Abstract
Objective
Assess long-term risk of revision surgery and its predictors after undergoing humeral head replacement (HHR).
Methods
We used prospectively collected data from the Mayo Clinic Total Joint registry and other institutional electronic databases. Revision-free survival for HHR at 5-, 10- and 20-years was calculated using Kaplan-Meier survival analysis. We used univariate and multivariable-adjusted Cox regression analyses to examine the association of age, gender, body mass index (BMI), comorbidity assessed by Deyo-Charlson index, American Society of Anesthesiologist (ASA) class, implant fixation (cemented versus not) and underlying diagnosis with the risk of revision surgery. Hazard ratio with 95% confidence interval (CI) and p-values are presented.
Results
1,359 patients underwent 1,431 shoulder HHRs during the study period, 1976–2008. The average age was 63 years, 63% were female, mean BMI was 28 kg/m2 and 60% implants were cemented. 114 HHRs were revised during the follow-up. At 5-, 10- and 20-years, the shoulder implant survival was 93.6% (95% CI, 92.1%–95%), 90% (95% CI, 88%–92%) and 85% (95% CI, 81.8%–88.4%) respectively. In multivariable-adjusted analyses, older age was associated with lower hazard of revision, hazard ratio, 0.97 (95% CI, 0.96–0.99; p-value<0.001) and higher BMI with higher hazard ratio of 1.04 (95% CI, 1.01–1.08; p-value=0.02).
Conclusions
Long-term survival of HHR at 20-years was excellent. Obesity and younger age are risk factors for higher revision rate after HHR. Further studies should investigate the biologic rationale for these important associations. Surgeons can discuss these differences in revision risk with patients, especially in young obese patients.
Keywords: Shoulder hemiarthroplasty, humeral head replacement, revision, obesity, age
Introduction
Humeral Head Replacement (HHR), also known as shoulder hemiarthroplasty, has been performed with good results for the treatment of patients with a variety of shoulder disorders 4; 8. In a recent systematic review, compared to total shoulder arthroplasty (TSA), patients with HHR had statistically significantly worse functional scores, no significant differences in pain scores, quality of life or adverse events and a non-statistically significant trend towards higher revision rate 11. Two recent systematic reviews, that included studies of both TSA and HHR, have summarized revision rates from 23 studies 9 and 40 studies 14. At mean follow-up of 43 and 59 months, combined revision rates were 10.2% 9 and 15% 14.
Only two studies have estimated the revision rates following HHR. The revision rates were 6–8% at 5-years 5; 13 and 8–17% at 10-years 5; 13. Only one study has reported 15-year revision rate of 27% 13. With low revision rates, a small sample size of 114 shoulders in one of the two previous studies limited the analyses 13.
To our knowledge, only two studies have examined the predictors of revision surgery after HHR 5,13. They included 74 revisions in 1,470 shoulders (6%) 5 and 11 revisions in 114 shoulders (11%) 13. An underlying diagnosis of trauma or fracture sequelae, previous surgery and an age <70 significantly increased the risk of revision 5,13, but gender was not significant 5,13. Only one study used multivariable analyses 5. Some findings were contradictory; for example, younger age was significantly associated with higher revision rate in one study 5, but not the other 13. None of the studies examined modifiable factors, such as comorbidity and body mass index (BMI). Given that higher BMI is associated with more unsatisfactory results in morbidly obese patients undergoing primary shoulder arthroplasty 7, one needs to examine if higher BMI is associated with higher revision rate.
Our objective was to examine the revision rate in a large cohort of patients who had undergone HHR from 1976–2008 at our medical center. We examined: (1) the revision rates at 5-, 10-, 20-year follow-up; and (2) whether higher comorbidity, BMI, age, gender and type of implant) were associated with risk of revision.
