Abstract
Oral epithelial cells detect the human pathogenic fungus Candida albicans via NF-κB and a bi-phasic mitogen-activated protein kinase (MAPK) signaling response. However, discrimination between C. albicans yeast and hyphal forms is mediated only by the MAPK pathway, which constitutes activation of the MAPK phosphatase MKP1 and the c-Fos transcription factor and is targeted against the hyphal form. Given that C. albicans is not the only Candida species capable of filamentation or causing mucosal infections, we sought to determine whether this MAPK/MKP1/c-Fos mediated response mechanism was activated by other pathogenic Candida species, including C. dubliniensis, C. tropicalis, C. parapsilosis, C. glabrata and C. krusei. Although all Candida species activated the NF-κB signaling pathway, only C. albicans and C. dubliniensis were capable of inducing MKP1 and c-Fos activation, which directly correlated with hypha formation. However, only C. albicans strongly induced cytokine production (G-CSF, GM-CSF, IL-6 and IL-1α) and cell damage. Candida dubliniensis, C. tropicalis and C. parapsilosis were also capable of inducing IL-1α and this correlated with mild cell damage and was dependent upon fungal burdens. Our data demonstrate that activation of the MAPK/MKP1/c-Fos pathway in oral epithelial cells is specific to C. dubliniensis and C. albicans hyphae.
Keywords: Candida albicans, Candida dubliniensis, Hypha formation, MAPK, MKP1, c-Fos, NF-κB, Oral epithelium, Innate immunity
Introduction
The most significant fungal pathogens of humans are Candida species, which are normal constituents of the oral and gut flora in approximately 50% of the population. However, under suitable predisposing conditions, Candida species cause a variety of mucosal diseases with significant morbidity [1]. Many Candida species are “polymorphic”, capable of growing either as yeasts, pseudohyphae or hyphae; however, only C. albicans and C. dubliniensis are thought to be capable of true hyphal growth. A number of studies have addressed the ability of Candida species to “infect” mucosal tissues and induce effector cytokine responses by using monolayer culture systems or reconstituted human epithelial models. These studies demonstrate that C. albicans is the most “pathogenic” Candida species, as determined by hyphal penetration and induction of epithelial cell damage [2–7] and is the most immunostimulatory [4, 5, 7–10]. In all cases, fungal viability was required to induce epithelial cell damage and effector responses [4, 7, 9, 11]. Candida albicans superior ability to induce damage and effector responses appears to be due to hypha formation, as all non-C. albicans species including C. dubliniensis fail to form hyphae in epithelial cell culture systems [2, 5].
Given the apparent importance of hypha formation in pathogenicity and epithelial activation [12–14], we recently investigated how oral epithelial cells discriminate between the yeast and hypha form of C. albicans [7]. We reported that oral epithelial cells orchestrate innate immunity to C. albicans via NF-κB and a bi-phasic MAPK signaling response. Activation of NF-κB and the first MAPK phase, constituting c-Jun transcription factor activation, is independent of the morphological form and is likely due to the recognition of fungal cell wall polysaccharides (chitin, mannan, β-glucan). Activation of the second MAPK phase, constituting stabilization of the MAPK phosphatase MKP1 and activation of the c-Fos transcription factor, is specifically associated with hypha formation and correlates with proinflammatory responses in a dose-dependent manner. We concluded that this MAPK/MKP1/c-Fos activation system may be part of a “danger response” mechanism that permits epithelial tissues to remain quiescent in the presence of low fungal burdens while responding specifically and strongly to damage-inducing hyphae when burdens increase, and may be critical in identifying when this normally opportunistic fungus has become pathogenic.
However, C. albicans is not the only Candida species capable of filamentation or causing mucosal infections. Therefore, in this study, we sought to determine whether the MAPK/MKP1/c-Fos response mechanism was activated by other Candida species, including C. dubliniensis, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and the non-pathogenic brewer’s yeast Saccharomyces cerevisiae. Of particular interest was C. dubliniensis, which is regarded as the only other Candida species (apart from C. albicans) that forms true hyphae. Thus, a specific aim was to determine whether the MAPK/MKP1/c-Fos pathway was a common pathway activated by both these Candida species or a unique mechanism activated only by C. albicans.
Materials and methods
Epithelial cell lines and fungal strains
Experiments were carried out using the TR146 buccal epithelial carcinoma cell line. Monolayer TR146 cultures were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and experiments carried out in serum-free DMEM. Reconstituted human oral epithelium (oral RHE: 5 days) were purchased from SkinEthic Laboratories (Nice, France) and used as previously described [7, 12]. This model is created from the same cell line (TR146) and constitutes several layers of stratified squamous epithelium permitting the direct analysis of pathogen–epithelial interactions that are not complicated by non-epithelial factors. Rabbit monoclonal antibodies to human phospho-MKP1, phospho-c-Jun, phospho-IκBα, IκBα and c-Fos were purchased from Cell Signaling Technologies (New England BioLabs). Mouse monoclonal antibody to human α-actin was purchased from Chemicon (Millipore) and goat anti-mouse and anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies were purchased from Jackson Immunologicals Ltd (Stratech Scientific). The fungal strains used included C. albicans SC5314 [15], C. dubliniensis (CD36), C. tropicalis (ATCC 750), C. parapsilosis (NCPF 3191), C. glabrata (ATCC 2001), C. krusei (clinical isolate 2,325) and Saccharomyces cerevisiae (NCPF 3139). All strains were grown in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) overnight at 30°C to stationary phase prior to experimentation. Candida dubliniensis was pre-induced to form germ tubes/hyphae in 10% serum/sterile water for 3 h at 37°C [16].
