Abstract
Background
Allergen measurements are widely used for environmental exposure assessments and for determining the potency of allergen vaccines, yet few purified allergen standards have been developed. The aim of the study was to develop a single standard containing multiple purified allergens that could be used in enzyme immunoassays and in multiplex arrays for standardization of allergen measurements.
Methods
Eight purified allergens were formulated into a single multi-allergen, or “universal”, standard based on amino acid analysis. Dose response curves were compared with previous individual ELISA standards and allergen measurements of house dust extracts to obtain correction factors. Measured allergen concentrations were also modeled using linear regression and the predictive accuracy was determined.
Results
Parallel dose response curves were obtained between the universal allergen standard and the individual ELISA standards, with close agreement between curves for 5/8 allergens. Quantitative differences of >2-fold were observed for Fel d 1, Can f 1 and Der f 1, which were confirmed by analysis of house dust extracts. Correction factors were developed that allowed ELISA data to be expressed in terms of the universal standard. Linear regression data confirmed the predictive accuracy of the universal standard.
Conclusion
This study shows that a single standard of eight purified allergens can be used to compare allergen measurements by immunoassay. This approach will improve continuity of environmental exposure assessments and provide improved standardization of allergy diagnostics and vaccines used for immunotherapy.
Keywords: allergen standardization, asthma, allergen exposure, immunoassays, allergy vaccines
Introduction
The widespread use of allergen measurements for research, diagnostics, development of new allergy vaccines and environmental exposure assessments requires well defined allergen standards. Manufacturers need standards for allergenic extracts that are used for in vitro immuno-diagnostic IgE tests and for purified allergens that are used in microarray based IgE tests[1-4]. Allergen therapeutics companies require standards to measure the major allergen content of sub-cutaneous or sub-lingual allergy vaccines and for new generations of recombinant allergen vaccines that have proved successful in recent clinical trials[5;6]. Epidemiologic studies of allergic diseases also rely extensively on allergen measurements for environmental exposure assessments[7].
The increasing use of purified natural and recombinant allergens for diagnostic and therapeutic use has spurred the need to develop purified allergen standards. To address these needs, the WHO/IUIS Allergen Standardization Committee initiated a program to develop purified allergen standards that could be used for calibration of in vitro allergen measurements. This initiative was funded through the European Union Fifth Framework Programme to develop certified reference materials for allergenic products and to validate ELISA methods for their quantification (acronym CREATE). The aims of CREATE were to develop international reference materials with verifiable allergen content. These aims were achieved by i) comparison of purified natural and recombinant allergens for protein purity, IgE antibody binding and biologic activity; and ii) evaluation of ELISA tests for measuring the purified allergens. Eight purified natural and recombinant allergens were compared in the CREATE study by a consortium of academic researchers and scientists from industry or regulatory authorities[8-12].
Our goal was to apply the principles of allergen standardization developed in CREATE to other purified allergens. We recently developed a fluorescent multiplex array for indoor allergens (MARIA) which enables eight (or more) allergens to be measured simultaneously[13]. The use of purified proteins in multiplex systems is essential to reduce non-specific interactions that could affect assay performance. The development of a single multi-allergen standard for use in MARIA required the formulation of a cocktail of purified natural allergens: Der p 1, Der f 1, Der p 2, Fel d 1, Can f 1, Rat n 1, Mus m 1 and Bla g 2. The present paper describes the validation of the multi-allergen standard by comparison with previous individual ELISA standards and the performance of this standard for allergen measurements by immunoassay.