Methods
Study Population
The Mayo Clinic Joint registry was used to conduct this study. The study was approved by the Mayo Clinic Institutional Review Board. This prospective registry has captured every arthroplasty performed at the Mayo Clinic, Rochester, Minnesota, USA, since 1969, including all shoulder arthroplasties performed since 1976. The Mayo Clinic medical center provides primary and specialty care to residents of Olmsted county and specialty care to referred patients. All patients who undergo shoulder arthroplasty are invited to return for a clinic visit at 1-, 2- and 5-years and then every 5 years for a standardized physician interview, clinical examination and radiographs. For patients who are unable to come for a clinic visit, patients are requested to complete a standardized shoulder questionnaire 12 and send radiographs. Those who fail to return the questionnaire are contacted by telephone by trained registry staff and interviewed using standardized shoulder questionnaire, including any additional surgery. In case a surgical procedure had been performed at an outside facility, data were requested including operative reports for indication, operative findings and revision. Study cohort was defined as patients who underwent HHR between January 1976 and December 2008.
Outcome and Predictors
The outcome of interest for this study was revision shoulder surgery for index HHR due to any reason, as documented in the Total Joint Registry. Observations were censored for patients at death. We used Total Joint Registry to obtain data on predictors of interest. Patient’s age at surgery, gender, underlying diagnosis and implant fixation (cementing of humeral and/or glenoid component versus not) are documented in total joint registry. Age was treated as a continuous variable. Underlying diagnosis was categorized as osteoarthritis, rheumatoid arthritis, rotator cuff disease, trauma, tumor and other (avascular necrosis, ankylosing spondylitis, psoriatic arthritis, dislocation, etc.). We used other institutional electronic databases to obtain data on BMI, comorbidity and American Society of Anesthesiologist (ASA) class at the time of HHR. BMI (in kg/m2) was considered as a continuous variable. ASA class, a validated measure of perioperative mortality and morbidity 3; 15, was categorized as class 1–2 versus 3–4 (higher class=worse physical status). Comorbidity was measured using Deyo-Charlson comorbidity index 2, a validated commonly used summative weighted scale of 17 comorbidities (including cardiac, pulmonary, renal, hepatic disease, diabetes, cancer, HIV etc.). All variables were available for the entire study duration, except BMI (available since 1987) and ASA class (available since 1988).
Statistical Analyses
We present summary statistics for patient demographic and clinical characteristics as mean (standard deviation) or proportions. Revision-free survival at 5-, 10- and 20-years was calculated by using Kaplan-Meier survival analysis method, censoring patients at death. We examined the association of each predictor of interest (age, gender, BMI, Deyo-Charlson in index, ASA class, implant fixation, underlying diagnosis) and revision using univariate Cox regression analyses. The main multivariable-adjusted Cox regression model (Model 1) included all variables significant at p<0.05 in univariate analyses and available for the entire study duration (all except BMI and ASA class). Model 2 included BMI in addition to model 1, and Model 3 included ASA class in addition to model 1. BMI and ASA class was entered in separate models since they were available since 1987 and 1988, respectively. We present hazard ratio with 95% confidence interval. A p-value <0.05 was considered statistically significant.
Results
Clinical Characteristics
The patient characteristics are summarized in Table 1. 1,359 patients underwent 1,431 shoulder HHRs during the study period, 1976–2008. The average age was 63 years, 63% were female, and mean BMI was 28 kg/m2; 60% implants were cemented. ASA class was 1 or 2 in 49% and 3 or 4 in 51%. Mean cohort follow-up was 7 years (standard deviation, 7 years; range, 1 day to 32 years) with median follow-up of 5 years.
Table 1.