Fungal infection of epithelium and morphological analysis
Candida species and S. cerevisiae were inoculated at 2 × 106 cells (in 50 μl PBS; 4 × 107 cells/ml stock) onto oral RHE, or at 107 cells/ml for signaling assays or between 104–8 cells/ml for cytokine and PCR assays on monolayer epithelial cultures. The multiplicity of infection (MOI) ranged from 0.01 to 100 (fungal cells per epithelial cell) depending on the experiment. Oral RHE and monolayers were incubated at 37°C in 5% CO2 for 30 min, 2 h, 6 h or 24 h as previously described [7, 12, 17]. Noninfected controls contained PBS alone. For morphological analysis, monolayers were fixed in 10% buffered formalin and examined by differential interference contrast (DIC) microscopy at ×400.
Western blotting
Epithelial cells were lyzed using a modified RIPA lysis buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton x-100, 1% Sodium deoxycholate, 0.1% SDS) containing protease (Sigma–Aldrich) and phosphatase inhibitors (Perbio), left on ice for 30 min, and then centrifuged for 10 min in a refrigerated microfuge. Supernatants were assayed for total protein using the BCA protein quantitation kit (Perbio). Twenty micrograms of protein were separated on 12% NuPAGE Bis/Tris minigels (Invitrogen) before transfer to PVDF membranes (GE Healthcare). After probing with primary (1:1,000 dilution) and secondary (1:10,000 dilution) antibodies, membranes were developed using Immobilon chemiluminescent substrate (Millipore) and exposed to ECL film (GE Health-care). α-actin was used a loading control.
Transcription factor DNA binding assay
c-Fos DNA binding activity was assessed using the TransAM transcription factor ELISA system (Active Motif). Briefly, nuclear proteins were isolated from epithelial cells after 3 h infection with Candida using a nuclear protein extraction kit as per manufacturer’s instructions (Active Motif). Protein concentration was determined as above and 5 μg of nuclear extract was assayed in the TransAM system according to the manufacturer’s protocol.
Cytokine determination
Cytokine levels in cell culture supernatants was determined at 24 h using the Fluorokine MAP cytokine multiplex kits (R&D Systems), coupled with the Luminex 100™ machine according to the manufacturer’s protocol. The trimmed median value was used to derive the standard curve and calculate sample concentrations.
Epithelial cell damage assay
Epithelial cell damage was determined at 24 h by measuring lactate dehydrogenase (LDH) activity in the culture supernatant as described previously [7, 12, 17]. This was performed using the Cytox 96 NonRadioactive Cytotoxicity Assay kit (Promega) according to the manufacturer’s protocol and using a recombinant porcine LDH (Sigma–Aldrich) to generate a standard curve. Sample values were then extrapolated from this curve.
RNA isolation and analysis
Total RNA was isolated from TR146 oral epithelial monolayers using the Nucleospin II kit (Macherey–Nagel, Thermoscientific) and treated with Turbo DNase-(Ambion) to remove genomic DNA. cDNA was transcribed from total RNA using 1 U HIV reverse transcriptase (Ambion) and real-time PCR was performed on cDNA using Jump-start SYBR green mastermix (Sigma–Aldrich) on a Rotorgene 6000 (Corbett Research). IL-6 primer sequences were: Forward 5′-AAC CTG AAC CTT CCA AAG ATG G-3′; Reverse 5′-TCT GGC TTG TTC CTC ACT ACT-3′; and cycling conditions were 95°C 5 min; 95°C 3 s, 58°C 10 s, 72°C 10 s for 40 cycles.
Statistics
TransAM and cytokine data were analyzed using a paired t tests. In all cases, P < 0.05 was taken to be significant.
Results
Activation of the NF-κB and MAPK signaling pathways
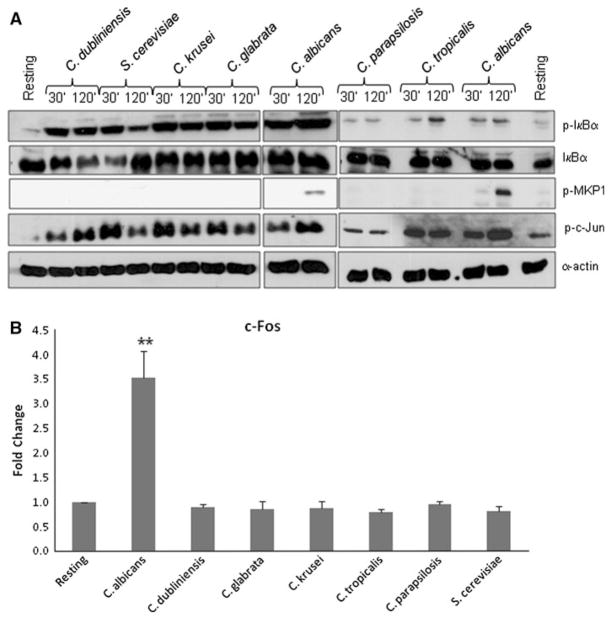
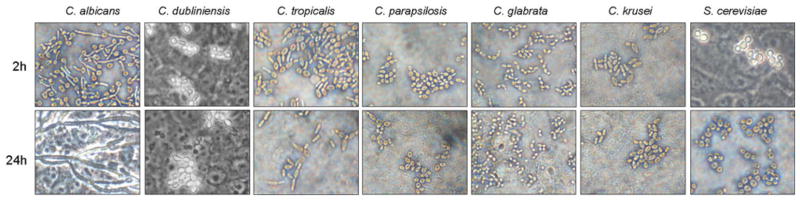
For comparative studies of signal protein activation, 30 min and 2 h were taken as representative time points to delineate the bi-phasic MAPK response against different Candida species and S. cerevisiae. Previously, we demonstrated that IκB-α phosphorylation (a key event in NF-κB pathway activation) and c-Jun transcription factor phosphorylation was activated by C. albicans yeasts and was not associated with the hyphal-mediated response [7]. Western blot analysis of TR146 oral epithelial cells demonstrated that all Candida species and S. cerevisiae induced IκB-α and c-Jun phosphorylation at both 30 min and 2 h postinfection, with little quantitative difference between time points or fungal species (Fig. 1a). The second stage of the bi-phasic MAPK response constitutes MKP1 phosphorylation and c-Fos activation at 2 h and is induced specifically by the hyphal form of C. albicans [7]. Figure 1 shows that C. dubliniensis, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei and S. cerevisiae were all unable to stimulate production of phosphorylated (stabilized) MKP1 or c-Fos at 2 h. Nor were they able to increase c-Fos DNA binding activity at 3 h (Fig. 1b). These findings correlated with the lack of hypha formation under standard assay conditions (Fig. 2).