MATERIALS AND METHODS
Individual ELISA Standards
The individual ELISA standards were produced by Indoor Biotechnologies Inc (Charlottesville, VA) and were those in use at the time of the study. These standards had been extensively used in prior studies on environmental allergen exposure e.g. for the NIH Inner-City Asthma Studies, the U.S. National Survey of Lead and Allergens in Housing, and the European Community Respiratory Health Survey.[14-18] Mite allergen standards (Der p 1, Der f 1, Der p 2) were prepared from D. pteronyssinus or D. farinae spent culture medium (kindly provided by Laboratorios Leti, Madrid, Spain). Source materials for Fel d 1, Can f 1, Mus m1, Rat n 1, and Bla g 2 were cat/dog hair, rodent urine, or Blattella germanica frass. Where possible, the allergen content of individual ELISA standards was determined by reference to national or international standards. The Der p 1 and Can f 1 standards were sub-standardized against WHO/IUIS International Reference Preparations obtained from the National Institute for Biological Standards and Control (Potters Bar, UK), designated NIBSC 82/518 and NIBSC 84/685, respectively.[19;20] The Fel d 1 standard was sub-standardized against the FDA standard, Cat E10, and was calibrated in FDA units Fel d 1/ml. The Mus m 1 standard was sub-standardized against a natural Mus m 1 standard (MUP E428) that had been used in previous studies and was kindly provided by Dr. Peyton Eggleston, The Johns Hopkins University (Baltimore, MD).[21;22] The other ELISA standards were calibrated using in house references of purified allergens. The Lot numbers of individual ELISA standards were as follows: Der p 1 (2901), Der f 1 (30065), Mite Group 2 (Der p 2, 2409), Fel d 1 (30002), Can f 1 (2832), Rat n 1 (2714), Mus m 1 (2508), Bla g 2 (2418).
“Universal” Allergen Standard (UAS)
A single multi-allergen standard (termed the Universal Allergen Standard, UAS) was prepared using natural allergens (Der p 1, Der f 1, and Der p 2, Fel d 1, Can f 1, Rat n 1, Mus m 1 and Bla g 2) that were purified by affinity chromatography, size exclusion HPLC or ion-exchange HPLC using previously published methods.[22-26] Mus m 1 and Rat n 1 were purified from male urine by gel filtration and ion-exchange chromatography. Purity of the allergens was >90%, as determined by SDS-PAGE analysis using silver stained 8-25% gradient gels in the Pharmacia PhastSystem (GE Life Sciences, Piscataway, NJ). The purity of the mite allergens was comparable to the preparations used in CREATE.[12] The protein concentration of the purified allergens was determined by amino-acid analysis, by Advanced Protein Assay (APA) (Cytoskeleton, Denver, CO), and by extinction coefficient (A280nm). Amino acid analysis was performed using the Pico-Tag method (Waters, Milford, MA). Measurements were performed in duplicate and concentrations were calculated based on analysis of the internal amino acid standard A. The Advanced Protein Assay is a sensitive colorimetric assay with low protein to protein variance. The one step procedure resulted in a green to blue color change which was detected by measuring absorbance at 570 to 615 nm within 1 minute. The UAS was formulated by mixing the purified allergens to achieve working concentrations of 250 - 2500ng/ml in phosphate buffered saline, pH 7.4, containing 1% bovine serum albumin and 50% glycerol.
Quantitative Comparisons of Allergen Standards by ELISA
Measurements of the eight allergens used in the study were made using previously published ELISA methods.[27] The quantitative relationship between individual ELISA standards and the UAS was established by i) comparing dose response curves for each allergen and ii) by comparing allergen levels in house dust extracts using ELISA standards or the UAS.
To compare dose response curves, serial doubling dilutions of the UAS (Lot 31012), individual ELISA standards and the natural allergen from which the UAS 31012 was made were tested in duplicate across a 96-well microtiter plate. Starting dilutions for the UAS and for the individual ELISA standards were 1/10, while the natural allergen was started at 1/1000. For the analysis, the natural allergen was set up as the control curve and concentrations of UAS 31012 and ELISA standards were calculated. The conversion factors were then calculated by dividing the mean (ng/ml) of the UAS 31012 by the mean (ng/ml) of the ELISA standard.
House dust extracts (n = 13-18) were analyzed for each allergen by ELISA using either the UAS 31012 or individual ELISA standards. Doubling dilutions of each extract were assayed, from 1/10-1/40. The concentration of each sample was calculated against the corresponding curve and correction factors were calculated (as above). The mean correction factor was calculated from all samples for each allergen.
Using these approaches, correction factors were developed that could be applied to convert allergen measurements made with individual ELISA standards to those made with the UAS and vice-versa.
Linear Regression
Measured allergen concentrations based on UAS 31012 and ELISA standards were plotted and the relationship between the two was modeled using linear regression: y = ax + b, where y = UAS result, as predicted by the formula; x = measured concentration based on ELISA standard; a = coefficient; b = intercept. To evaluate the predictive accuracy for each allergen, the formula was applied to the measured concentration obtained with individual ELISA standards. As before, the so generated predicted UAS results were compared with measured UAS results using CV%.
