SUMMARY
The regulation of telomere length (TL) is a complex process, requiring the telomerase enzyme complex and numerous regulatory proteins. Epigenetic regulation may also be important in telomere maintenance. Specifically, methylation at subtelomeres is associated with changes in TL in vitro and in mouse models. Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome characterized by exceedingly short telomeres and mutations in telomere biology genes. In order to understand the interaction between methylation and TL in humans, we measured LINE-1, pericentromeric (NBL2), and subtelomeric (D4Z4) methylation in peripheral blood DNA derived from 40 patients with DC and 51 mutation-negative relatives. Pearson’s correlation coefficient and linear regression models were used to evaluate the relationship between age-standardized lymphocyte TL measured by flow-FISH and % DNA methylation.
No differences in % subtelomeric, LINE-1, or pericentromeric methylation between DC patients and relatives were noted except for an increase in % subtelomeric methylation in DC patients with a telomerase-complex mutation (TERC, TERT, DKC1, or TCAB1) (63.0% in DC vs. 61.8% in relatives, p=0.03). Positive correlations between TL and DNA methylation at LINE-1 (r=0.39, p=0.01) and subtelomeric (r=0.32, p=0.05) sites were present in patients with DC. The positive correlation between TL and % LINE-1 methylation was restricted to TINF2 mutations. In contrast, statistically non-significant inverse correlations between TL and % LINE-1 (r=−0.17), subtelomeric (r= −0.20) were present in unaffected relatives. This study suggests an interaction between TL, and both subtelomeric and LINE-1 methylation which may be altered based on mutation status of telomere biology genes.
INTRODUCTION
Human telomeres consist of long (TTAGGG)n nucleotide repeats and an associated protein complex at chromosome ends that are essential for maintaining chromosomal stability. They shorten with each cell division due to the inability of DNA polymerases to replicate the ends of linear DNA. Thus, telomeres are markers of aging and cellular replicative history (Aubert & Lansdorp, 2008). Telomeres are elongated by the telomerase ribonucleoprotein complex which consists of the telomerase reverse transcriptase (TERT), its RNA component (TERC), and other regulatory proteins such as dyskerin (DKC1). In addition, telomeric length and structure are maintained, in part, by six core proteins called the “shelterin” complex (Palm & de Lange, 2008). Epigenetic regulation also appears to be an important component of telomere maintenance. Mammalian telomeres are enriched with histone markers but lack CpG islands and thus are not methylated. In contrast, subtelomeres, the adjacent regions of repetitive DNA, are rich in both histone markers and CpG islands, reviewed in (Blasco, 2007).
Mouse models and in vitro studies suggest that histone modifications and/or DNA methylation patterns at the subtelomere may be important in telomere length (TL) regulation (Schoeftner & Blasco, 2009). Subtelomeric hypomethylation and telomere elongation via the recombination-based alternative lengthening of telomeres (ALT) pathway were noted in DNA methyltransferase (DNMT) deficient mice (Gonzalo et al., 2006). This suggests a negative regulatory role for DNA methylation on TL. In contrast, studies of lymphoblastoid or fibroblast cell lines derived from patients with immunodeficiency, centromeric region instability, and facial anomalies (ICF) syndrome - caused by a germline mutation in the DNA methyltransferase 3b (DNMT3b) gene - showed that the DNA hypomethylation status, particularly in subtelomeric DNA repeats, did not activate ALT and was associated with short telomeres. Telomere repeat-containing RNA (TERRA) was overexpressed in these cell lines (Yehezkel et al., 2008; Deng et al., 2010).
Gradual telomere shortening was associated with a reduction in levels of subtelomeric DNA methylation in TERC deficient mice (Benetti et al., 2007). However, it is important to recognize that murine telomeres are significantly longer than human telomeres; murine phenotypes due to defects in telomere biology genes are often not seen until late generations of knockout mice are created (Riethman, 2008). In a panel of human cancer cell lines, a negative correlation between subtelomeric methylation and TL was observed (Vera et al., 2008). A subsequent study suggested that the inverse relationship between subtelomeric methylation and TL was chromosomal dependent (Lee et al., 2009).