Fig. 1.
Activation of NF-κB and MAPK signaling by different Candida species and S. cerevisiae. Different Candida species and S. cerevisiae were added to TR146 oral epithelial cells under standard culture conditions for 2 h. a Total protein was isolated and phosphorylation of IκB-α and MKP1 and c-Jun assessed as well as production of c-Fos. b Nuclear extracts were isolated and c-Fos DNA binding activity measured by TransAm ELISA. Bands are shown relative to α-actin loading control. A fungal/epithelial cell MOI of 10:1 was used. Data are representative (assembled from different experiments) (a) or mean (b) of three independent experiments ±SEM. **P < 0.01
Fig. 2.
Morphology of different Candida species and S. cerevisiae. Different Candida species and S. cerevisiae were added to TR146 oral epithelial cells for 2 or 24 h and formalin fixed. Morphology was assessed by DIC microscopy at ×400
C. dubliniensis germ tubes/hyphae can activate MKP1 and c-Fos
Under standard cell culture conditions only C. albicans was able to form hyphae in the presence of epithelial cells and this directly correlated with MKP1 and c-Fos activation (Figs. 1, 2). However, given that C. dubliniensis is able to form hyphae under nutrient poor conditions (and not nutrient rich conditions e.g., culture medium), this raised the possibility that C. dubliniensis may also be able to activate the MKP1/c-Fos response if first pre-induced to form hyphae. Therefore, we incubated C. dubliniensis in sterile water:10% serum for 3 h at 37°C to induce germ tube formation [16] prior to incubation with oral epithelial cells. Under these conditions, C. dubliniensis germ tubes were able to activate both MKP1 and c-Fos to a similar degree as C. albicans after 2 h (Fig. 3; Note: at 30 min, C. albicans is negative as it only starts forming germ tubes between 45 and 60 min). To confirm the transcription factor data, we additionally assessed c-Fos for DNA binding by TransAM ELISA and again demonstrated that C. dubliniensis germ tubes were equally efficient at activating c-Fos as C. albicans germ tubes. As expected, c-Jun phosphorylation was equal between the species and was morphology independent. Interestingly, after 4–6 h incubation, C. dubliniensis germ tubes reverted back to pseudohyphae and yeast cells as a result of nutrient-rich culture conditions (Fig. 4). Given this, we hypothesized that reversion to pseudohyphae/yeast cells may result in dephosphorylation or partial deactivation of MKP1 and c-Fos. Therefore, we analyzed MKP1 and c-Fos activation at 2, 6 and 24 h post-addition of C. albicans and C. dubliniensis, with and without pre-induction of germ tubes. For c-Fos, this was performed by Western blotting in order to directly compare data visually with MKP1. After 2 h, only C. albicans and C. dubliniensis pre-induced hyphal cells activated MKP1 and c-Fos (Fig. 4). By 6 h, C. albicans either markedly increased or sustained MKP1 phosphorylation and c-Fos, respectively, presumably as a result of increased hypha formation. In contrast, C. dubliniensis, which had reverted back to pseudohyphae/yeast cells by 6 h, demonstrated a marked or moderate reduction in MKP1 phosphorylation and c-Fos, respectively. By 24 h, MKP1 phosphorylation and c-Fos was still evident with C. albicans but absent with C. dubliniensis. Candida dubliniensis, when applied as yeast, did not significantly activate MKP1 and c-Fos at any stage.
Fig. 3.
