Abstract
Side chains of Lys/Arg near transmembrane domain (TMD)1–3 membrane-water interfaces can “snorkel” placing their positive charge near negatively-charged phospholipid head groups4–6; however, snorkeling's functional effects are obscure. Integrin β TMDs exhibit such conserved basic amino acids; here we used nuclear magnetic resonance (NMR) spectroscopy7, 8 to show that integrin β3(Lys716) helps determine β3 TMD topography. The αIIbβ3 TMD structure suggests that precise β3 TMD crossing angles enable the assembly of outer and inner membrane “clasps” (OMC and IMC) that hold the αβ TMD together to limit transmembrane signalling9 . Mutation of β3(Lys716) caused dissociation of αIIbβ3 TMDs and integrin activation. To confirm that altered topography of β3(Lys716) mutants activated αIIbβ3, we utilized directed evolution of β3(K716A) to identify substitutions restoring default state. Introduction Pro(711) at the midpoint of β3 TMD (A711P) increased αIIbβ3 TMD association and inactivated integrin αIIbβ3(A711P,K716A). β3(Pro711) introduced a TMD kink of 30 ± 1° precisely at the OMC/IMC border, thereby decoupling the tilt between these segments. Thus, widely-occurring snorkeling residues in TMDs can help maintain TMD topography and membrane-embedding thereby regulating transmembrane signalling.
Integrins are composed of α and β Type I transmembrane subunits10; association of the α and β TMDs regulates bidirectional transmembrane signal transduction11. Most metazoan integrin β subunits contain a positively charged Lys or Arg (Fig. 1a) near the inner TMD boundary that precedes an additional hydrophobic patch, termed the ‘membrane proximal region’12. The membrane proximal region of integrin β3 and, in particular, Lys716, is protected from paramagnetic Mn2+EDDA2−, and is therefore membrane-embedded. This region in other integrin β subunits is also embedded13 ; hence, the membrane proximal domain is the C-terminal limb of a long α-helical TMD that is tilted at an angle of ~25°, thus enabling the ε-amino group of β3(Lys716) to snorkel near phospholipid head groups7.
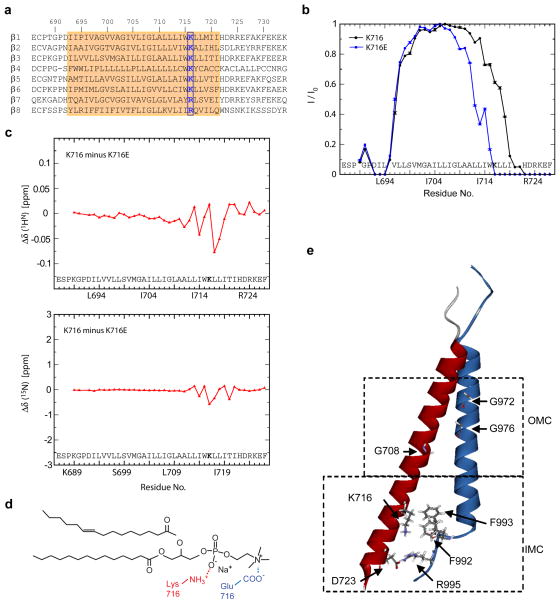
Figure 1. Loss of snorkeling lysine changes lipid embedding of β3 TMD.
a, Sequence alignment of TMD regions of integrin βsubunits indicated with β3 numbering. Transmembrane domains are highlighted in orange. Conserved positive charged amino acids at position 716 (of β3) are bolded and boxed with blue line. b, Mutation of β3(Lys716) changes TMD membrane embedding. The TROSY H-N cross-peak signal intensity of a residue in the presence and absence of 1 mM Mn2+EDDA2− in the aqueous phase, I/I0, was measured to quantitatively express protection from the paramagnetic reagent. Experiments were performed in duplicates using independently prepared samples and quote the standard error between datasets. c, Chemical shift changes of K716E relative to the wild type. d, The predicted interaction of Lys716 side chain ε-NH3+ with a lipid’s PO4− group (red) and the interaction of glutamate’sγ-COO− with a POPS lipid’s amino NH3+ group (blue) are illustrated. e, NMR structure of integrin αIIbβ3 TMD (PBD ID 2K9J). Side chains of residues essential to forming the OMC (Gly972, G976 in αIIb, and Gly708 in β3) and the IMC (Phe992, Phe993, Arg995 in αIIb, Asp723 in β3) are indicated.