Dyskeratosis congenita (DC) is an inherited bone marrow failure and cancer predisposition syndrome caused by defects in telomere biology (reviewed in Savage & Alter, 2009; Walne & Dokal, 2009). This disorder is defined clinically by the presence of the diagnostic triad of lacy reticular skin pigmentation, nail dystrophy, and oral leukoplakia. Multiple other medical complications, such as esophageal stenosis, developmental delay, pulmonary fibrosis, or liver disease may also be present. Patients with DC have very short telomeres (typically less than the 1st percentile for their age) (Alter et al., 2007; Du et al., 2009b; Vulliamy et al., 2001). Germline mutations in genes important in telomere biology have been identified in about 60% of patients with DC. All forms of inheritance have been noted in DC families: X-linked recessive (DKC1), autosomal dominant (TERC, TERT, or TINF2), and autosomal recessive (TERT, NOP10, NHP2, or WRAP53 [protein: TCAB1]) (Savage & Alter, 2009; Walne & Dokal, 2009; Zhong et al., 2011).
In order to better understand the role of methylation in TL regulation, we studied the relationship between TL and subtelomeric, global (LINE-1), and pericentromeric DNA methylation levels in patients with aberrant telomere biology due to DC and their mutation-negative, unaffected relatives.
RESULTS
Characteristics of Study Participants
Patients with DC were significantly younger than their unaffected relatives (median age in years [range]; 14.4 [1.3–71.1] vs. 45.6 [4.9–87.1], respectively, p<0.0001), more likely to be males (p<0.001), and had very short telomeres for their age (p<0.0001). The median age standardized TL (measured by Z-score) for relatives was − 0.5 standard deviation from the population mean of the same age (range= −4.1 to 1.5) compared with a median of −4.5 in patients with DC (range= −7.9 to −1.5) (Table 1). As expected, we observed a statistically significant inverse correlation between age and TL in the unaffected relatives (r= −0.59, p<0.0001). The inverse correlation between age and TL in patients with DC was present but not significant (r= −0.17, p=0.30, data not shown). No statistically significant (p<0.05) correlations between age and % subtelomeric, LINE-1, or pericentromeric DNA methylation were observed in DC patients or relatives (data not shown).
Table 1.
Demographic characteristics and telomere length of dyskeratosis congenita patients by genotype and their unaffected, mutation-negative family members
| Unaffected Relatives N=51 | Dyskeratosis Congenita Patients By Genotype, N=40 | |||
|---|---|---|---|---|
| Mutations in Telomerase-Complex Genes* N=18 | Mutations in TINF2 N=11 | Unknown Gene N=11 | ||
|
Age Median (range) |
45.6 (4.9–87.1) | 24.3 (3.04–47.76) | 21.2 (3.4–71.05) | 7.9 (1.32–16.9) |
|
Gender Male: Female |
23:28 | 13:5 | 9:2 | 9:2 |
|
Absolute telomere length (Kb) Median (range) |
6.4 (3.4–10.3) | 4.0 (3.2–6.0) | 3.4 (3.0–5.2) | 3.8 (2.8–6.6) |
|
Age standardized (z-scores) Median (range) |
−0.5 (−4.1–1.5) | −3.8 (−5.7–1.5) | −4.6 (−7.1–2.2) | −5.7 (−7.8–2.4) |
|
% LINE-1 methylation Median (range) |
77.9 (68.0–83.6) | 78.1 (75.3–82.5) | 78.0 (71.7–79.7) | 79.1 (67.2–82.3) |
|
% subtelomeric methylation Median (range) |
61.8 (49.8–73.2) | 63.0 (52.7–72.5) | 59.3 (53.7–73.9) | 61.0 (52.8–73.5) |
|
% pericentromeric methylation Median (range) |
87.4 (59.4–90.9) | 86.9 (66.9–91.6) | 86.7 (83.4–89.7) | 85.3 (81.0–89.5) |
4 patients with TERT mutations, 5 with TERC, 7 with DKC1, and 2 with TCAB1
The Relationship between Subtelomeric Methylation, Disease Status, and TL
Overall, the level of subtelomeric methylation in patients with DC was not different from that of their healthy relatives (median=61.9% vs. 61.8%, p=0.5). Stratification of DC patients by genotype subgroup showed that the median % subtelomeric methylation was higher in patients with a telomerase-complex mutation (TERC, TERT, DKC1, or TCAB1) than in unaffected relatives (all cases 63.0% vs. 61.8% in relatives, p=0.03) (Table 1). The relationship between telomerase-complex mutations in DC and the presence of subtelomeric hypermethylation remained statistically significant after adjusting for age and gender, and accounting for the correlation between family members (odds ratio [OR]=1.15, 95% confidence interval [CI]=1.01–1.30, p=0.03). Within the telomerase-complex group, there was a trend toward higher levels of subtelomeric methylation in TERT mutations (67.2%), than in DKC1 (63.3%) or TERC (62.8%) (data not shown). However, these gene-specific differences were not statistically significant.