RESULTS
The results showed good agreement between the protein concentrations of the purified allergens as determined by amino-acid analysis, APA and extinction coefficient. Most of the allergens showed <40% variation in total protein levels using the three methods (Table 1). In formulating the UAS, protein concentration values obtained by amino acid analysis were used to be consistent with the methods used in CREATE. Each allergen showed the expected molecular weight band(s) on SDS-PAGE, with purity of >90% (Figure 1). The Can f 1, Rat n 1 and Bla g 2 allergens showed trace levels of dimers which is consistent with previous data[22-26].
TABLE 1. Protein Measurements of Purified Allergens Used in the Universal Allergen Standard.
| Allergen |
Advanced Protein Assay (mg/ml) |
Amino Acid Analysis (mg/ml) |
A280 (mg/ml) |
|---|---|---|---|
| nDer p 1 | 1.40 | 1.07 | 1.15 |
| nDer f 1 | 1.10 | 0.69 | 0.91 |
| nDer p 2 | 1.10 | 0.85 | 0.98 |
| nFel d 1 | 1.10 | 1.38 | 1.08 |
| nCan f 1 | 0.56 | 0.65 | 1.01 |
| nMus m 1 | 2.00 | 1.20 | 1.41 |
| nRat n 1 | 1.30 | 0.80 | 1.15 |
| nBla g 2 | 3.10 | 3.60 | 5.97 |
Figure 1.
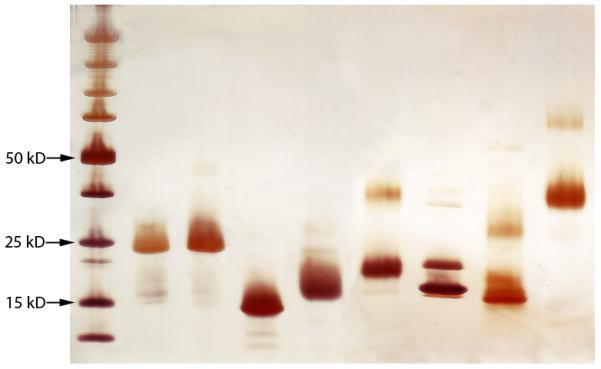
SDS-PAGE analysis of purified allergens. Left to right: molecular weight markers, Der p 1, Der f 1, Der p 2, Fel d 1, Can f 1, Mus m 1, Rat n 1, Bla g 2.
Experimental comparison of individual ELISA standards and the UAS
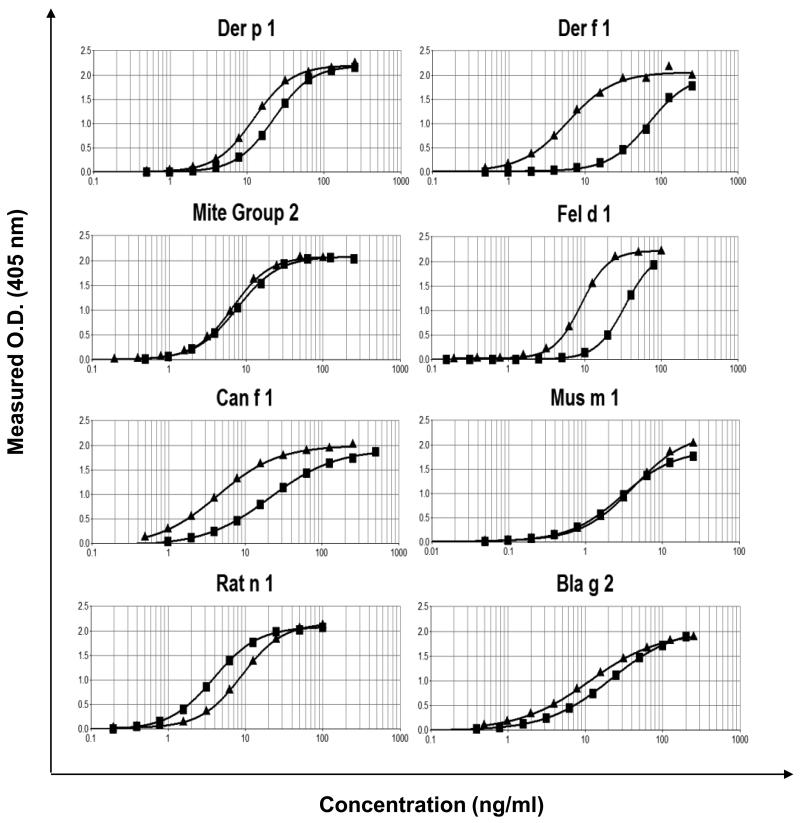
Parallel dose response curves were obtained when comparing the UAS with individual ELISA standards (Figure 2). The ELISA comparison showed close agreement (<2-fold difference) between the UAS and ELISA standards for Der p 1, Der p 2, Mus m 1, Rat n 1 and Bla g 2. For Fel d 1 and Can f 1, the UAS curves were three fold and 4-5 fold lower than the curves using the ELISA standards, respectively. The UAS curves for Der f 1 were ~10 fold lower than the ELISA standard. This suggested that the concentrations of Der f 1, Fel d 1 and Can f 1 were significantly over-estimated using previous ELISA standards, as compared to the purified allergen standards used in the UAS. To confirm these results, the allergen levels of 13-18 dust samples were compared using the UAS and ELISA standards. The comparison showed similar differences between allergen concentrations as were observed using the control curves. The fold differences were consistent with the differences seen with the standard dose response curves, i.e. UAS values were 3.4-fold. 5.9-fold and 12.7-fold lower than ELISA standards for Fel d 1, Can f 1 and Der f 1, respectively (Table 2). The results obtained from the dose response curves and house dust extracts provided conversion factors that could be used to express UAS results in terms of ELISA or to express ELISA standard values in terms of the UAS.