To assess the role of β3(Lys716) in TMD topography, we mutated it to a Glu residue and assessed embedding in phospholipid bicelles by measuring protection of the backbone amide protons from the electroneutral paramagnetic Mn2+EDDA2− agent7, 8. Lipid embedding on the extracellular side, defined by the protection pattern of Leu694-Val696, was unchanged in β3(K716E) (Fig. 1b). In contrast, β3(K716E) reduced protection on the intracellular side by approximately five residues, shifting the membrane border from residue 721 to 716 and decreasing membrane crossing angle. The absence of significant 13Cα chemical shift changes between β3 and β3(K716E) indicated no change in secondary structure as a consequence of the mutation (sFig. 1a). At the level of HN shifts, which are sensitive to surrounding chemical environment, relatively small HN chemical changes between β3 and β3(K716E) indicated limited rearrangements in bicelle structure (Fig. 1c), suggesting that Glu716 still localized within the lipid-water interface. In analogy to the K716(ε-NH3+)-lipid(PO4−) snorkeling interaction, glutamate’s γ-COO− group may engage a POPC lipid’s choline N(CH3)3+ group or amino NH3+ group within the lipid head group region (Fig. 1d). β3(K716E) TMD-tail was not aggregated (sFig. 1b), indicating that displacement of negatively charged Glu716 from the hydrophobic core shifted Leu(717)-Ile(721) into a more polar environment. Thus, Lys716 substitutions perturb β3 TMD membrane embedding and crossing angle.
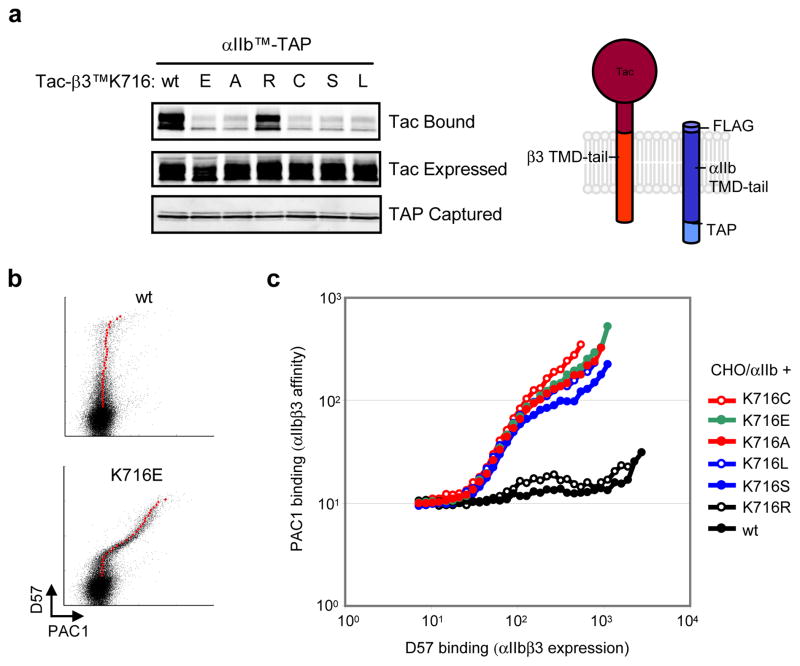
Disruption of the interaction between integrin α and β TMDs leads to allosteric rearrangements that result in increased ligand-binding affinity of the extracellular domain (integrin activation)10, 11, 14 and activation of cytosolic signalling pathways15. A stable αβ TMD association, which is crucial in the regulation of and physiological functions of integrins, requires the simultaneous formation of two discrete assemblies, an inner and outer membrane clasp (IMC and OMC), respectively (Fig. 1e)9. Since the β3 TMD forms a continuous α-helix, its crossing angle appears critical for the simultaneous assembly of these clasps9. To examine the effect of β3(Lys716) mutations on the αIIbβ3 TMD association in mammalian cell membranes, we used a mini-integrin affinity capture assay16 and chemical cross-linking. The latter experiments showed that the αIIbβ3 TMD interaction is primarily a 1:1 heterodimer (sFig.2). For the capture assay, an αIIb mini-integrin bait containing the TMD and cytoplasmic tail of αIIb (Fig. 2a) joined to a C-terminal tandem affinity purification (TAP) tag16 for rapid efficient purification was expressed with preys comprising the extracellular domain of the Tac (IL-2 receptor α) joined to the TMD and tail of β3 or β3 bearing Lys716 substitutions (Fig. 2a). When the cells were lysed and baits were captured using calmodulin beads, we found that the αIIb bait captured the β3 prey, as expected; however, neutral (Ala), polar (Cys, Ser), Acidic (Glu), or hydrophobic (Leu) substitutions at β3(Lys716) blocked the αIIbβ3 TMD association. In contrast, a basic amino acid substitution (Arg) did not disrupt the association consistent with the idea that a snorkeling residue in this position is required to the formation of the αIIbβ3 TMD complex. To examine the potential effects of β3(Lys716) mutations on transmembrane signalling, we assayed their effects on the affinity state of integrin αIIbβ3 by measuring binding to activation-specific αIIbβ3 antibody (PAC1)17 as in Fig. 2b. The results precisely correlated with the effects onαβ TMD interaction; all substitutions with the exception of Arg led to spontaneous integrin activation (Fig. 2c). The importance of this highly conserved Lys seems general because mutation of the paralogous residue in integrin β1A (K732E) activated integrin α5β1 (sFig. 3a–d) and inhibited the association of the β1 TMD with either the α5 or αV TMD (sFig. 3e). Thus, loss of a conserved basic residue in integrin β TMDs leads to disruption of the α-β TMD interaction and spontaneous transmembrane signalling.
