Abstract
A method for five- and six-membered heterocycle formation by palladium-catalyzed C-H/N-H coupling is presented. The method employs a picolinamide directing group, PhI(OAc)2 oxidant, and toluene solvent at 80–120 °C. Cyclization is effective for sp2 as well as aliphatic and benzylic sp3 C-H bonds.
Transition-metal-catalyzed functionalization of carbon-hydrogen bonds is a topic of recent intense interest. During the last few years, significant advances have been made in conversion of C-H bonds to C-C and C-O functionalities.1 However, direct non-nitrene amination reactions are rare. Most of the reports demonstrate functionalization of either sp2 or activated (benzylic) sp3 C-H bonds.2a–k Only a few papers describe palladium-catalyzed amination of alkane C-H bonds. Functionalization of methyl groups adjacent to quartenary centers is described in most cases. An early paper by Sames describes amino acid cyclization under platinum catalysis.3a Glorius has developed an elegant method for intramolecular, palladium-catalyzed amination of unactivated sp3 C-H bonds that results in the formation of indolines.3b The reaction proceeds by an oxidative coupling of an amide NH with a C(sp3)-H bond that is the most straightforward way for generating a N-C bond. A stoichiometric Ag(I) oxidant was employed and no examples of pyrrolidine formation were presented. Buchwald has shown that palladium-catalyzed intermolecular amination of 2-bromo-t-butylbenzenes by using aryl amines as nitrogen source is possible. In this example, an ortho-C(sp2)-Br bond is used as an internal oxidant.4 In two examples, palladium-catalyzed formation of indolines has been accomplished by C-Br/sp3 C-H coupling.5 In these cases, functionalization of C-H bonds that are not part of t-butyl groups is also possible. Several examples describe the formation of indolines or carbazoles by a C(sp2)-H/NH coupling.6a–c,e Notably, Yu has demonstrated palladium-catalyzed N-triflated phenethylamine conversion to indolines by employing a range of oxidants.6a Buchwald and Gaunt have formed carbazoles from 2-arylanilines by employing palladium