Clinical and Demographic characteristics of study population
| Shoulder Hemiarthroplasty/Humeral Head Replacement (n=1,349 patients with 1,431 shoulders) | |
|---|---|
|
| |
| Mean (SD) or %
|
|
| Age (years) | 63 (16) |
| Male/Female | 497 (37%)/852 (63%) |
| Underlying Diagnosis | |
| Rheumatoid arthritis | 233 (16%) |
| Trauma | 493 (35%) |
| Tumor | 145 (10%) |
| Osteoarthritis | 348 (24%) |
| Rotator Cuff | 137 (10%) |
| Othera | 75 (5%) |
| Body Mass Indexb | 28 (6) |
| ASA classc | |
| 1 or 2 | 489 (49%) |
| 3 or 4 | 501 (51%) |
| Deyo-Charlson index | 1.4 (2) |
| Cement status | |
| Cemented | 857 (60%) |
| Uncemented | 574 (40%) |
Other category for underlying diagnosis includes: avascular necrosis, ankylosing spondylitis, psoriatic arthritis, gout, Charcot arthropathy, dislocation, old injury, prior history of septic arthritis
available from 9/1/1987 to present
available from 11/1/1988 to present; for 9 patients with TSA and 7 patients with hemiarthroplasty, ASA class was missing
Revision Rates
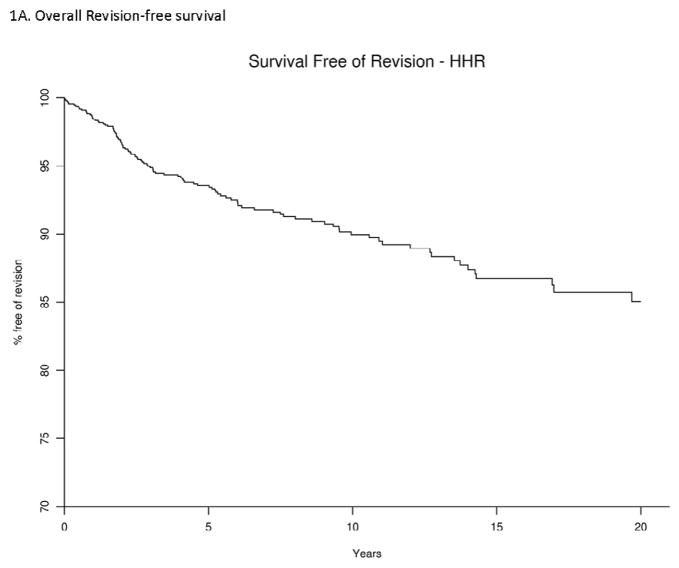
Of the 1,413 shoulders that underwent humeral head replacement, 114 were revised during the follow-up. At 5-, 10- and 20-years, the implant survival was 93.6% (95% CI, 92.1%–95%), 90% (88%–92%) and 85% (81.8%–88.4%), respectively. The K-M curve depicts the overall revision-free survival rate during follow-up (Figure 1A). The causes for revision were as follows: loosening (n=17); instability (n=23); infection (n=9); fracture (n=6); osteolysis or wear (n=5); pain of unknown etiology (n=41); soft tissue problems (n=5); metastatic cancer (n=3); and other causes (n=5).
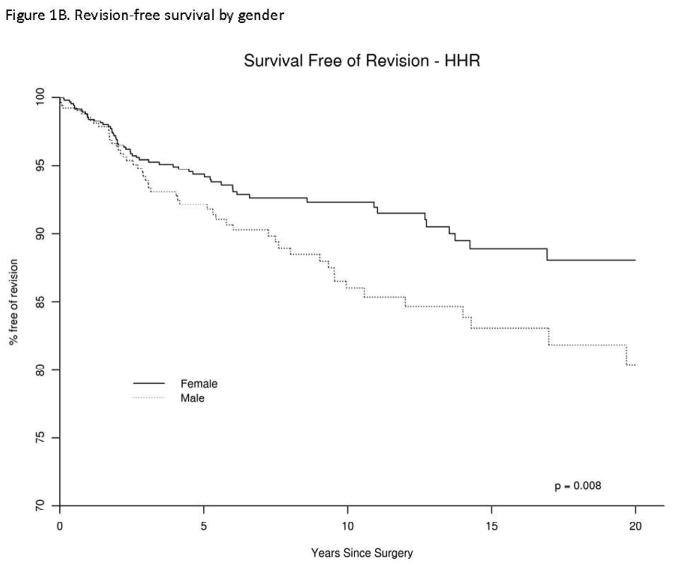
Figure 1. Revision-free survival in patients with Humeral Head Replacement (HHR), overall (1A) and by gender (1B).