Figure 2. Mutations in the snorkeling lysine induce integrin activation and disrupt α- β TMD interaction.
a, Mutations of β3(Lys716) disrupt the αIIbβ3 TMD interaction. CHO cells were co- transfected with αIIb TMD-tail construct fused with C-terminal TAP tag, αIIbTM-TAP, and β3 TMD-tail constructs fused with N-terminal Tac extracellular domain, Tac-β3TM, bearing various mutations at β3(K716) as indicated. αIIbTM-TAP proteins were isolated and associated Tac-β3TM was detected by western blotting (upper panel). Expressed Tac-β3TM proteins (middle panel) and captured αIIbTM-TAP proteins (bottom panel) are also shown. b, β3(K716E) activates integrin αIIbβ3. CHO cells stably expressing integrin αIIb (CHO/αIIb) were transiently transfected with wild type (wt) integrin β3 orβ3(K716E). 18 hour later, surface expression (D57 binding) and affinity of αIIbβ3 (PAC1 binding) were analyzed. Geometric means of PAC1 binding in cells expressing different quantities of αIIbβ3 were plotted as larger red dots. c, Multiple β3(K716) mutations activate integrin αIIbβ3. CHO/αIIb cells were transfected with integrin β3 bearing different mutations in the K716 residue as indicated. The geometric means of PAC1 binding to those CHO/αIIb cells were plotted against D57 binding.
The findings that β3(Lys716) substitutions that prohibit snorkeling alter TMD embedding, that such mutations dissociate the αIIbβ3 TMD complex and activate integrins, and the predicted importance of the crossing angle in maintenance of the two clasps that stabilize the αβ TMD association support the idea that the snorkeling Lys controls transmembrane signalling by specifying the embedding and crossing angle of the β3 TMD. Nevertheless, Rosetta modeling with sparse restraints provided by Cystine cross-linking predicted seven clusters of integrin αIIbβ3 TMD structures18, some of which resembled the calculated NMR structure9. The Rosetta structures suggested that β3(Lys716) can form hydrogen bonds with αIIb backbone carbonyl groups of Phe992 and Phe993, thereby stabilizing the α-β interaction18 and the same paper reported that mutations at β3(Lys716) resulted in integrin activation18, a result we confirmed above. However, NMR-based structural restraints9 preclude β3(Lys716/ε-NH3+)-αIIb(Phe993/CO) interactions (sFig. 4). Furthermore, the embedding of the isolated β3 TMD was similar to that observed in the αIIbβ3 complex9 and, as shown above, loss of the snorkeling β3(Lys716) alters β3 TMD embedding. We therefore sought a positive experimental confirmation that the effects of β3(Lys716) mutations on αIIbβ3 TMD association and on integrin activation could be ascribed to changes in the β3 membrane topography.
If β3(Lys716) mutations changed topography to prevent the simultaneous formation of the IMC and OMC, thereby leading to integrin activation, we reasoned that compensating mutations within the β3 TMD might exist that would correct the crossing angle. Conversely, if β3(Lys716) formed hydrogen bonds with the αIIb, then a compensating mutation might introduce additional basic residues that could form such bonds.
