counterparts at the 15, 90, and 180 min time points following challenge with saline or ethanol. Given these interactions, to further explore age-related differences in ethanol effects on CORT and their time course, ANOVAs were conducted separately for each sex. Data are shown in Figure 1.
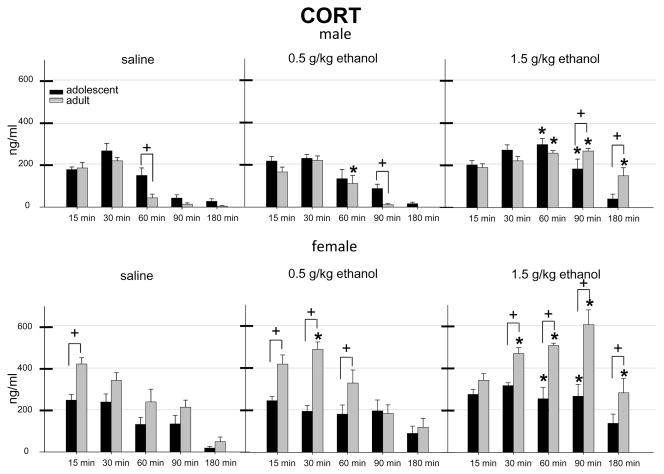
Fig. 1.
CORT values at each age, sex, dose, and time point. * indicates a significant difference from the comparable saline group. + indicates a significant age difference within a given sex, dose, and time point. Each bar represents a group size of 6–8 animals.
Analysis of the male CORT data revealed significant main effects of age [F(1,195) = 4.60, p < 0.05], dose [F(2,195) = 47.43, p < 0.001], and time point [F(4,195) = 60.12, p < 0.001]. Dose interacted with both age [F(2,195) = 4.38, p < 0.05] and time point [F(8,195) = 8.26, p < 0.001]; these effects, however, were tempered by a significant interaction of all three factors [F(8,195) = 2.22, p < 0.05]. Post-hoc analysis of this interaction revealed that CORT levels were higher in adolescent males than adults at 60 min following saline injection and at 90 min following 0.5 g/kg ethanol injection, whereas adult males conversely had higher CORT values than adolescents at 90 and 180 min following 1.5 g/kg ethanol. When comparing ethanol-exposed animals to their saline counterparts at each age, adult males were found to show significant ethanol-induced increases in CORT levels at 60 min following a 0.5 g/kg ethanol injection, and at 60, 90 and 180 min following 1.5 g/kg ethanol. Adolescent males only showed significant ethanol-induced elevations in CORT over levels seen in saline injected animals at 60 and 90 min following 1.5 g/kg ethanol.
Analysis of the female CORT data revealed significant effects of age [F(1, 198) = 84.93, p < 0.001], dose [F(2, 198) = 31.44, p < 0.001], and time point [F(4,198) = 26.71, p < 0.001], main effects that were tempered by a significant interaction of all three variables [F(8, 198) = 3.44, p < 0.01]. Post-hoc tests revealed that female adults had significantly higher CORT levels than female adolescents at 15 min following challenge with saline, at 15, 30 and 60 min after administration of 0.5 g/kg ethanol, and at all time points except 15 min following challenge with 1.5 g/kg ethanol. Ethanol-induced increase in CORT over saline control animals was seen in adolescent females only at 60 and 90 min following 1.5 g/kg ethanol, whereas significant increases were seen in adult females at 30 min following 0.5 g/kg ethanol, and at 30–180 min following 1.5 g/kg ethanol.