catalysis and O2/Cu(OAc)2 or PhI(OAc)2 oxidants.6b,c Copper-catalyzed and transition-metal-free synthesis of carbazoles has been reported.2i,k Hofmann-Löffler reaction is a classical way to form pyrrolidines.6f,g Picolinamide directing group has been used for acetoxylation of sp2 C-H bonds.6d A general method for C-H/N-H bond coupling that could utilize both sp2 and sp3 C-H bonds has not yet been disclosed. We report here a method for picolinamide auxiliary assisted, palladium-catalyzed pyrrolidine, indoline, and isoindoline synthesis by a C-H/N-H coupling.
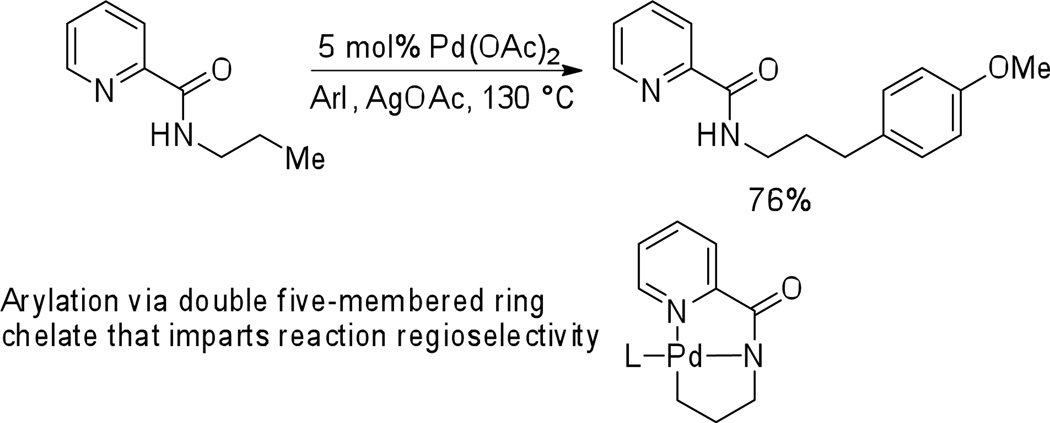
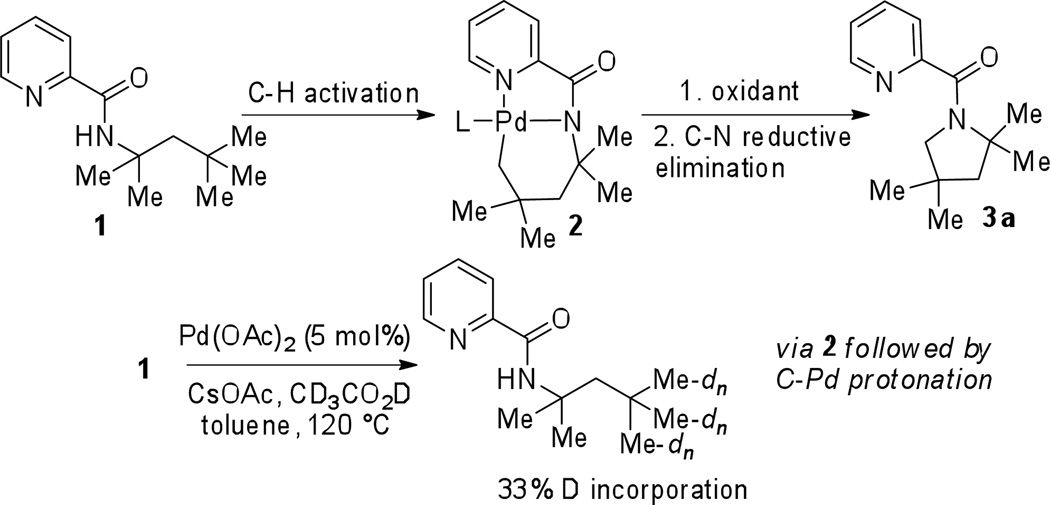
In 2005, we reported the β-arylation of carboxylic acid and γ-arylation of amine derivatives by employing an 8-aminoquinoline or picolinic acid auxiliary, catalytic Pd(OAc)2, stoichiometric AgOAc, and an aryl iodide coupling partner (Scheme 1).7 Subsequently, a number of auxiliaries were investigated for carboxylic acid β-arylation and it was shown that silver salts can be replaced by simple inorganic bases.8 Several other groups have used this methodology for synthetic purposes. Corey has used the 8-aminoquinoline auxiliary to arylate sp3 C-H bonds in amino acid derivatives.9 Chen has employed the methodology in total synthesis of Celogentin C.10a Picolinic acid directing group has also been used by Chen in arylation of γ-positions of amines culminating in the synthesis of obafluorin.10b Total syntheses of piperarborenines were recently reported by Baran by employing a 2-thiomethylaniline directing group.10c The arylation reactions proceed via a favored double five-membered ring chelate (Scheme 1). If C-H activation in δ-positions would be possible, oxidation of the palladacycle 2 to form a high-valent palladium species followed by C-N reductive elimination would afford a pyrroline derivative 3. Treatment of 1 with 5% Pd(OAc)2 in toluene/CD3CO2D at 120 °C showed 33% deuterium incorporation at the terminal methyl groups. No other C-H bonds were deuterated. Hence, C-H δ–activation is possible by employing a picolinic acid directing group.3b,11,12
Scheme 1.