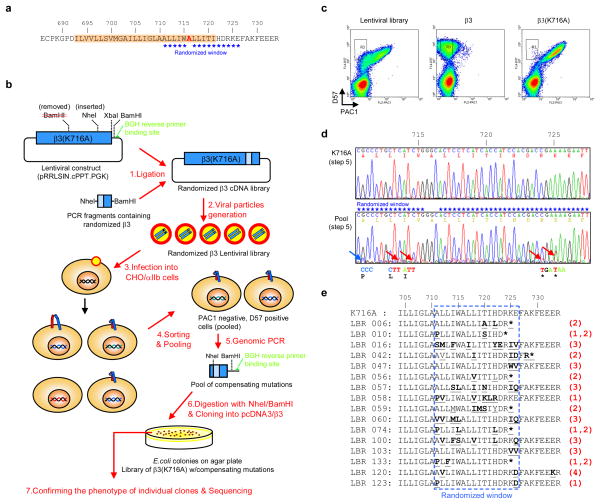
We used random mutagenesis in a window of 5 residues N-terminal and 10 residues C-terminal to the β3(K716) substitution (Fig. 3a and sFig. 6a) to identify mutations that would complement the activating effects. We chose β3(K716A) as the activating mutation; its effects may be less profound than β3(K716E), thus favoring the discovery of weakly compensating mutations. Lentivirus particles carry two genomes 20, making it possible that a single particle might encode two mutants. To test this possibility, we transfected packaging cells with a mixture of lentiviral plasmids encoding integrin β3 and β3(K716A). When CHO cells bearing integrin αIIb were infected with the resulting viruses, we found only populations containing either fully active or fully inactive αIIbβ3, with no intermediate phenotype (sFig. 5). Thus, the packaging cells incorporated two copies of identical genomes into each viral particle. We performed PCR using a primer that was synthesized with 9% contamination of incorrect nucleotides in the randomized windows. The contamination level was predicted by computer simulation to cover most single amino acid changes within the window (sFig. 6b and sTable 1). We ligated the PCR fragments containing random mutations into a lentiviral vector encoding full-length integrin β3, to create a randomized β3 cDNA library (Fig. 3b).
Figure 3. Directed evolution of the β3 integrin to identfy mutations which complement the activating effect of snorkeling lysine mutation.
a, Mutational window. The randomized amino acid sequence in integrin β3(K716A) is indicated by stars. b–e, Strategy and results of random mutagenesis. After removing BamHI and inserting NheI restriction enzyme sites by silent mutations, integrinβ3(K716A) was cloned into lentiviral vector. TMD-tail fragments of integrin β3 containing random mutations in the mutational window were produced by performing PCR with randomized forward primer and reverse primer containing a BamHI linker (sFig. 6). The lentiviral β3(K716A) construct and PCR fragments were digested with NheI and BamHI, and ligated to generate a randomized β3 cDNA library containing 310,000 independent clones. By transfecting the cDNA library into HEK293T cells with packaging vectors, viral particles were generated. CHO/αIIb cells were infected with the lentiviral particles, so that 10 % of cells were infected to ensure that single lentiviral particle can be introduced into each cell (sFig. 5). Cells infected with the lentiviral library, and cells infected with wild type integrin β3 or β3(K716A) lentivirus, were tested for D57 binding and PAC1 binding (c). Among 107 cells screened, ~7,000 cells with PAC1-negative and D57-positive phenotypes (cells in R3 in panel c) were sorted and pooled. Their genomic DNAs were prepared and TMD-tail regions of introduced integrin β3 were amplified by PCR using integrin β3 specific forward primer and BGH reverse primer. The genomic PCR products from cells infected with lentiviral library or integrin β3(K716A) mutant lentivirus were sequenced in bulk (d) to identify predominant mutations in the pool of compensating mutations. The genomic PCR products were ligated into integrin β3 to obtain individual compensating clones. Those individual clones (140 clones) were transiently transfected into CHO/αIIb cells to verify that they exhibited reduced PAC1 binding (sFig. 7), and compensating clones were sequenced. Those clones were grouped according to the major mutation present (e). Clones containing proline at A711 position (group 1), stop mutations at R724 or K725 positions (group 2), neutral residue at E726 position (group 3), and spontaneous mutations that did not fall within the target window (group 4).