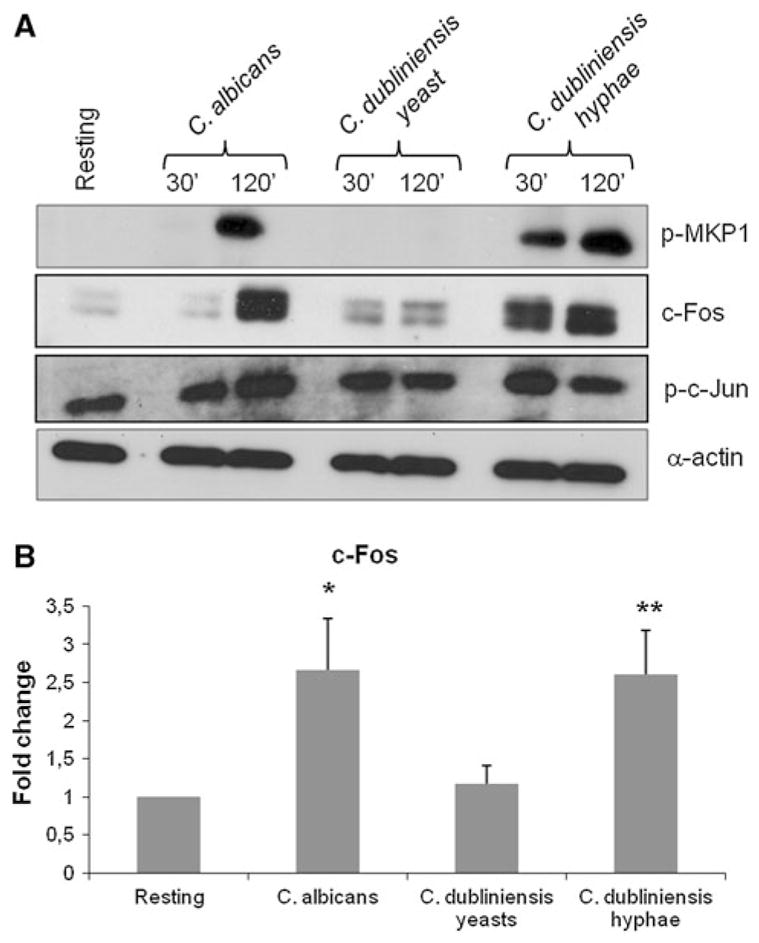
Activation of MKP1 and c-Fos by C. dubliniensis hyphae. Candida dubliniensis was pre-induced to form hyphae in water/10% serum for 3 h at 37°C and added to TR146 cells for 2 h together with control strains (C. dubliniensis and C. albicans in the yeast phase). Total protein and nuclear extracts were isolated and assessed for a MKP1 and c-Jun phosphorylation as well as c-Fos production by Western blot (relative to α-actin loading control) or b c-Fos DNA binding activity by TransAm ELISA. A fungal/epithelial cell MOI of 10:1 was used. Data are representative of three (assembled from different experiments) (a) or the mean of 5 (b) independent experiments (±SEM). *P < 0.05; **P < 0.01
Fig. 4.
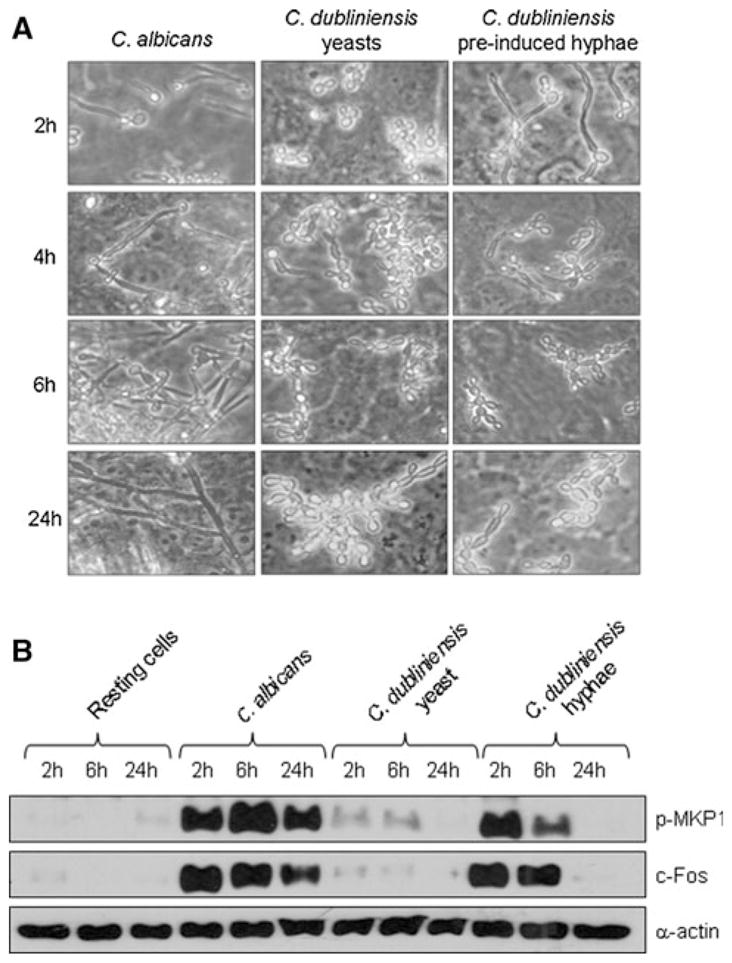
Reduction of MKP1 and c-Fos activation upon reversion of C. dubliniensis hyphae to pseudohyphal/yeast cells. Candida dubliniensis was pre-induced to form hyphae in water/10% serum for 3 h at 37°C and added to TR146 cells. At 2, 6 and 24 h postinfection a the morphology of C. dubliniensis or b MKP1 phosphorylation and c-Fos production was assessed by microscopy or Western blot, respectively. Bands are shown relative to α-actin loading control. A fungal/epithelial cell MOI of 10:1 was used for the 2 and 6 h time points and 0.01 for the 24 h time point. Data are representative of three independent experiments
Cytokine activation and cell damage in organotypic epithelial models
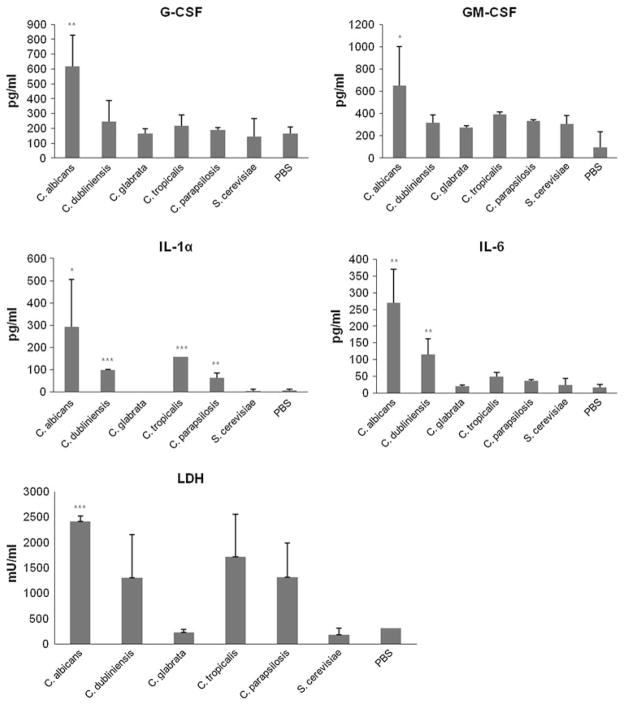
Previously, using oral epithelial TR146 cell monolayers, we found that MKP1 and c-Fos activation correlated with cytokine induction by C. albicans [7]. As only C. albicans formed hyphae under standard assay conditions, we hypothesized that none of the other fungal species would significantly activate cytokine production. Using TR146 organotypic oral RHE models, we confirmed this as only C. albicans was capable of inducing high amounts of G-CSF, GM-CSF or IL-6 (P < 0.05 or < 0.01), although C. dubliniensis was capable of inducing low amounts of IL-6 (Fig. 5). Interestingly, in addition to C. albicans, C. dubliniensis, C. tropicalis and C. parapsilosis were all capable of inducing epithelial damage (Fig. 5) despite not forming hyphae (Fig. 2), and this correlated with an increase in secretion of the damage-associated cytokine IL-1α (P < 0.01; Fig. 5).
Fig. 5.