Picolinic Acid Directing Group
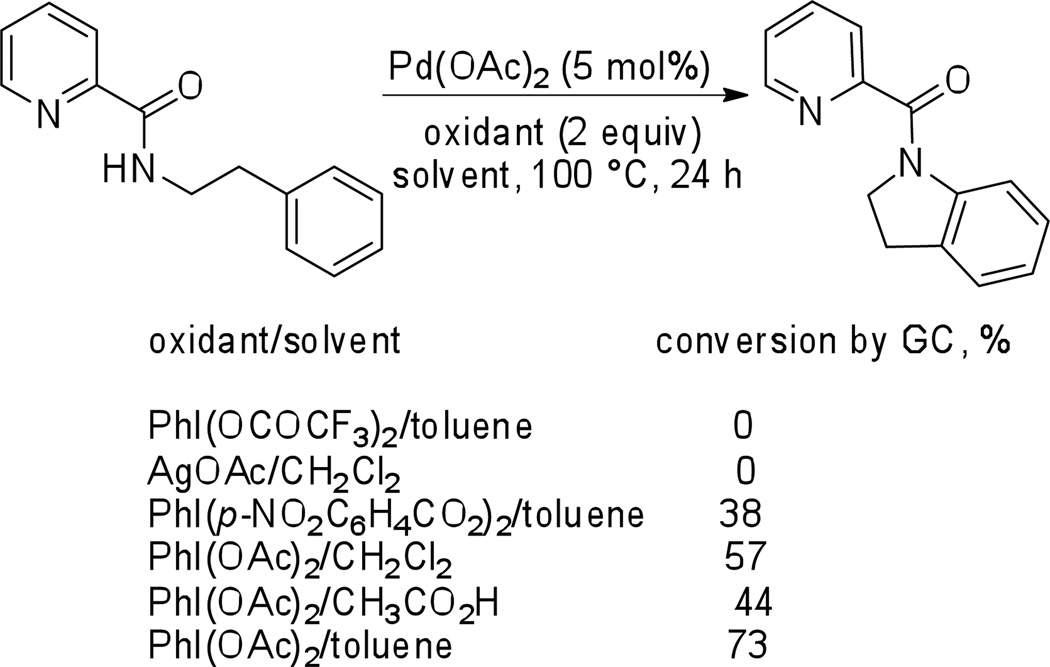
Subsequent reaction steps require oxidant capable of converting palladium to a higher oxidation state followed by reductive elimination that would afford cyclized product and regenerate the Pd(II) catalyst. Several oxidants were investigated and the best results were obtained by employing PhI(OAc)2 in toluene (Scheme 3).
Scheme 3.
Optimization of Oxidant
Cyclization results are presented in Table 1. Direct sp2 C-H/N-H coupling is possible, generating indolines in fair to good yields (entries 1–5). Chloro substitution on aromatic ring is tolerated (entry 2), as is ester on indoline (entry 3). 2,2-Diphenylethylamine picolinamide is cyclized in a good yield affording 3-phenylindoline (entry 4). Low yield is observed for cyclization of 3,4-dimethoxyphenetylamine picolinamide (entry 5). In addition to the cyclized product, the corresponding indole and sp2 C-H bond acetoxylation products are obtained. Interestingly, formation of a six-membered ring structure is also possible in a good yield (entry 6), showing that cyclopalladation via a seven-membered ring is feasible.3b,12 In addition to dihydrophenanthridine derivative, a minor amount of 7-phenylphenanthridine was also isolated.
Table 1.