CHO/αIIb cells were infected with the mutant β3 lentiviral particles and the infected cells were analyzed for integrin expression (D57 antibody binding) and activation (PAC1 antibody binding) by using flow cytometry (Fig. 3b). In contrast to the β3(K716A)-infected cells, cells infected with the mutant β3 library exhibited a population of cells that expressed inactive αIIbβ3 (R3 region in Fig. 3c). To identify those mutations, we collected ~7,000 cells in the R3 region and purified genomic DNAs from those cells and used PCR to isolate the region of integrin β3 containing the mutagenized window (Fig. 3b). Sequencing of the bulk product revealed that a β3(A711P) mutation and stop codons at residue 724 and 725 were found in the mutagenized window (Fig. 3d). We performed NheI and BamHI digestion to isolate individual fragments containing the mutations and, after ligation into wild type integrin β3, confirmed the compensating effect of the mutations by transient transfection into cells expressing wild-type integrin αIIb and measuring PAC1 binding (sFig. 7). The clones that showed a compensating effect were sequenced and fell into 3 major groups (Fig. 3e): clones containing Pro substitutions at Ala711 position (group 1), stop codons at Arg724 or Lys725 positions (group 2), or a neutral residue substitution at Glu726 position (group 3).
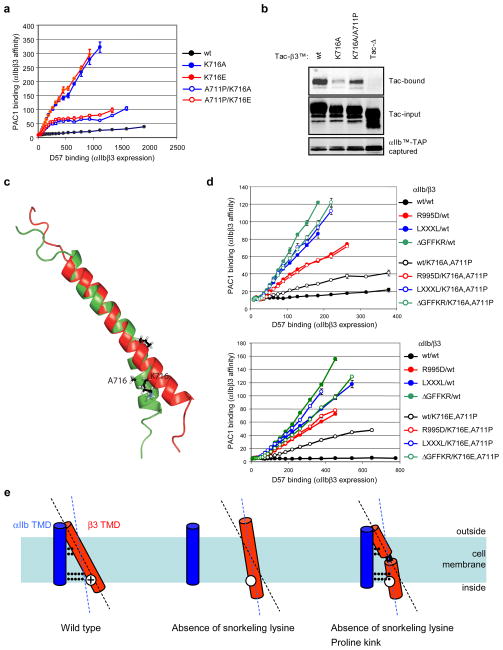
The only compensating mutation consistently observed in the TMD was β3(A711P) (Group 1 in Fig. 3e). This represents an integrin TMD point mutation that inhibits transmembrane signaling. This residue is inaccessible to talin or other cytoplasmic proteins and is unlikely to provide a direct interaction with αIIb since it is not in the α-β TMD binding interface9. We confirmed that β3(A711P) compensates both β3(K716A) and β3(K716E) mutations with respect to integrin activation. (Fig. 4a, sFig. 8b). In addition, β3(A711P) increased interaction of the β3(K716A) TMD with that of αIIb (Fig. 4b). In contrast, the two other groups of compensating mutations were in the β3 cytoplasmic domain. The truncated β3 caused by β3(724X or 725X) (group 2) deletes the talin binding site in the β3 tail21 . Furthermore, β3 Glu726 can contribute to talin binding via electrostatic interactions with Lys317 and Lys364 in talin122. Thus, we suspected that talin binding might amplify the activating effect of β3(K716A); to test this idea we examined its effects in conjunction with β3(Y747A) a mutation that disrupts talin binding15. Indeed the αIIbβ3(K716A,Y747A) mutant was less active than αIIbβ3(K716A) (sFig. 8a). Thus, the only consistent TMD mutation that compensated for β3(Lys716) substitutions was the introduction of a Pro at position 711.