Cytokine activation and cell damage by different Candida species and S. cerevisiae. Different Candida species and S. cerevisiae were added (2 × 106 cfu/ml) to organotypic oral RHE models for 24 h and the cell culture medium collected and assessed for cytokine proteins by multiplex microbead assay (luminex) or cell damage by LDH (lactate dehydrogenase) release. Data are representative of three independent experiments. *P < 0.05; ***P < 0.001 (compared with PBS control)
Cytokine induction and cell damage by C. dubliniensis germ tubes is dose-dependent
Given that C. dubliniensis germ tubes were able to induce MKP1/c-Fos at 2 h but were unable to induce cytokines by 24 h due to a reversion back to pseudohyphae/yeasts, we explored whether an epithelial cytokine response could be forced by exposing epithelial cells to long hyphae of C. dubliniensis or by increasing the fungal burden. Unfortunately, we found that long hyphae could not be induced in C. dubliniensis, with maximal germ tube length peaking about 3–6 h post-induction in water:10% serum (data not shown). Also, it made no difference whether germ tubes were induced for 3 h or overnight (similar or shorter in length than at 3 h) because all the germ tubes reverted back to pseudohyphae/yeast soon after addition to the epithelial culture system. Thus, the transcriptional program from germ tubes back to pseudohyphae/yeast appears to be initiated very early in C. dubliniensis and so this hypothesis could not be fully tested.
We next hypothesized that since C. dubliniensis germ tube formation is maintained for the first 4–6 h, early induction of cytokine mRNA might be observed even though this does not ultimately result in secretion of a significant amount of cytokine protein at 24 h. This would indicate that the epithelial cells were activated in the early time points when C. dubliniensis germ tubes were present. To test this, we stimulated TR146 monolayer epithelial cells for 6 h with a high dose (108 cells/ml) of pre-induced (3 h) C. dubliniensis germ tubes and looked for IL-6 mRNA transcripts. As hypothesized, pre-induced C. dubliniensis germ tubes strongly increased production of IL-6 mRNA (~30-fold) as compared with C. dubliniensis yeasts (Fig. 6), confirming that C. dubliniensis germ tubes were capable of activating epithelial cells. However, C. albicans increased IL-6 mRNA transcripts to a much higher extent (~500-fold) than C. dubliniensis germ tubes. The difference in IL-6 mRNA induction between C. albicans and C. dubliniensis, together with the reversion of C. dubliniensis germ tubes back to pseudohyphae/yeasts by 4–6 h, probably explain why C. dubliniensis only induced minimal amounts of IL-6 protein at 24 h (Fig. 5).