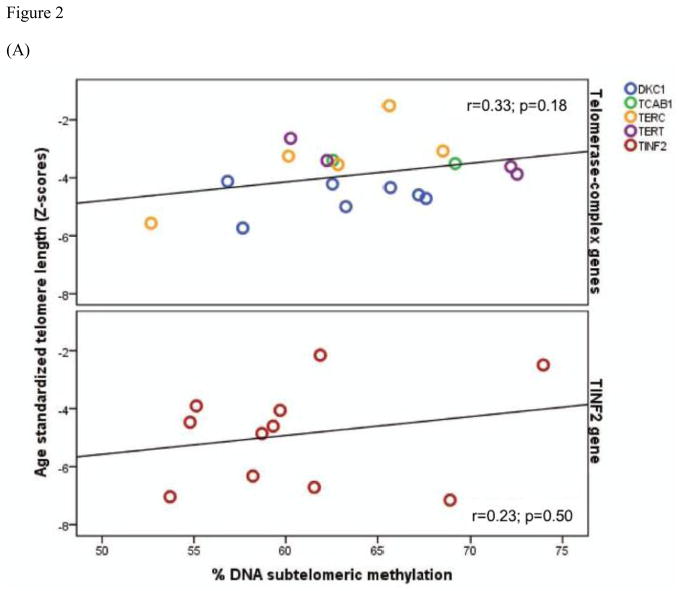
We further evaluated the correlation between subtelomeric methylation and TL in DC patients and relatives separately. In DC, subtelomeric methylation and TL were positively correlated (r=0.32, p=0.05) (Figure 1A). This suggests that within DC, subtelomeric hypermethylation is associated with relatively longer telomeres (although still very short for age). The positive relationship between TL and subtelomeric methylation remained statistically significant after accounting for the correlation between affected family members and adjusting for gender and age (β=0.07, 95% CI=0.02–0.13, p=0.01). Furthermore, this positive relationship did not vary by genotype group in DC patients (r=0.23, and 0.33, in TINF2 and telomerase-complex, respectively) (Figure 2A). In contrast, there was an inverse correlation between TL and subtelomeric methylation (r= −0.20, p=0.20), although not statistically significant, in the unaffected relatives (Figure 1A).
Figure 1. Correlation between age standardized telomere length and methylation in patients with dyskeratosis congenita (DC) and their mutation-negative, unaffected relatives.
A) % subtelomeric, B) % LINE-1, and C) % pericentromeric DNA methylation in DC patients (upper panel) and their unaffected relatives (lower panel). Telomere length was age standardized using Z-scores; r values represent the Pearson’s correlation coefficient between telomere length and methylation.
Figure 2. Correlation between age standardized telomere length and methylation in patients with dyskeratosis congenita (DC) by genotype.
A) % subtelomeric, B) % LINE-1 methylation in DC patients by genotype group; telomerase-complex genes (upper panel) and the TINF2 gene (lower panel). Telomere length was age standardized using Z-scores; r values represent the Pearson’s correlation coefficient between telomere length and methylation.