Figure 4. Proline introduced in the TMD forms a flexible kink that stabilizes the αIIbβ3(K716A) TMD interaction and reduces integrin activation.
a, β3(A711P) complements β3(Lys716) mutations in integrin activation. CHO/αIIb cells were transiently transfected with various integrin β3 constructs as indicated, and their binding to PAC1 and D57 was analyzed as described above. Error bars indicate standard errors of the mean (n=3). b, β3(A711P) stabilizes the TMD interactions of αIIbβ3(K716A). CHO cells were co-transfected with αIIbTM-TAP constructs and wild type, or mutant Tac-β3TM constructs as indicated, and the interaction between those integrin TMDs were analyzed as previously reported16. c, Structure of the β3(K716A,A711P) TMD. Structure of the bicelle-embedded integrin β3(A711P,K716A) TMD segment (shown in green, PDB ID 2L91) in comparison to the wild-type β3 TMD structure (shown in red) 7. To illustrate the proline-induced kink, the structures were superimposed on the backbone heavy atom coordinates of Ile693-Leu709. Average structures are shown. d, The inactive state of αIIbβ3(A711P,K716A/E) requires formation of both IMC and OMC. αIIb mutations that disrupt either the OMC (αIIb G972L,G976L) (LXXXL) or IMC (αIIbR995D or αIIb(ΔG991FFKR) reactivate the αIIbβ3(K716A,A711P) integrin (upper panel) or αIIbβ3(K716E,A711P) integrin (lower panel). Error bars indicate standard errors (n=3). e, Mechanism of proline-mediated compensation of snorkeling mutations. Snorkeling of positive charged residue fixes the crossing angle of β3 TMD (left). Loss of snorkeling changes the crossing angle and preventing simultaneous formation of IMC and OMC, thereby disrupting TMD interaction (middle). The Proline-induced kink restores the angle of N-terminal half of the helix (right), enabling reformation of both clasps.
The structure of the β3(A711P,K716A) TMD peptide embedded in phospholipid bicelles was determined. In accordance with the capacity of Pro to introduce kinks in TM helices23, A711P induced a 30 ± 1° kink between preceding and succeeding helical segments (Fig. 4c) caused by the disruption of helix-stabilizing hydrogen bonds between Ile707 and the proline residue and also between Gly707 and Leu712 (sFig. 9). This kink precisely separated the α-helical segments of β3 that constitute the inner and outer clasps. Moreover, A711P introduced a tilt between these elements that is independent of the overall membrane β3 TMD crossing angle. Thus, even if the tilt angle of the inner helix is perturbed, this kink can aid the formation of both inner and outer membrane clasps9 to stabilize the αβ dimer. To verify that both clasps still partake in maintaining the inactive state of the integrin, mutations that disrupted either clasp were examined. The OMC involves packing interactions of αIIb(Gly972 and Gly976) and β3(Gly708)9, 24. αIIb(G972,976A) substitutions that disrupt the OMC overcame the compensating effect of β3A711P in both of K716A and K716E background (Fig. 4d). Similarly, the αIIb(R995D) mutation or αIIb(ΔGFFKR) deletion, which disrupt the IMC, also overcame compensation (Fig. 4d). Thus, the kink introduced by β3(A711P) allows the formation of the two membrane clasps required to stabilize the integrin in the off state (Fig. 4e).
In sum, we demonstrated that the loss of a snorkeling residue in integrin β TMDs can change membrane embedding and, thus, membrane-crossing angle, providing direct evidence that snorkeling can specify the topography of TMDs. Furthermore, we showed that the snorkeling can affect transmembrane signalling by altering the stability of interactions between integrin TMDs. We developed an efficient random lentiviral mutagenesis screening method, which was used to discover that a Pro-induced helix kink led to the stabilization of integrin α and β TMDs interaction by facilitating the formation of the inner and outer membrane clasps. Thus, the long-appreciated snorkeling of basic residues in TMDs1–3 can play an important role in their topography and lateral association and therefore in signal transduction.
Methods summary
NMR spectroscopy
Peptides encompassing human integrin β3(P685-F727), either wild type or K716E mutant, were produced enriched in 15N or 2H/13C/15N isotopes as described previously7. Defined amounts of freeze-dried peptide were reconstituted in 25 mM HEPES•NaOH, pH 7.4, 6 % D2O, 0.02 % w/v NaN3 solution containing 200 mM 1,2-Dihexanoyl-sn-Glycero-3-Phosphocholine (DHPC), 40 mM 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphocholine (POPC) and 20 mM 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-[Phospho-L-Serine] (Avanti Polar Lipids, Inc.). 2D TROSY H-N experiments were recorded in the absence and presence of 1 mM Mn2+EDDA2− using peptide concentrations of 0.2 mM8. K716E assignment was transferred from the wild type using HNCA experiments.