Blood Ethanol Concentration
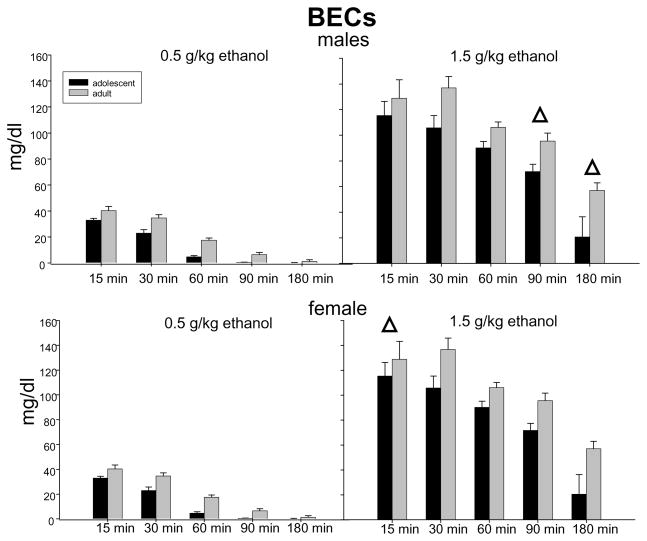
Results of a 2 age × 2 sex × 2 dose by 5 time point ANOVA of BECs revealed main effects of age [F(1, 262) = 53.97, p < 0.001], dose [F(1,262) = 1506.45, p < 0.001], and their interaction [F(1,262) = 14.20, p < 0.001]. Post-hoc analysis of this interaction revealed that adolescent BECs were lower than adult BECs at both doses, with this difference more marked following 1.5 g/kg ethanol (80.94 ± 5.49 vs 101.07 ± 5.03 mg/dl) than after 0.5 g/kg (12.46 ± 1.63; 20.25 ± 1.89 mg/dl, respectively). A main effect of time point [F(4,262) = 183.14, p < 0.001] interacted with both sex [F(4,262) = 6.62, p < 0.001] and dose [F(4,262) = 48.77, p < 0.001], effects that were all tempered by an interaction involving all three factors [F(4,262) = 5.38, p < 0.001]. Post-hoc investigation of data collapsed across age to explore this interaction revealed no sex differences at any time point following administration of 0.5 g/kg ethanol, whereas females were found to have significantly higher BECs than males at 15 min post-injection following 1.5 g/kg ethanol, but lower BECs than males at 90 and 180 min post-injection. Data are shown in Figure 2.
Fig. 2.
BECs at each age, sex, dose and time point. Δ indicates significantly higher BECs than opposite sex counterparts at the same post-injection time interval collapsed across age. Each bar represents a group of 7–8 animals.
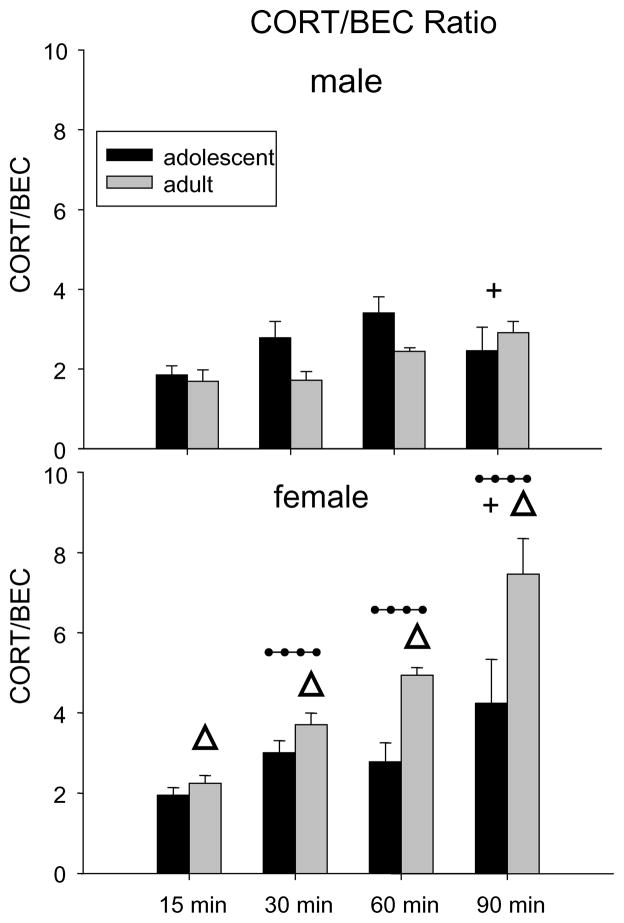
In order to determine if age and sex differences in BECs influenced CORT levels, CORT/BEC ratios were also analyzed in ethanol challenged animals by dividing each animal’s CORT level by its BEC. Due to the large number of zero values for BECs following the 0.5 g/kg dose and the minimal age differences seen at this dose, this analysis focused only on data following the 1.5 g/kg challenge dose (excluding the 180 min time point to again avoid BEC values of 0). This 2 age × 2 sex × 4 time point ANOVA of the CORT/BEC ratio data after 1.5 g/kg ethanol revealed significant effects of age [F(1,105) = 6.87, p < 0.05], sex [F(1,105) = 40.01, p < 0.001], and time point [F(3,105) = 19.84, p < 0.001]. These main effects were tempered by interactions of age × sex [F(1,105) = 21.18, p < 0.001], with adult females (4.59 ± 0.22) having significantly higher CORT/BEC ratios than adult males (2.18 ± 0.22), and age × time point [F(3,105) = 4.03, p < 0.01], with adults having significantly greater CORT/BEC ratios (5.19 ± 0.77) than adolescents (3.35 ± 0.64) only at 90 min