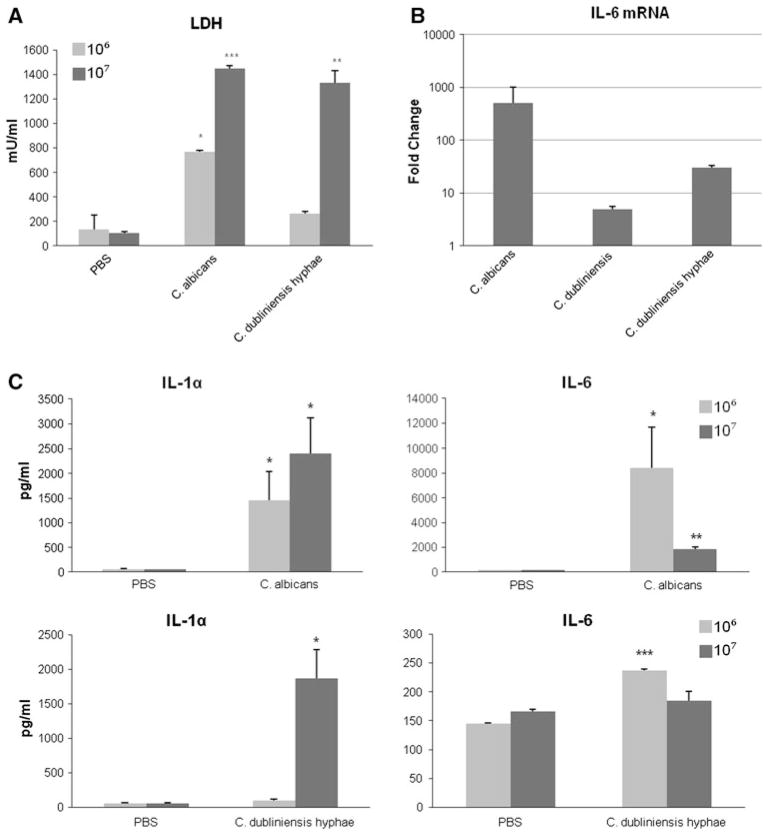
Fig. 6.
Cytokine activation and cell damage is dependent upon fungal burdens. Increasing concentrations of C. albicans and C. dubliniensis were added to TR146 monolayers and incubated at 37°C. a Cell damage was assessed 24 h postinfection with either C. albicans or pre-induced C. dubliniensis germ tubes (3 h in water:10% serum at 37°C) at 106 or 107 cfu/ml (MOI of 1 and 10, respectively). b IL-6 mRNA production was assessed 6 h postinfection with C. albicans, C. dubliniensis and pre-induced C. dubliniensis germ tubes. Results are expressed as fold change in mRNA transcripts relative to the PBS control. c IL-1α and IL-6 protein production in culture supernatants 24 h postinfection with C. albicans or pre-induced C. dubliniensis germ tubes at 106 or 107 cfu/ml (MOI of 1 and 10, respectively). Data are the mean of two experiments. *P < 0.05; ***P < 0.001 (compared with PBS control)
Our previous work indicated that fungal burdens and threshold levels of activation were crucial for the induction of cytokine responses and damage from epithelial cells [7]. With C. albicans, cytokine and damage assays are normally performed with a start dose of 104 cells/ml (MOI of 0.01) as this gives the fungus an opportunity to grow, produce hyphae and induce cytokine responses before the epithelial cells are killed. Candida dubliniensis was unable to induce cytokine production at this MOI due to the reversion of the germ tubes back to pseudohyphae/yeast at 4–6 h post-addition. Therefore, TR146 cells were stimulated with higher doses of 106 or 107 C. dubliniensis germ tubes/ml (MOI of 1 or 10) and cytokine and damage induction was assessed at 24 h. We found that C. dubliniensis germ tubes at 106 cells/ml induced a significant increase in IL-6 protein release, but minimal induction of LDH and the damage-associated cytokine IL-1α (Fig. 6). However, at a dose of 107 germ tubes/ml C. dubliniensis also significantly induced LDH and IL-1α to similar levels as C. albicans. It should be noted that IL-6 release by C. albicans and C. dubliniensis germ tubes at 107 cells/ml was markedly reduced by 24 h because at these high doses significant damage to the epithelial cells occurs early in the assay, thereby preventing de novo synthesis of IL-6 protein as a result of cell death. This does not necessarily apply to IL-1α as significant stores of IL-1α protein are constitutively present in epithelial cells and are released rapidly upon stimulation.
Discussion
C. albicans is regarded as the most pathogenic Candida species and one of its key virulence attributes is hypha formation. Previously, we reported that a bi-phasic innate immune MAPK response constituting activation of MKP1 and c-Fos discriminates between the yeast and hyphal forms of C. albicans in oral epithelial cells [7], which leads to the induction of a proinflammatory cytokine response and ultimately protection against infection [7, 11]. Since C. albicans is not the only Candida species capable of filamentation or causing mucosal infections, we sought to determine whether this MAPK/MKP1/c-Fos mediated mechanism was also activated by other pathogenic Candida species, particularly C. dubliniensis, which is also a hypha-forming species.
Under standard cell culture conditions (DMEM, serum-free) all Candida species and S. cerevisiae were able to induce the NF-κB pathway and the first MAPK phase constituting c-Jun activation, demonstrating that the epithelial cells detect and respond to the presence of fungus. Only C. albicans was able to activate the second MAPK phase constituting MKP1/c-Fos activation and proinflammatory cytokines, and given that C. albicans was the only fungal species able to form hyphae under these culture conditions this supports the conclusion that the MAPK/MKP1/c-Fos response mechanism is hypha associated. However, when pre-induced to form germ tubes prior to addition to epithelial cells, C. dubliniensis was also able to induce MKP1/c-Fos activation, but since C. dubliniensis germ tubes reverted back to the pseudohyphal/yeast form after 4–6 h post-addition to epithelial cells this provided insufficient time for the production and secretion of cytokine proteins. Notably, the reversion of C. dubliniensis germ tubes back to pseudohyphae/yeast correlated with the subsequent deactivation of the MKP1/c-Fos response. The data indicate that sustained epithelial activation and cytokine induction requires the maintenance of Candida hypha formation together with continual activation of the MAPK/c-Fos pathway.