Random β3 library construction
Integrin β3(K716A) cDNA was mutated to remove BamHI site and introduce NheI site with silent mutations. The cDNA was cloned into lentiviral vector which is modified from pRRLSIN.cPPT.PGK-IRES-GFP.WPRE (Addgene) to have BamHI site and BGH reverse primer binding site between PGK promoter and IRES-GFP. The region between NheI and BamHI site were cut out, and replaced with NheI and BamHI restricted mutagenized PCR products by ligation. The mutagenized PCR products were generated using BGH reverse primer and mutagenized forward primer (sFig. 6a). A total ~310,000 E.coli colonies on plates from the ligation reaction were harvested and pooled. Plasmids from those cells were purified to make the mutagenized β3 cDNA library.
Methods
Plasmids, antibody, and cell lines
αIIbTM-TAP, Tac-β3TM constructs16, and lentiviral cloning vector (pRRLSIN.cPPT.PGK-IRES-GFP.WPRE)26 were described previously. Site specific mutagenesis was performed using the Quick change site-directed mutagenesis kit (Stratagen). Anti-Tac N19 (Santa Cruz Biotechnology), anti-Flag M2 (Sigma-aldrich), and anti-HA 16B12 (Covance) antibodies were obtained commercially. Mouse monoclonal antibody specific for the human integrin αIIbβ3 (D57) and activation-specific anti αIIbβ3 antibody (PAC1) have been described previously13. Chinese hamster ovary (CHO) cell were maintained as described previously13. CHO/αIIb cells were prepared by infecting CHO cells with lentiviral particle containing integrin αIIb. Lipofectamine and Lipofectamine Plus reagents (Invitrogen) were used according to the manufacturer’s recommendation for transient transfections.
Affinity capture
αIIbTM-TAP were co-transfected into CHO cells with Tac-β3TM bearing various mutations, and their affinities were analyzed as described previosly16.
Flow cytometry
PAC1 binding assay were performed as described16, 27. In brief, one day after transfection, suspended cells were incubated with D57 in combination with PAC1, followed by staining with allophycocyanin (APC)-conjugated anti-mouse IgG and with R-phycoerythrin (PE)-conjugated anti-mouse IgM. Five min prior to analysis, propidium iodide (PI) was added, and PI-negative cells were analyzed on FACSCalibur (BD Biosciences). The data were imported into MATLAB R2009a software, and the geometric means of PAC1 binding in cells expressing specified levels of αIIbβ3 expression were calculated. Those mean values were indicated in the dot plots as larger red dots, and also used for the line graphs.
NMR spectroscopy
Peptide encompassing human integrin β3(P685-F727), including β3(C687S), was produced enriched in 15N or 2H/13C/15N isotopes as described previously7. K716E substitution was introduced using QuikChange mutagenesis (Stratagene, Inc.) and the peptide was produced analogously to wild-type β3. Defined amounts of freeze-dried peptide was reconstituted in 25 mM HEPES·NaOH, pH 7.4, 6% D2O, 0.02% w/v NaN3 solution containing 200 mM 1,2-Dihexanoyl-sn-Glycero-3-Phosphocholine (DHPC), 40 mM 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphocholine (POPC) and 20 mM 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-[Phospho-L-Serine] (Avanti Polar Lipids, Inc.). 2D TROSY H-N experiments were recorded in the absence and presence of 1 mM Mn2+EDDA2− using peptide concentrations of 0.2 mM8. K716E assignment was transferred from the wild type using HNCA experiments. The structure of β3(A711P, K716A) was determined in 350 mM DHPC, 105 POPC as described previously for the corresponding wild-type peptide7. Structural statistics and spectral quality are summarized in supplemental Tables 2–3 and sFig. 10). All NMR experiments were conducted on a cryoprobe-equipped Bruker Avance 700 spectrometer at 35 °C.
Random β3 library construction
Integrin β3(K716A) cDNA was mutated to remove BamHI site and introduce NheI site with silent mutations. The cDNA was cloned into lentiviral vector which is modified from pRRLSIN.cPPT.PGK- IRES-GFP.WPRE (Addgene) to have BamHI site and BGH reverse primer binding site between PGK promoter and IRES-GFP. The region between NheI and BamHI site (corresponding to the integrin β3 region containing C-terminal TMD and full cytoplasmic tail, sFig. 6a) were cut out, and replaced with NheI and BamHI restricted mutagenized PCR products by ligation. The mutagenized PCR products were generated using BGH reverse primer and mutagenized forward primer (5’-cattgggctagccGCCCTGCTCATCTGGgcaCTCCTCATCACCATCCACGACCGAAAAGAAttcgctaaatttg aggaag-3’, where capital letter represents the position containing 9% of contaminating nucleotide, sFig. 6a). A total ~310,000 E.coli colonies on plates from the ligation reaction were harvested and pooled. Plasmids from those cells were purified to make the mutagenized β3 cDNA library.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health of the USA. TSU acknowledges support from the National Institutes of Health (HL089726) and MHG was supported by HL078784, HL57900, and AR27214. CK is a recipient of postdoctoral fellowship from American Institute for Cancer Research.