Figure 2.
Dose response curves of individual ELISA standards (■) and the UAS (▲) for eight allergens measured by ELISA.
TABLE 2. Conversion Factors between Allergen Standards Derived from Dose Response Curves or Analyses of House Dust Extracts.
| Conversion Factors of Dose Response Curves (n=2)* |
Conversion Factors of Dust Extract Results** |
Mean Conversion Factors for ELISA Stds and UAS (#31012) |
||||
|---|---|---|---|---|---|---|
| Allergen | ELISA Std Lot # | UAS vs ELISA (i) | UAS vs ELISA (ii) | UAS vs ELISA | UAS to ELISA Std | ELISA to UAS Std |
| Der p 1 | 2901 | 1.8 | 1.9 | 1.5 | 1.7 | 0.59 |
| Der f 1 | 2762 or 30065 | 11.6 | 13.6 | 13.0 | 12.7 | 0.08 |
| Der p 2 | 2409 | 1.0 | 1.0 | 2.3 | 1.4 | 0.71 |
| Fel d 1 | 2853 or 30002 | 4.0 | 3.6 | 2.6 | 3.4 | 0.29 |
| Can f 1 | 2832 | 5.4 | 6.3 | 5.9 | 5.9 | 0.17 |
| Mus m 1 | 2508 | 1.0 | 1.0 | 1.2 | 1.1 | 0.91 |
| Rat n 1 | 2714 | 0.4 | 0.5 | 0.5 | 0.5 | 2.10 |
| Bla g 2 | 2418 | 1.8 | 1.8 | 1.4 | 1.7 | 0.59 |
ELISA dose response curves using the Universal Allergen Standard (Lot # 31012) or individual ELISA standards were compared in two separate assays (i) and (ii).
Dust extracts (n=13 to 18) were analyzed by ELISA using either UAS or individual ELISA standard. Values represent the mean correction factor of all the dust samples tested.
Linear regression approach
Previously measured allergen results based on the UAS and ELISA Standards were plotted and fit with a linear regression line. In some cases, very high results were excluded from the fitting process to reduce influence of dilution errors and improve fit. Formulas and R2 for the linear regression were used to evaluate the predictive accuracy for each allergen. In most cases, the linear regression approach produced predicted UAS results within 20% of the measured UAS results (Table 3). Results based on the experimental conversion factor only (Table 2) were within 30% when the lower limit of detection was taken into account. This level of reproducibility is comparable to the level of inter-laboratory variability of ELISA’s for allergens (typically 30%). While the predictive accuracy of both conversion methods was satisfactory, the linear regression approach produced more accurate results for Der f 1, Mite Group 2, Fel d 1 and Mus m 1. As both approaches have been shown to provide satisfactory predictive accuracy, either the linear regression formulas or simple correction factors could be used to convert allergen measurements based on individual ELISA standards to those obtained by using the UAS, or vice versa.