Cyclization of aryl and alkyl picolinamidesa
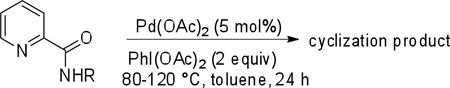 | |||
|---|---|---|---|
| entry | R | cyclized product | yield, % |
| 1 | 2-phenylethyl | 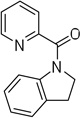 |
80 |
| 2 | 2-chlorophenylethyl | 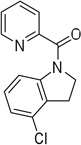 |
80 |
| 3 | 1-(CO2tBu)-2-phenylethyl | 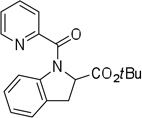 |
77 |
| 4 | 2,2-diphenylethyl | 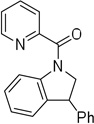 |
76 |
| 5b | 3,4-dimethoxyphenylethyl | 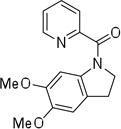 |
16 |
| 6c | 2,6-diphenylbenzyl | 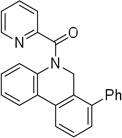 |
86 |
| 7 | 2,2,4-trimethylpent-2-yl | 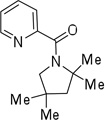 |
88 |
| 8d | 3,3-dimethylbutyl | 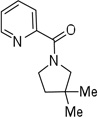 |
40 |
| 9d,e | 2-CO2tBu-3-methylbutyl | 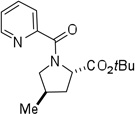 |
36 |
| 10f | 4-methyl-pent-2-yl | 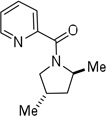 |
59 |
| 11d | 2,6-dimethylbenzyl | 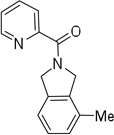 |
68 |
| 12d | 2,6-dimethyl-4-bromo-α-methylbenzyl | 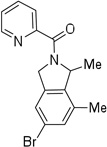 |
42 |
| 13 | 1-(CO2Me)-2-phenylethyl | 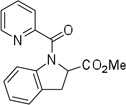 |
67 |
| 14g | 2-t-butylphenyl | 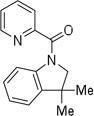 |
36 |
Yields are isolated yields. See Supporting Information for details.
5,6-Dimethoxyindole picolinamide (18%) and 2-acetoxy-4,5-dimethoxyphenethylamine picolinamide (59%) also isolated.
7-Phenylphenanthridine (7%) also isolated.
Toluene/acetonitrile solvent.
Trans/cis ratio 9/1.
>10/1 trans/cis.
Tolene/DMF solvent. Entries 1, 3–7, 13 were run at 80 °C; 2, 9 at 100 °C, and 10–12, 14 at 120 °C.
More challenging sp3 C-H/N-H cyclizations also proceed smoothly (entries 7–12). t-Octylamine picolinamide is cyclized in a good yield (entry 7), and 3,3-dimethylbutylamine derivative affords the product in moderate yield (entry 8). Leucine picolinamide gives cyclized product in fair yield (entry 9). 4-Methyl-2-aminopentane picolinamide cyclizes in 59% yield (entry 10). Benzylic sp3 C-H bonds are also reactive. 2,6-Dimethylbenzylamine picolinamide affords a 4-methylisoindoline derivative (entry 11). A 2,4,6-trisubstituted isoindoline can be obtained if 2,6-dimethyl-4-bromo-α-methylbenzylamine picolinamide is cyclized (entry 12). 2-t-Butylaniline derivative is cyclized in a modest yield (entry 14). For sp3 C-H bond amination, addition of acetonitrile solvent is sometimes beneficial (entries 8, 9, 11, 14). Reasonable functional group tolerance is observed, with t-butyl and methyl esters (entries 3 and 13) as well as aromatic chloride and bromide substituents (entries 2 and 12) tolerated. The directing group can be removed by LiEt3BH (eq 1). In general, amination is preferable to acetoxylation which is observed if a six-membered chelate such as 2 cannot be formed. Amination of methylene groups was not successful.