Footnotes
Author Contributions: The project was conceived by CK and MHG. All experiments with the exception of the NMR studies were performed by CK. The NMR studies were conducted by TS under the supervision of TSU. EC and FY contributed valuable reagents. MHG and CK wrote the paper which was edited by TS and TSU.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interest.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Killian JA, von Heijne G. How proteins adapt to a membrane-water interface. Trends Biochem Sci. 2000;25:429–34. doi: 10.1016/s0968-0004(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 2.von Heijne G. Membrane proteins: from sequence to structure. Annu Rev Biophys Biomol Struct. 1994;23:167–92. doi: 10.1146/annurev.bb.23.060194.001123. [DOI] [PubMed] [Google Scholar]
- 3.Sipos L, von Heijne G. Predicting the topology of eukaryotic membrane proteins. Eur J Biochem. 1993;213:1333–40. doi: 10.1111/j.1432-1033.1993.tb17885.x. [DOI] [PubMed] [Google Scholar]
- 4.Krishnakumar SS, London E. The control of transmembrane helix transverse position in membranes by hydrophilic residues. J Mol Biol. 2007;374:1251–69. doi: 10.1016/j.jmb.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strandberg E, Killian JA. Snorkeling of lysine side chains in transmembrane helices: how easy can it get? FEBS Lett. 2003;544:69–73. doi: 10.1016/s0014-5793(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 6.Strandberg E, et al. Lipid dependence of membrane anchoring properties and snorkeling behavior of aromatic and charged residues in transmembrane peptides. Biochemistry. 2002;41:7190–8. doi: 10.1021/bi012047i. [DOI] [PubMed] [Google Scholar]
- 7.Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry. 2008;47:4008–16. doi: 10.1021/bi800107a. [DOI] [PubMed] [Google Scholar]
- 8.Lau TL, Dua V, Ulmer TS. Structure of the integrin alphaIIb transmembrane segment. J Biol Chem. 2008;283:16162–8. doi: 10.1074/jbc.M801748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau TL, Kim C, Ginsberg MH, Ulmer TS. The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. Embo J. 2009;28:1351–61. doi: 10.1038/emboj.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 11.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–16. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Stefansson A, Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of N- and C-terminal borders of the transmembrane domain of integrin subunits. J Biol Chem. 2004;279:21200–5. doi: 10.1074/jbc.M400771200. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, et al. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside-in signaling. Blood. 2007;110:2475–83. doi: 10.1182/blood-2007-03-080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C, Lau TL, Ulmer TS, Ginsberg MH. Interactions of platelet integrin alphaIIb and beta3 transmembrane domains in mammalian cell membranes and their role in integrin activation. Blood. 2009;113:4747–53. doi: 10.1182/blood-2008-10-186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–14. [PubMed] [Google Scholar]
- 18.Zhu J, et al. The structure of a receptor with two associating transmembrane domains on the cell surface: integrin alphaIIbbeta3. Mol Cell. 2009;34:234–49. doi: 10.1016/j.molcel.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tugarinov V, Kay LE. Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J Am Chem Soc. 2003;125:13868–78. doi: 10.1021/ja030345s. [DOI] [PubMed] [Google Scholar]
- 20.Frankel AD, Young JA. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Tadokoro S, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 22.Anthis NJ, et al. The structure of an integrin/talin complex reveals the basis of inside- out signal transduction. Embo J. 2009;28:3623–32. doi: 10.1038/emboj.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senes A, Engel DE, DeGrado WF. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr Opin Struct Biol. 2004;14:465–79. doi: 10.1016/j.sbi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Berger BW, et al. Consensus motif for integrin transmembrane helix association. Proc Natl Acad Sci U S A. 2010;107:703–8. doi: 10.1073/pnas.0910873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G- protein-coupled receptors. Nature. 2009;459:356–63. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye F, et al. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188:157–73. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.