post-injection. Finally, post-hoc analyses of the interaction of sex × time point [F(3,105) = 7.58, p < 0.001] revealed effects similar to those seen in the CORT analyses, with data collapsed across age showing females to have significantly higher ratios than males at all time points except 15 min. As can be seen in Fig. 3, when controlling for BEC levels by utilizing a ratio, similar effects were seen as when CORT levels alone were analyzed.
Fig. 3.
Ratio of CORT/BEC following 1.5 g/kg ethanol for each age and sex across time point. The 180 min time point was excluded to avoid values of zero in the ratio. Δ indicates that adult females had greater ratios than adult males regardless of time point. + indicates when collapsed across sex, adults had a greater ratio than adolescents at 90 min. Dotted line indicates that when collapsed across age, females had a greater ratio than males at that time point. Each bar represents a group size of 7–8 animals.
Discussion
Following administration of 1.5 g/kg ethanol, animals of both sexes and ages had a higher and more prolonged CORT response than following saline injection; however, adult females showed an overall higher CORT response to both saline and ethanol injections than any other age/sex group. These findings are in line with previous research, given that studies in both humans and laboratory animals have found adult females to respond to various natural and drug stimuli with higher CORT secretion than males (Greenspan et al., 1993; Jenkins and Connolly, 1968; Pericic and Pivac, 1995), including in response to challenge with ethanol (Jenkins and Connolly, 1968; Ogilvie and Rivier, 1996; 1997; Silveri and Spear, 2004; Rivier, 1993). The CORT levels in adult males following saline injection are very similar to those reported previously in similarly mildly stressed rodents (e.g., Adriani and Laviola, 2000; Ryabinin et al., 1999). The lack of sex differences during adolescence and the attenuated CORT response to saline injection in P35–37 females compared with adult females is consistent with other work showing that females show consistently greater CORT responses than males only in adulthood (Ogilvie and Rivier, 1996). These findings are reminiscent of findings from previous studies (Ogilvie and Rivier, 1996; Rivier, 1993) where animals underwent shipping stress and surgical manipulation prior to testing, thereby demonstrating that the age and sex differences in the CORT response invoked by ethanol administration are reliable and transcend notable procedural differences across studies.