TABLE 3. Linear Regression Formulae for Conversion of Allergen Values Using ELISA standards or the UAS.
| Allergen | ELISA Std Lot# |
Linear Regression Conversion ELISA Std to UAS (#31012) |
Linear Regression Conversion UAS (#31012) to ELISA Std |
R2 Value | Mean CV% (predicted vs. measured results) |
|---|---|---|---|---|---|
| Der p 1 | 2901 | y = 0.66x + 22.08 | x = (y−22.08)/0.66 | 0.94 | 17.4 |
| Der f 1 | 30065 | y = 0.07x +13.11 | x = (y−13.11)/0.07 | 0.99 | 9.2 |
| Mite Group 2 | 2409 | y = 0.43x − 1.88 | x = (y+1.88)/0.43 | 0.99 | 15.5 |
| Fel d 1 | 30002 | y = 0.38x + 7.56 | x = (y−7.56)/0.38 | 0.99 | 10.9 |
| Can f 1 | 2832 | y = 0.22x − 4.56 | x = (y+4.56)/0.22 | 0.99 | 15.9 |
| Mus m 1 | 2508 | y = 1.20x − 0.52 | x = (y+0.52)/1.20 | 0.99 | 8.8 |
| Rat n 1 | 2714 | y = 1.65x + 39.17 | x = (y−39.17)/1.65 | 0.99 | 11.2 |
| Bla g 2 | 2418 | y = 0.67x + 22.59 | x = (y−22.59)/0.67 | 0.99 | 13.5 |
DISCUSSION
The essential principle of the CREATE study was that use of mass units of purified allergens, and accurate measurement thereof, provided the most objective approach to harmonize allergen standardization worldwide.[10-12] Allergens should elicit IgE responses in a majority of allergic patients, have biologic activity, satisfy criteria of protein purity and be important for allergy diagnosis and treatment. The allergens used in the UAS are among the most important allergens associated with asthma and occupational allergic disease. Their protein structure and allergenic importance has been well documented[28-30]. Previously, measurements of these allergens by ELISA were based on in house standards that were extracts of source materials in which the level of allergens was estimated. The source materials were not purified allergens. The results show that purified natural allergens can be formulated into a single standard that can be used in both ELISA and MARIA, or potentially in other applications involving immunoassay. The advantages of a multi-allergen standard are that all of the allergens are measured under the same assay conditions, which increases the reproducibility of immunoassays. Most of the allergens in the UAS were comparable to previous individual ELISA standards. Three allergens (Fel d 1, Can f 1, Der f 1) were significantly over-estimated using the previous ELISA standards. This could be explained by drift that occurred over time during sub-standardization, variability of protein estimates and/or a lack of well defined primary reference preparations.
The transition to a multi-allergen standard may affect ongoing exposure studies in epidemiologic studies, e.g. birth cohorts and population surveys, involving allergen measurements that have been using individual ELISA standards. Switching standards has the potential to disrupt the continuity of exposure assessments and can also affect measurements of specific allergens in commercial allergen source materials and products for immunotherapy. This problem is compounded by the current lack of suitable international reference preparations of purified allergens. The quantitative relationship between the UAS and the previous ELISA standards has been defined in the present study to facilitate the transition from extract based standards to purified allergens. The data show that simple conversion factors or linear regression formulae can be applied to express results either in terms of individual ELISA standards or the multi-allergen standard.
Advances in allergen manufacturing and the use of “component resolved” diagnostics means that use of allergen extracts as standards will be discontinued for specific allergen measurements. Natural or recombinant allergens with defined protein content will be used for standardization purposes. The protein content of the standards used in the UAS was determined by amino acid analysis to be consistent with the CREATE project. We were able to use the UAS to determine the allergen content of national and international allergen reference preparations (data to be published elsewhere). The results demonstrate the feasibility of using multi-allergen standards as calibrators for immunoassays, similar to those used for multiplexed cytokine measurements, and suggest that this approach could be applied to other sources where purified allergens are available, e.g. tree, grass and weed pollens, molds and foods. The use of a single allergen standard improves the reproducibility of multiplex assays. Preliminary data from a multi-center trial of MARIA™ showed a high level intra- and inter-laboratory reproducibility which appeared to be related to use of homogeneous reagents and controlled assay conditions.[31]
For standardization purposes, it is vital that regulatory agencies generate purified natural or recombinant allergen standards can be used as international biological reference preparations. As part of the BSP090 program, two allergens used in CREATE (Bet v 1 and Phl p 5) are being tested as biological reference preparations by the Biological Standardization Programme of the European Directorate for the Quality of Medicines (EDQM)[32]. It is anticipated that the EDQM will extend its standardization program to include purified dust mite, cat and other allergens. The European Medicines Agency has issued guidelines that measurement of allergen exposure should be included in the clinical development of products for specific immunotherapy and that quantification of individual allergens should be included in the characterization of allergen extracts.[33] These measurements will be facilitated by using reference preparations and assays approved by the EDQM and included in the European Pharmacopoeia. This will facilitate improved standardization of allergen vaccines for use in subcutaneous and sub-lingual immunotherapy, as well as precise formulation of recombinant allergen diagnostic and therapeutic products.