In addition to hypha formation, fungal burdens and threshold levels of activation are also crucial for the induction of cytokine responses and epithelial damage by C. albicans [7]. Given this, we hypothesized that stimulating epithelial cells with higher doses of C. dubliniensis germ tubes may force a cytokine response since the exposure to the “hyphal” form would be greater. Indeed this is what we found, as stimulating epithelial cells with between 106 and 108 C. dubliniensis germ tubes/ml induced transcription of cytokine (IL-6) mRNA and protein, albeit lower than that induced by C. albicans. At the high doses (107 cells/ml), C. dubliniensis germ tubes were also capable of causing epithelial damage and inducing secretion of the damage-associated cytokine IL-1α (see below). The data indicates that C. dubliniensis germ tubes/hyphae have the ability to activate epithelial cells and induce cytokine secretion if present in sufficient quantity and confirms the importance of fungal burdens in mediating mucosal innate responses. It also suggests that C. dubliniensis possesses an ortholog of the C. albicans hyphal factor that activates epithelial cells, but that it may be present in lower quantities (or is modified) and is thus less immunostimulatory. However, we would like to stress that such high burdens of C. dubliniensis are unlikely to be present during infection in vivo. Therefore, since hyphal burdens are key for activation of the MAPK/c-Fos pathway and that C. dubliniensis cannot maintain hyphal formation, this may in part explain why C. dubliniensis is a relatively poor activator of epithelial cells and a weak pathogen of humans.
Apart from C. albicans and C. dubliniensis, C. tropicalis and C. parapsilosis were also able to induce IL-1α and cell damage despite not forming hyphae. Given the established link between IL-1α and cell damage in myeloid cells [18, 19], it is likely that IL-1α production in oral epithelial cells also correlates with cell damage. This is probably induced independently of hypha formation or MKP1/c-Fos activation, with IL-1α production most likely mediated via the NF-κB pathway as previously demonstrated [7]. However, precisely how IL-1α production, cell damage and NF-κB activation are linked in epithelial cells is unclear and requires more detailed investigation. The data also indicate that the mechanisms associated with cell damage are, in part, separate from the mechanisms inducing cytokines since C. tropicalis and C. parapsilosis were unable to induce G-CSF, GM-CSF or IL-6 despite causing mild damage.
Our data are in line with some studies demonstrating minimal or no invasion of oral epithelial cells [2, 3] and lack of cytokine production [5] with non-C. albicans species, but in contrast to others that have demonstrated invasion and/or cytokine production by C. tropicalis, C. parapsilosis or C. glabrata [4, 20–23]. An important and common feature of these latter studies, though, is that invasion and cytokine production by the non-C. albicans species was significantly lower than that induced by C. albicans. Although these non-C. albicans species are capable of causing human mucosal infections, it is unclear whether they induce epithelial responses in vivo. One possibility is that C. tropicalis, C. parapsilosis and C. krusei may form “hyphal-like” structures in vivo to activate epithelial cells. However, it is unlikely that these structures will parallel the true hyphae produced by C. albicans and C. dubliniensis and are thus unlikely to possess the same hyphal moiety that seems to be shared by C. albicans and C. dubliniensis to activate epithelial cells via the MAPK/MKP1/c-Fos pathway. Unfortunately, the morphology of these non-C. albicans species cannot be efficiently manipulated to test this hypothesis using in vitro systems. Another possibility is that other Candida species might not be as metabolically active as C. albicans hyphae, which may lead to minimal epithelial responses. However, this is also unlikely given that heat-killed C. albicans hyphae, which are also metabolically inactive, are able to induce MKP1 and c-Fos activation, albeit to a lesser degree than viable C. albicans hyphae [7]. These arguments, together with the reduction in MKP1 and c-Fos activation that accompanied the loss of hyphal growth of C. dubliniensis and the lack of MKP1 and c-Fos activation by C. albicans strains that are unable to form true hyphae (such as 529L) [7], strongly indicate that epithelial immune activation via the MAPK/MKP1/c-Fos pathway is specifically associated with a hyphal moiety that is shared by C. albicans and C. dubliniensis hyphae. Conceptually, this is important as it means that both the epithelial mechanism and the hyphal moiety could be essential components in Candida pathogenesis and induction of host immunity.
The identity of the shared C. albicans and C. dubliniensis hyphal moiety responsible for epithelial activation via the MAPK/MKP1/c-Fos pathway is unknown, but cell wall associated chitin, mannan or β-glucan are not the activatory factors [7]. The C. dubliniensis genome has recently been sequenced, and comparative analysis demonstrates that it possesses a near identical gene repertoire to C. albicans, with C. dubliniensis possessing only 29 species-specific genes and C. albicans 168 species-specific genes [24]. Notably, C. dubliniensis lacks certain hypha-specific genes of interest, including SAP4/5 and HYR1; therefore, these proteins are unlikely to be the epithelial activating factors. This leaves a host of potential hyphal genes/proteins shared between the two species that could activate the MAPK-based MKP1/c-Fos mediated discriminatory response. Future studies will endeavor to identify this common hyphal component, which appears to be a crucial target of oral epithelial cells and a key activator of mucosal innate immunity.