Age differences in the CORT response to ethanol injection were most apparent in females. Adolescent females shared an attenuated CORT response to ethanol relative to adult females beginning 15 min post-injection at the lower dose and 30 min after administration of the higher dose of ethanol. Age differences in males, however, were minimal and were apparent primarily in a delayed recovery from CORT elevations seen following the higher dose of ethanol among adult males, a delay reminiscent of earlier findings (Silveri and Spear, 2004). These different time courses for expression of age differences in CORT between males and females may reflect different processes. Increased levels of estradiol in adult compared to adolescent females may underlie the age difference in CORT release in females. In a study by Ogilvie and Rivier (1997), intact adult females and castrated males implanted with estradiol had a more robust CORT response to ethanol administration than either intact males or castrated males that did not receive estradiol. In contrast, the delayed post-ethanol CORT recovery in adult males compared to adolescents may reflect increased fat which may accumulate steroids and release them more gradually (Davis and Squires, 1999; Stimson et al., 2009). Further studies are necessary to determine if the age and sex differences in CORT response to ethanol administration are due to differences at the level of the hypothalamus, the pituitary, or both. There is some data implicating age differences in the pituitary may be responsible for this effect. Adults were found to have a greater percentage of corticotrophin releasing hormone (CRH) responsive cells in the pituitary than adolescents (Senovilla et al., 2005), a difference which could underlie the lower CORT levels seen in adolescents compared to adults following ethanol injection.
Adolescents were shown to have slightly but significantly lower BECs than adults, with this age difference more notable at 1.5 g/kg than 0.5 g/kg ethanol. Similar results have sometimes (Silveri and Spear, 2000; Brasser and Spear, 2002; Hefner and Holmes, 2007), but not always (Varlinskaya and Spear, 2006; Silveri and Spear, 2000), been reported by others, and may be related to slightly faster ethanol metabolism rates (Holsteadt et al., 1977, see also Spear and Varlinskaya, 2005; Spear, 2007 for discussion) or more rapid ethanol distribution (Wiberg et al., 1970; York, 1983) in adolescents than adults. Yet, the CORT differences observed do not appear to be simply related to alterations in BECs given that age and sex differences persisted when CORT levels were analyzed as a ratio with BECs. That is, if the differences in CORT levels following ethanol administration were attributable to BEC differences across groups, the ratio of CORT/BEC should have eliminated these effects. Also, the patterns of BECs and CORT are different from each other, i.e. adolescent males show significantly lower BECs than adult males, whereas notable age differences were not seen in CORT levels. Also, although adult females were shown to have BECs that peaked higher but cleared faster than adult males, the CORT levels of adult females remained higher than adult males at 180 min.
Results of the present study revealed age differences in CORT levels following ethanol injections primarily in female animals. Yet, prior work has yielded little evidence of notable sex differences in the developmental changes seen in the behavioral response to ethanol between adolescents and adults (eg. Varlinskaya et al., 2010; Varlinskaya and Spear, 2006; 2010). Collectively, these findings suggest that it is unlikely that age differences in CORT elevations provide a major contribution to the often dramatic ontogenetic differences seen in both sexes to alcohol’s behavioral effects.