Acknowledgements
We are grateful to Audrey Koid for additional technical support. This work was supported in part by National Institutes of Health Small Business Innovation and Research (SBIR) Award ES55545C from the National Institute of Environmental and by the European 5th Framework Programme CREATE project (G6RD-CT-2001-00582). Elizabeth Matsui was supported by the National Institute of Allergy and Infectious Diseases grants R01AI070630 and R01AI081845, National Institute of Environmental Health Sciences grants P50ES015903 and P01ES018176 and by the Environmental Protection Agency.
Abbreviations used
- APA
Advanced Protein Assay
- EDQM
European Directorate for the Quality of Medicines
- ELISA
Enzyme-linked immunosorbent assay
- FDA
U.S. Food and Drug Administration
- HPLC
High Performance Liquid Chromatography
- NIBSC
National Institute for Biological Standards and Control
- MARIA
Multiplex array for indoor allergens
- WHO/IUIS
World Health Organization and International Union of Immunological Societies
Footnotes
Conflict of Interest
Martin Chapman is a co-owner and has a financial interest in Indoor Biotechnologies Inc. Fatima Ferreira serves on the Scientific Advisory Board of Indoor Biotechnologies.
References
- 1.Chapman MD, Smith AM, Vailes LD, Arruda LK, Dhanaraj V, Pomés A. Recombinant allergens for diagnosis and therapy of allergic disease. J Allergy Clin Immunol. 2000;106:409–418. doi: 10.1067/mai.2000.109832. [DOI] [PubMed] [Google Scholar]
- 2.Wohrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006;61:633–639. doi: 10.1111/j.1398-9995.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 3.Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–426. doi: 10.1096/fj.01-0711fje. [DOI] [PubMed] [Google Scholar]
- 4.King EM, Vailes LD, Tsay A, Satinover SM, Chapman MD. Simultaneous detection of total and allergen-specific IgE by using purified allergens in a fluorescent multiplex array. J Allergy Clin Immunol. 2007;120:1126–1131. doi: 10.1016/j.jaci.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HS. Allergen immunotherapy: Where is it now? J Allergy Clin Immunol. 2007;119:769–79. doi: 10.1016/j.jaci.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 8.Becker WM, Vogel L, Vieths S. Standardization of allergen extracts for immunotherapy: where do we stand? Curr Opin Allergy Clin Immunol. 2006;6:470–475. doi: 10.1097/01.all.0000246622.34247.21. [DOI] [PubMed] [Google Scholar]
- 9.van Ree R. A new start for allergen references and standardization based on purified/recombinant allergens and monoclonal and monospecific polyclonal antibodies. In: Kurth R, Haustein D, editors. Regulatory Control and Standardization of Allergenic Extracts. GIT VERLAG GMBH; Darmstadt, Germany: 2007. pp. 87–92. [PubMed] [Google Scholar]
- 10.van Ree R. Indoor allergens: relevance of major allergen measurements and standardization. J Allergy Clin Immunol. 2007;119:270–277. doi: 10.1016/j.jaci.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 11.van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–326. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 12.Chapman MD, Ferreira F, Villalba M, Cromwell O, Bryan D, Becker WM, et al. The European Union CREATE Project: A model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008;122:822–829. doi: 10.1016/j.jaci.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Earle CD, King EM, Tsay A, Pittman K, Saric B, Vailes L, et al. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol. 2007;119:428–433. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 15.Arbes SJ, Jr., Cohn RD, Yin M, Muilenberg ML, Burge HA, Friedman W, et al. House dust mite allergen in US beds: results from the First National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2003;111:408–414. doi: 10.1067/mai.2003.16. [DOI] [PubMed] [Google Scholar]