Acknowledgments
We thank Gary Moran and Stephen Challa-combe for helpful discussions. This work was supported by the NIDCR (DE017514). The authors also acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London. DM is supported by a Wellcome Trust Value In People (VIP) award, CM by a FEMS Advanced Fellowship and AI by a King’s College London Overseas Research Studentship.
References
- 1.Odds FC. Candida and Candidosis. Bailliere Tindall; Philadelphia: 1988. [Google Scholar]
- 2.Jayatilake J, Samaranayake Y, Samaranayake L. A comparative study of candidal invasion in rabbit tongue mucosal explants and reconstituted human oral epithelium. Mycopathologia. 2008;165:373–380. doi: 10.1007/s11046-008-9096-1. [DOI] [PubMed] [Google Scholar]
- 3.Jayatilake JAMS, Samaranayake YH, Cheung LK, Samaranayake LP. Quantitative evaluation of tissue invasion by wild type, hyphal and SAP mutants of Candida albicans, and non-albicans Candida species in reconstituted human oral epithelium. J Oral Pathol Med. 2006;35:484–491. doi: 10.1111/j.1600-0714.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 4.Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118:652–657. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 5.Jayatilake JA, Samaranayake LP, Lu Q, Jin LJ. IL-1alpha, IL-1ra and IL-8 are differentially induced by Candida in experimental oral candidiasis. Oral Dis. 2007;13:426–433. doi: 10.1111/j.1601-0825.2007.01318.x. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Dongari-Bagtzoglou A. Oral epithelium-Candida glabrata interactions in vitro. Oral Microbiol Immunol. 2007;22:182–187. doi: 10.1111/j.1399-302X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaller M, Boeld U, Oberbauer S, Hamm G, Hube B, Korting HC. Polymorphonuclear leukocytes (PMNs) induce protective Th1-type cytokine epithelial responses in an in vitro model of oral candidosis. Microbiology. 2004;150:2807–2813. doi: 10.1099/mic.0.27169-0. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Kashleva H, Dongari-Bagtzoglou A. Cytotoxic and cytokine-inducing properties of Candida glabrata in single and mixed oral infection models. Microb Pathog. 2007;42:138–147. doi: 10.1016/j.micpath.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naglik JR, Moyes D. Epithelial cell innate response to Candida albicans. Adv Dent Res. 2011;23:50–55. doi: 10.1177/0022034511399285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest. 2007;117:3664–3672. doi: 10.1172/JCI28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, Tappuni AR, Rodgers CA, Woodman AJ, Challa-combe SJ, Schaller M, Hube B. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology. 2008;154:3266–3280. doi: 10.1099/mic.0.2008/022293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naglik JR, Fostira F, Ruprai J, Staab JF, Challacombe SJ, Sundstrom P. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J Med Microbiol. 2006;55:1323–1327. doi: 10.1099/jmm.0.46737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007;9:2938–2954. doi: 10.1111/j.1462-5822.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 15.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor L, Caplice N, Coleman DC, Sullivan DJ, Moran GP. Differential filamentation of Candida albicans and Candida dubliniensis is governed by nutrient regulation of UME6 expression. Eukaryot Cell. 2010;9:1383–1397. doi: 10.1128/EC.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaller M, Zakikhany K, Naglik JR, Weindl G, Hube B. Models of oral and vaginal candidiasis based on in vitro reconstituted human epithelia. Nat Protoc. 2006;1:2767–2773. doi: 10.1038/nprot.2006.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 19.Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30:1075–1079. doi: 10.1016/s1357-2725(98)00081-8. [DOI] [PubMed] [Google Scholar]
- 20.Silva S, Hooper SJ, Henriques M, Oliveira R, Azeredo J, Williams DW. The role of secreted aspartyl proteinases in Candida tropicalis invasion and damage of oral mucosa. Clin Microbiol Infect. 2011;17:264–272. doi: 10.1111/j.1469-0691.2010.03248.x. [DOI] [PubMed] [Google Scholar]
- 21.Silva S, Henriques M, Oliveira R, Azeredo J, Malic S, Hooper SJ, Williams DW. Characterization of Candida parapsilosis infection of an in vitro reconstituted human oral epithelium. Eur J Oral Sci. 2009;117:669–675. doi: 10.1111/j.1600-0722.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 22.Gacser A, Schafer W, Nosanchuk JS, Salomon S, Nosanchuk JD. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44:1336–1341. doi: 10.1016/j.fgb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Dongari-Bagtzoglou A. Epithelial GM-CSF induction by Candida glabrata. J Dent Res. 2009;88:746–751. doi: 10.1177/0022034509341266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson AP, Gamble JA, Yeomans T, Moran GP, Saunders D, Harris D, Aslett M, Barrell JF, Butler G, Citiulo F, Coleman DC, De Groot PW, Goodwin TJ, Quail MA, McQuillan J, Munro CA, Pain A, Poulter RT, Rajandream MA, Renauld H, Spiering MJ, Tivey A, Gow NA, Barrell B, Sullivan DJ, Berriman M. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231–2244. doi: 10.1101/gr.097501.109. [DOI] [PMC free article] [PubMed] [Google Scholar]