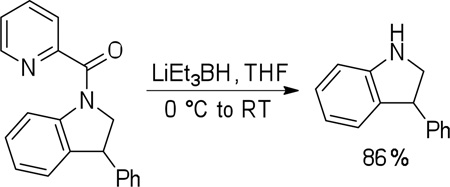 |
(1) |
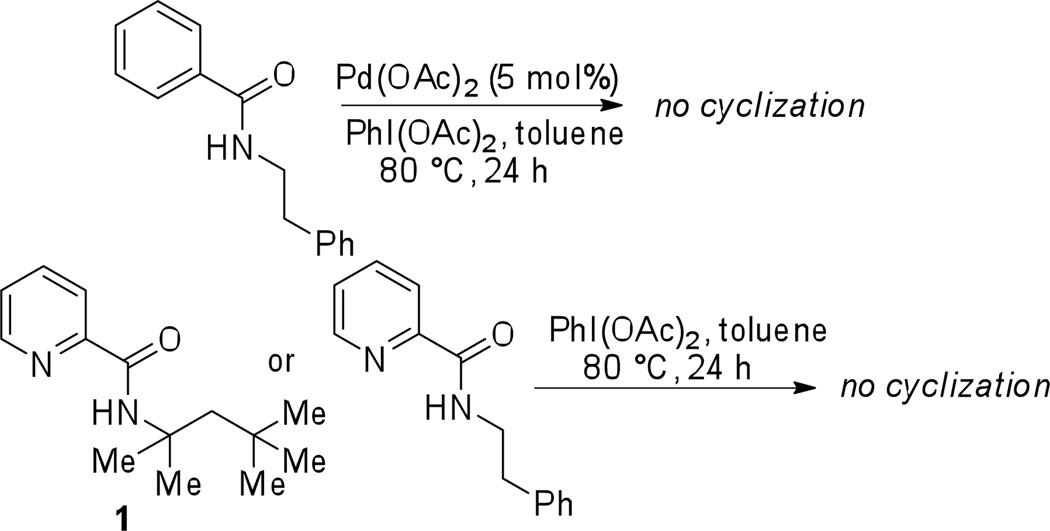
Several control experiments were run to exclude the possibility of other reaction pathways. First, subjecting 2-phenylethylamine benzamide to the cyclization conditions afforded no product. Consequently, picolinamide directing group is essential for achieving cyclization. (Scheme 4). Second, the reaction of phenylethylamine picolinamide and N-(2,4,4-trimethylpentan-2-yl)picolinamide with PhI(OAc)2 in toluene at 80 °C did not afford cyclization product, showing that palladium catalyst is required.
Scheme 4.
Control experiments
In conclusion, we have developed a palladium-catalyzed method for pyrrolidine, indoline, and isoindoline formation by a C-H/N-H coupling. The method employs a picolinamide directing group, PhI(OAc)2 oxidant, and toluene solvent at 80–120 °C. Cyclization is effective for sp2 as well as aliphatic and benzylic sp3 C-H bonds.
Supplementary Material
Scheme 2.
Activation of C-H Bonds in δ-Positions
ACKNOWLEDGMENT
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Sciences (Grant No. R01GM077635), A. P. Sloan Foundation, and Camille and Henry Dreyfus Foundation for supporting this research.
Footnotes
ASSOCIATED CONTENT
Supporting Information Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Reviews: Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. Ackermann L, Vicente R, Kapdi AR. Angew. Chem., Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996. Chen X, Engle KM, Wang DH, Yu JQ. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. Daugulis O, Do HQ, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Messaoudi S, Brion JD, Alami M. Eur. J. Org. Chem. 2010:6495. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. Sun CL, Li BJ, Shi ZJ. Chem. Commun. 2010:677. doi: 10.1039/b908581e. Li CJ. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n. Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem.-Eur. J. 2010;16:2654. doi: 10.1002/chem.200902374. Catellani M, Motti M, Della Ca’ N, Ferraccioli R. Eur. J. Org. Chem. 2007:4153.