Acknowledgments
The research presented in this article was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R37-AA012525 and R01-AA017355) to Linda P. Spear. Special thanks to Judy Sharp for her assistance with BECs and CORT assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CC, Kitchen I. Developmental responses to opioids reveals a lack of effect on stress-induced corticosterone levels in neonatal rats. Br J Pharmacol. 1987;91:119–125. doi: 10.1111/j.1476-5381.1987.tb08990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Davis SM, Squires EJ. Association of cytochrome b5 with 16-androstene steroid synthesis in the testis and accumulation in the fat of male pigs. J Anim Sci. 1999;77:1230–1235. doi: 10.2527/1999.7751230x. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Kashawny SK, Thiebes KP, Charles DY. Decreased sensitivity to ethanol reward in adolescent mice as measured by conditioned place preference. Alcohol Clin Exp Res. 2009;33:1246–1251. doi: 10.1111/j.1530-0277.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Fahlke C, Engel JA, Eriksson CJ, Hard E, Soderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;11:195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Thomasson R, Engel JA, Hansen S. Metyrapone-induced suppression of corticosterone synthesis reduces ethanol consumption in high-preferring rats. Pharmacol Biochem Behav. 1994;48:977–981. doi: 10.1016/0091-3057(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Rowe JW, Maitland LA, McAloon-Dyke M, Elahi D. The pituitary-adrenal glucocorticoid response is altered by gender and disease. J Gerontol. 1993;48:M72–77. doi: 10.1093/geronj/48.3.m72. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. The effect of alcohol on the developing organism. Genetical, teratological and physiological aspects. Med Biol. 1977;55:1–14. [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Jenkins JS, Connolly J. Adrenocortical response to ethanol in man. Br Med J. 1968;2:804–805. doi: 10.1136/bmj.2.5608.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Lee S, Rivier C. Long-term influence of an initial exposure to alcohol on the rat hypothalamic-pituitary axis. Alcohol Clin Exp Res. 2003;27:1463–1470. doi: 10.1097/01.ALC.0000086065.06203.DD. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res. 1997;21:467–476. doi: 10.1111/j.1530-0277.1997.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in alcohol-evoked hypothalamicpituitary- adrenal activity in the rat: ontogeny and role of neonatal steroids. Alcohol Clin Exp Res. 1996;20:255–261. doi: 10.1111/j.1530-0277.1996.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol. 2005;37:157–166. doi: 10.1016/j.alcohol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericic D, Pivac N. Sex differences in conflict behaviour and in plasma corticosterone levels. J Neural Transm Gen Sect. 1995;101:213–221. doi: 10.1007/BF01271558. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology (Berl) 2010;212:501–512. doi: 10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin Exp Res. 2008;32:1807–1815. doi: 10.1111/j.1530-0277.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM, Finn DA. Different levels of Fos immunoreactivity after repeated handling and injection stress in two inbred strains of mice. Pharmacol Biochem Behav. 1999;63:143–151. doi: 10.1016/s0091-3057(98)00239-1. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Senovilla L, Garcia-Sancho J, Villalobos C. Changes in expression of hypothalamic releasing hormone receptors in individual rat anterior pituitary cells during maturation, puberty and senescence. Endocrinology. 2005;146:4627–4634. doi: 10.1210/en.2005-0836. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in corticosterone. Alcohol. 2004;32:145–155. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Impaired adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress in aged rats. Neuroendocrinology. 1997;65:353–359. doi: 10.1159/000127195. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl) 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson RH, Andersson J, Andrew R, Redhead DN, Karpe F, Hayes PC, Olsson T, Walker BR. Cortisol release from adipose tissue by 11beta-hydroxysteroid dehydrogenase type 1 in humans. Diabetes. 2009;58:46–53. doi: 10.2337/db08-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic-pituitary-adrenal axis: exploration of the mechanism of action. Neuroendocrinology. 1989;50:427–432. doi: 10.1159/000125259. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague- Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats after repeated ethanol exposure. Alcohol. 2010;44:99–110. doi: 10.1016/j.alcohol.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The Effects of Gonadectomy on Age- and Sex- Typical Patterns of Ethanol Consumption in Sprague-Dawley Rats. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01555.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg GS, Trenholm HL, Coldwell BB. Increased ethanol toxicity in old rats: changes in LD50, in vivo and in vitro metabolism, and liver alcohol dehydrogenase activity. Toxicol Appl Pharmacol. 1970;16:718–727. doi: 10.1016/0041-008x(70)90077-3. [DOI] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202:122–129. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL. Increased responsiveness to ethanol with advancing age in rats. Pharmacol Biochem Behav. 1983;19:687–691. doi: 10.1016/0091-3057(83)90346-5. [DOI] [PubMed] [Google Scholar]