The Relationship between LINE-1 Methylation, Disease Status, and TL
Methylation at LINE-1 sites was determined as a measure of global methylation. Overall, LINE-1 methylation was not different between patients with DC and their relatives (78.2% vs. 77.9, respectively, p=0.7). DC patient genotype was not associated with LINE-1 methylation. We next evaluated the correlation between LINE-1 methylation and TL in order to evaluate whether methylation throughout the genome has an effect on TL. Within the setting of very short telomeres in DC, we found that higher levels of LINE-1 methylation correlated with having relatively longer telomeres (r=0.39, p=0.01) (Figure 1B), This positive correlation was restricted to individuals with TINF2 mutations; no correlation between TL and % LINE-1 methylation was noted in patients with telomerase-complex mutations (TINF2 r=0.79, p=0.004 vs. telomerase-complex r= −0.05, p=0.83, p-interaction <0.0001) (Figure 2). In unaffected relatives, we observed a weak statistically non-significant negative correlation between TL and levels of LINE-1 (r= −0.17, p=0.30) (Figure 1B),
The Relationship between Pericentromeric Methylation, Disease Status, and TL
The level of pericentromeric methylation was measured as a negative control, since it is not expected to have a direct effect at the telomeres. Levels of pericentromeric methylation were very similar in patients with DC and their relatives (86.7 vs. 87.4, respectively, p=0.4). There were no genotype-specific associations. There was no correlation between levels of pericentromeric methylation and TL in DC patients (r=0.01, p=0.93) or in their relatives (r= −0.16, p=0.32) (Figure 1C).
DISCUSSION
The complex process of telomere maintenance is typically thought to primarily require the telomerase holoenzyme complex and numerous interacting proteins. However, several studies suggest that epigenetic regulation, in the form of DNA methylation and/or histone modification, is also very important in telomere biology (reviewed in Schoeftner and Blasco, 2009). We sought to better understand the relationship between DNA methylation and telomere biology in humans by evaluating the relationship between methylation levels at subtelomeric, LINE-1, and pericentromeric sites in a well-characterized cohort of patients with the telomere biology disorder DC and their healthy, mutation-negative relatives
Our case-control comparison of DNA methylation at subtelomeric, LINE-1, and pericentromeric sites did not identify methylation differences in patients with DC overall compared with their healthy, mutation-negative relatives. We did find that DC patients with telomerase-complex mutations had elevated levels of subtelomeric methylation compared with DC patients with TINF2 mutations or with the relatives. The exact reason for this difference is not clear, but it could be related to differences in the expression level of the telomere repeat-containing RNA (TERRA). TERRA expression was suggested to mediate subtelomeric methylation in telomerase-positive cancer cells (Ng et al., 2009), as well as in lymphoblastoid and fibroblast cells isolated from ICF patients (Yehezkel et al., 2008). However, the role of TERRA in DC has not yet been elucidated.
The inverse correlations between TL and subtelomeric, LINE-1, or pericentromeric methylation in mutation-negative healthy relatives observed in our study are in agreement with previously reported relationships in a panel of more than 20 different human cancer cell lines (Vera et al., 2008). Our study provides additional data suggesting a negative regulatory mechanism of DNA methylation on TL (Blasco, 2007). Notably, we found that DC-associated telomere biology abnormalities appear to change the direction of this regulatory mechanism. In patients with DC, TL and methylation at subtelomeric and LINE-1 sites were positively correlated. In other words, elevated levels of methylation were associated with longer telomeres, (albeit still exceedingly short for age) in DC. This correlation between subtelomeric hypermethylation and relatively longer telomeres in DC was independent of genotype.
Several studies suggest that telomere shortening caused by TERT or TERC mutations is less severe than what has been observed in patients with TINF2 mutations (Walne et al., 2008; Du et al., 2009a; Sasa et al., 2011;Vulliamy et al., 2010). It is possible that the relatively longer telomeres (although still very short for age) in DC patients who have mutations in telomerase-complex genes can be explained, at least partially, by the subtelomeric hypermethylation observed in those patients.