- 2. Yin G, Wu Y, Liu G. J. Am. Chem. Soc. 2010;132:11978. doi: 10.1021/ja1030936. Vickers CJ, Mei T-S, Yu J-Q. Org. Lett. 2010;12:2511. doi: 10.1021/ol1007108. Rice GT, White MC. J. Am. Chem. Soc. 2009;131:11707. doi: 10.1021/ja9054959. Tan Y, Hartwig JF. J. Am Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. Ng K-H, Chan ASC, Yu W-Y. J. Am. Chem. Soc. 2010;132:12862. doi: 10.1021/ja106364r. Wasa M, Yu J-Q. J. Am. Chem. Soc. 2008;130:14058. doi: 10.1021/ja807129e. Du H, Zhao B, Shi Y. J. Am. Chem. Soc. 2008;130:8590. doi: 10.1021/ja8027394. Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Org. Lett. 2007;9:2931. doi: 10.1021/ol0711117. Cho S-H, Yoon J, Chang S. J. Am. Chem. Soc. 2011;133:5996. doi: 10.1021/ja111652v. Fan R, Pu D, Wen F, Wu J. J. Org. Chem. 2007;72:8994. doi: 10.1021/jo7016982. Antonchick AP, Samanta R, Kulikov K, Lategahn J. Angew. Chem., Int. Ed. 2011;50:8605. doi: 10.1002/anie.201102984. Amination via nitrenoids: Du Bois J. Chemtracts. 2005;18:1. Lu H, Subbarayan V, Tao J, Zhang XP. Organometallics. 2010;29:389. Ichinose M, Suematsu H, Yasutomi Y, Nishioka Y, Uchida T, Katsuki T. Angew. Chem., Int. Ed. 2011;50:9884. doi: 10.1002/anie.201101801.
- 3.Amino acid cyclization: Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149. doi: 10.1021/ja016280f. Neumann JJ, Rakshit S, Dröge T, Glorius F. Angew. Chem., Int. Ed. 2009;48:6892. doi: 10.1002/anie.200903035.
- 4.Pan J, Su M, Bucwald SL. Angew. Chem., Int. Ed. 2011;50:8764. [Google Scholar]
- 5.(a) Watanabe T, Oishi S, Fujii N, Ohno H. Org. Lett. 2008;10:1759. doi: 10.1021/ol800425z. [DOI] [PubMed] [Google Scholar]; (b) Nakanishi M, Katayev D, Besnard C, Kündig EP. Angew. Chem., Int. Ed. 2011;50:7438. doi: 10.1002/anie.201102639. [DOI] [PubMed] [Google Scholar]
- 6.(a) Mei TS, Wang X, Yu J-Q. J. Am. Chem. Soc. 2009;131:10806. doi: 10.1021/ja904709b. [DOI] [PubMed] [Google Scholar]; (b) Tsang WPC, Zheng N, Buchwald SL. J. Am. Chem. Soc. 2005;127:14650. doi: 10.1021/ja055353i. [DOI] [PubMed] [Google Scholar]; (c) Jordan-Hore JA, Johansson CCC, Gulias M, Beck EM, Gaunt MJ. J. Am Chem. Soc. 2008;130:16186. doi: 10.1021/ja806543s. [DOI] [PubMed] [Google Scholar]; (d) Gou F-R, Wang X-C, Huo P-F, Bi H-P, Guan Z-H, Liang Y-M. Org. Lett. 2009;11:5726. doi: 10.1021/ol902497k. [DOI] [PubMed] [Google Scholar]; (e) Youn SW, Bihn JH, Kim BS. Org. Lett. 2011;13:3738. doi: 10.1021/ol201416u. [DOI] [PubMed] [Google Scholar]; (f) Hofmann AW. Ber. Dtsch. Chem. Ges. 1879;12:984. [Google Scholar]; (g) Majetich G, Wheless K. Tetrahedron. 1995;51:7095. [Google Scholar]
- 7.Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]
- 8.Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- 10.(a) Feng Y, Chen G. Angew. Chem., Int. Ed. 2010;49:958. doi: 10.1002/anie.200905134. [DOI] [PubMed] [Google Scholar]; (b) He G, Chen G. Angew. Chem., Int. Ed. 2011;50:5192. doi: 10.1002/anie.201100984. [DOI] [PubMed] [Google Scholar]; (c) Gutekunst WR, Baran PS. J. Am. Chem. Soc. 2011;133:19076. doi: 10.1021/ja209205x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J-J, Giri R, Yu J-Q. Tetrahedron. 2008;64:6979. [Google Scholar]
- 12.Lafrance M, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2007;129:14570. doi: 10.1021/ja076588s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.