- 16.Arbes SJ, Jr., Cohn RD, Yin M, Muilenberg ML, Friedman W, Zeldin DC. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2004;114:111–117. doi: 10.1016/j.jaci.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Zock JP, Heinrich J, Jarvis D, Verlato G, Norback D, Plana E, et al. Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006;118:682–690. doi: 10.1016/j.jaci.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich J, Bedada GB, Zock JP, Chinn S, Norback D, Olivieri M, et al. Cat allergen level: its determinants and relationship to specific IgE to cat across European centers. J Allergy Clin Immunol. 2006;118:674–681. doi: 10.1016/j.jaci.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Ford AW, Rawle FC, Lind P, Spieksma FT, Lowenstein H, Platts-Mills TA. Standardization of Dermatophagoides pteronyssinus: assessment of potency and allergen content in ten coded extracts. Int Arch Allergy Appl Immunol. 1985;76:58–67. doi: 10.1159/000233662. [DOI] [PubMed] [Google Scholar]
- 20.Larsen JN, Ford A, Gjesing B, Levy D, Petrunov B, Silvestri L, Lowenstein H. The collaborative study of the international standard of dog, Canis domesticus, hair/dander extract. J Allergy Clin Immunol. 1988;82:318–330. doi: 10.1016/0091-6749(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 21.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari E, Tsay A, Eggleston PA, Spisni A, Chapman MD. Environmental detection of mouse allergen by means of immunoassay for recombinant Mus m1. J Allergy Clin Immunol. 2004;114:341–346. doi: 10.1016/j.jaci.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Chapman MD, Aalberse RC, Brown MJ, Platts-Mills TA. Monoclonal antibodies to the major feline allergen Fel d I. II. Single step affinity purification of Fel d I, N-terminal sequence analysis, and development of a sensitive two-site immunoassay to assess Fel d I exposure. J Immunol. 1988;140:812–818. [PubMed] [Google Scholar]
- 24.Lombardero M, Heymann PW, Platts-Mills TA, Fox JW, Chapman MD. Conformational stability of B cell epitopes on group I and group II Dermatophagoides spp. allergens. Effect of thermal and chemical denaturation on the binding of murine IgG and human IgE antibodies. J Immunol. 1990;144:1353–1360. [PubMed] [Google Scholar]
- 25.Arruda LK, Vailes LD, Mann BJ, Shannon J, Fox JW, Vedvick TS, et al. Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g 2. Sequence homology to the aspartic proteases. J Biol Chem. 1995;270:19563–19568. doi: 10.1074/jbc.270.33.19563. [DOI] [PubMed] [Google Scholar]
- 26.de Groot H, Goei KG, Van SP, Aalberse RC. Affinity purification of a major and a minor allergen from dog extract: serologic activity of affinity-purified Can f I and of Can f I-depleted extract. J Allergy Clin Immunol. 1991;87:1056–1065. doi: 10.1016/0091-6749(91)92150-y. [DOI] [PubMed] [Google Scholar]
- 27.Chapman MD, Tsay A, Vailes LD. Home allergen monitoring and control--improving clinical practice and patient benefits. Allergy. 2001;56:604–610. doi: 10.1034/j.1398-9995.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 28.Chapman MD, Pomés A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119:414–420. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Gronlund H, Saarne T, Gafvelin G, van HM. The Major Cat Allergen, Fel d 1, in Diagnosis and Therapy. Int Arch Allergy Immunol. 2009;151:265–274. doi: 10.1159/000250435. [DOI] [PubMed] [Google Scholar]
- 30.Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010;16:321–328. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 31.King EM, Filep S, Smith B, Metwali N, Thorne PS, Versteeg S, et al. Performance Evaluation of Allergen Exposure Assessment using Fluorescent Multiplex Array Technology: - A Multi-Center Ring Trial. J Allergy Clin Immunol. 2009;123(2):S210. [Google Scholar]
- 32.Neske F, Schorner C, Buchheit KH, Costanzo A, Hanschmann KM, Himly M, et al. BSP090--the follow-up to CREATE. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 2009;96:12–19. [PubMed] [Google Scholar]
- 33.Kaul S, May S, Luttkopf D, Vieths S. Regulatory environment for allergen-specific immunotherapy. Allergy. 2011;66:753–764. doi: 10.1111/j.1398-9995.2011.02552.x. [DOI] [PubMed] [Google Scholar]