Surprisingly, the positive relationship between % LINE-1 methylation and TL was observed only in patients with TINF2 mutations. A recent study suggests that heterochromatin protein 1γ (HP1γ) binds to the C-terminal domain of the TINF2 protein (TIN2) and that DC-associated TINF2 mutations disrupt this binding (Canudas et al., 2011). The extra-telomeric functions of TIN2 have yet to be elucidated, but it is theoretically possible that interactions between TIN2 and heterochromatin proteins are related to changes in methylation across the genome.
In conclusion, the genetic and phenotypic heterogeneity of DC which occurs as a consequence of telomere biology defects provides an excellent human model in which to explore the epigenetic regulation of telomeres. Subtelomeric methylation in DC patients is positively correlated with TL independent of genotype whereas the converse is true in healthy individuals. In addition, the correlation between LINE-1 methylation and TL was only present with TINF2 mutations. Our results suggest that the relationship between TL and both LINE-1 and subtelomeric methylation is dependent upon telomere biology gene function.
EXPERIMENTAL PROCEDURE
Study Participants
This study included 40 patients with DC and 51 unaffected mutation-free relatives who are enrolled in the National Cancer Institute’s Institutional Review Board approved protocol 02-C-0052 (NCT00056121, www.marrowfailure.cancer.gov) (Alter et al., 2010). Patients were classified as DC patients in the current analyses if they had a mutation in one of the known genes or if they had at least 2 features of the diagnostic triad and other clinical findings consistent with DC, such as hematologic or neoplastic complications. All patients had telomeres <1st percentile for age in lymphocytes as measured by automated multicolor fluorescence in situ hybridization combined with flow cytometry (flow FISH) (Alter et al., 2007). All 40 patients with DC were tested for mutations in the known DC genes, DKC1 (males only), TERC, TERT, TINF2, NOP10, NHP2, and TCAB1. Throughout the manuscript, we refer to patients with DC who do not have a mutation in one of the known genes as patients with unknown gene. Patients with DC were stratified according to their affected gene based on the gene biological function into: 1) telomerase-complex mutations: patients with a mutation in one of the genes critical to telomerase function (5 TERC, 4 TERT, 7 DKC1, and 2 TCAB1); 2) patients with a mutation in the shelterin gene TINF2 (n=11); and 3) unknown gene patients (n= 11). The healthy relatives were tested for the mutation associated with disease in their family and included as controls only if they did not carry the mutation. Family members of patients in whom the causative gene is not yet known were excluded from the analyses to avoid possible misclassification bias.
Methylation Assays
DNA was extracted from peripheral blood cells of patients and relatives by manual Gentra Puregene procedure (Qiagen Inc., Valencia, CA). Lymphocyte TL was measured by automated multicolor flow-FISH, as previously described (Baerlocher et al., 2006). Genomic DNA was bisulphate converted and methylation at LINE-1, subtelomeric, and pericentromeric sites was quantified using PCR-pyrosequencing (EpigenDx, Worcester, MA), as described (Tost & Gut, 2007). Pericentromeric methylation was determined at the NBL2 locus (chromosomes 9, 13, 14, 21), and subtelomeric methylation was measured at the D4Z4 repeat sequences of chromosomes 4 and 10. The level of DNA methylation is presented as the mean percentage of methylated CpGs across all sequenced alleles to the total number of CpGs.
Statistical Analyses
Pearson’s correlation coefficient (r) was used to evaluate the strength of association between TL and levels of DNA methylation at the different sites. Multivariable linear regression models were used to adjust for potential confounders and test for interactions. All models were adjusted for age and gender. Interaction terms between participant disease status (DC vs. unaffected relative) and methylation level were included in the models to test whether the relationship between TL and levels of DNA methylation was modified by DC status. The robust variance estimator (Zeger & Liang, 1986) was used to account for the correlations between observations from participants of the same family. TL in this study was age standardized using Z-scores. The Z-scores standardize each subject’s absolute TL to the population mean and standard deviation for TL within the same age and are calculated as follows:
Population estimates were derived from TL measurement on 400 normal individuals who ranged in age between 0–100 years (Alter et al., 2007).
Acknowledgments
This work was funded by the intramural research program of the National Cancer Institute, National Institutes of Health. We thank the patients and their families for their generous participation in the study. Lisa Leathwood, RN, Westat, Inc. (NIH contracts N02-CP-11019, N02-CP-65504, and N02-CP-65501) provided outstanding study support.
Footnotes
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: SAS, SMG. Analyzed the data: SMG, HAK, FMS. Evaluated patients and collected clinical data: SAS, NG, BP. Wrote the paper: SMG, SAS. All authors reviewed, edited, and approved the final manuscript.
References
- Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110 (5):1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, Carr AG, Greene MH, Rosenberg PS. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150 (2):179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88 (2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1 (5):2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- Benetti R, Garcia-Cao M, Blasco MA. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat Genet. 2007;39 (2):243–250. doi: 10.1038/ng1952. [DOI] [PubMed] [Google Scholar]
- Blasco MA. The epigenetic regulation of mammalian telomeres. Nat Rev Genet. 2007;8 (4):299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- Canudas S, Houghtaling BR, Bhanot M, Sasa G, Savage SA, Bertuch AA, Smith S. A role for heterochromatin protein 1{gamma} at human telomeres. Genes Dev. 2011 doi: 10.1101/gad.17325211. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Campbell AE, Lieberman PM. TERRA, CpG methylation and telomere heterochromatin: lessons from ICF syndrome cells. Cell Cycle. 2010;9 (1):69–74. doi: 10.4161/cc.9.1.10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du HY, Mason PJ, Bessler M, Wilson DB. TINF2 mutations in children with severe aplastic anemia. Pediatr Blood Cancer. 2009a;52 (5):687. doi: 10.1002/pbc.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, Chirnomas D, Shimamura A, Vlachos A, Lipton JM, Goyal RK, Goldman F, Wilson DB, Mason PJ, Bessler M. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009b;113 (2):309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Jaco I, Fraga MF, Chen T, Li E, Esteller M, Blasco MA. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol. 2006;8 (4):416–424. doi: 10.1038/ncb1386. [DOI] [PubMed] [Google Scholar]
- Lee ME, Rha SY, Jeung HC, Chung HC, Oh BK. Subtelomeric DNA methylation and telomere length in human cancer cells. Cancer Lett. 2009;281 (1):82–91. doi: 10.1016/j.canlet.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Cropley JE, Pickett HA, Reddel RR, Suter CM. Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res. 2009;37 (4):1152–1159. doi: 10.1093/nar/gkn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- Riethman H. Human telomere structure and biology. Annu Rev Genomics Hum Genet. 2008;9:1–19. doi: 10.1146/annurev.genom.8.021506.172017. [DOI] [PubMed] [Google Scholar]
- Sasa G, Ribes-Zamora A, Nelson N, Bertuch A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01658.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23 (2):215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA. A ‘higher order’ of telomere regulation: telomere heterochromatin and telomeric RNAs. EMBO J. 2009;28 (16):2323–2336. doi: 10.1038/emboj.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nat Protoc. 2007;2 (9):2265–2275. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- Vera E, Canela A, Fraga MF, Esteller M, Blasco MA. Epigenetic regulation of telomeres in human cancer. Oncogene. 2008;27 (54):6817–6833. doi: 10.1038/onc.2008.289. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Beswick R, Kirwan M, Hossain U, Walne A, Dokal I. Telomere length measurement can distinguish pathogenic from non-pathogenic variants in the shelterin component, TIN2. Clin Genet. 2010 doi: 10.1111/j.1399-0004.2010.01605.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27 (2):353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- Walne AJ, Dokal I. Advances in the understanding of dyskeratosis congenita. Br J Haematol. 2009;145 (2):164–172. doi: 10.1111/j.1365-2141.2009.07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112 (9):3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehezkel S, Segev Y, Viegas-Pequignot E, Skorecki K, Selig S. Hypomethylation of subtelomeric regions in ICF syndrome is associated with abnormally short telomeres and enhanced transcription from telomeric regions. Hum Mol Genet. 2008;17 (18):2776–2789. doi: 10.1093/hmg/ddn177. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42 (1):121–130. [PubMed] [Google Scholar]
- Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25 (1):11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]