Univariate and Multivariable-adjusted predictors of risk of revision
Univariate analyses revealed the male gender, older age, very high BMI, higher ASA class of 3 or 4, were each significantly associated with higher risk of revision (Table 2). Differences in survival by gender are shown in Figure 1B. An underlying diagnosis of tumor was associated with 3-fold higher hazard of revision, compared to rheumatoid arthritis (Table 2). Deyo-Charlson index and cement status were not associated with revision risk.
Table 2.
Univariate association of patient and implant factors with risk of revision after hemiarthroplasty (humeral head replacement)
| Variable | # shoulders | # events | K-M estimates (95% CI) | Cox proportional hazards | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 5 years | 10 years | 20 years | Hazard Ratio (95% CI) | p-value | overall p-value | |||
| Female | 898 | 58 | 94.4 (92.7, 96.1) | 92.3 (90.2, 94.5) | 88.1 (84.5, 91.8) | 1.0 (ref) | ||
| Male | 533 | 56 | 92.1 (89.5, 94.9) | 86.0 (82.1, 90.1) | 80.4 (74.7, 86.5) | 1.65 (1.14, 2.40) | 0.008 | |
| Age at surgery | 0.97 (0.96, 0.98) | <0.001 | ||||||
| Body Mass Index in kg/m2 a | 1.04 (1.01, 1.08) | 0.03 | ||||||
| ASA (1, 2)b | 489 | 36 | 91.7 (88.9, 94.7) | 89.1 (85.5, 92.8) | 85.6 (79.9, 91.8) | 1.0 (ref) | ||
| ASA (3, 4) | 501 | 16 | 96.7 (94.8, 98.7) | 94.6 (91.5, 97.7) | N/A | 0.47 (0.26, 0.84) | 0.01 | |
| Deyo-Charlson index | 1.06 (0.96, 1.17) | 0.22 | ||||||
| No cement | 575 | 45 | 93.0 (90.6, 95.5) | 89.3 (86.0, 92.7) | 84.0 (78.9, 89.4) | 1.0 (ref) | ||
| Cement | 870 | 69 | 93.9 (92.1, 95.8) | 90.4 (87.9, 93.0) | 86.0 (82.2, 90.0) | 0.86 (0.58, 1.26) | 0.43 | |
| Diagnosis | 0.02 | |||||||
| Rheumatoid arthritis | 233 | 15 | 93.9 (90.5, 97.3) | 93.0 (89.4, 96.8) | 91.8 (87.5, 96.3) | 1.0 (ref) | ||
| Trauma | 493 | 44 | 93.7 (91.4, 96.1) | 89.8 (86.6, 93.2) | 84.0 (78.6, 89.8) | 1.51 (0.84, 2.70) | 0.17 | |
| Tumor | 145 | 15 | 84.3 (76.4, 92.9) | 80.9 (71.3, 91.8) | 74.1 (60.0, 91.7) | 3.33 (1.58, 7.02) | 0.002 | |
| Osteoarthritis | 348 | 26 | 94.4 (91.5, 97.3) | 90.3 (85.9, 94.9) | 79.9 (71.5, 89.3) | 1.71 (0.89, 3.27) | 0.11 | |
| Rotator cuff | 137 | 5 | 96.8 (93.3, 100) | 93.6 (88.1, 99.4) | 93.6 (88.1, 99.4) | 0.78 (0.28, 2.15) | 0.63 | |
| Otherc | 75 | 9 | 95.3 (90.2, 100) | 85.5 (75.9, 96.4) | 85.5 (75.9, 96.4) | 1.97 (0.83, 4.63) | 0.12 | |
available from 9/1/1987 to present
available from 11/1/1988 to present; for 9 patients with TSA and 7 patients with hemiarthroplasty, ASA class was missing
Other category for underlying diagnosis includes: avascular necrosis, ankylosing spondylitis, psoriatic arthritis, gout, Charcot arthropathy, dislocation, old injury, prior history of septic arthritis
N/A, not applicable
The multivariable-adjusted model, that adjusted for age, gender and diagnosis, found that only age was significantly associated with revision risk, where as gender and underlying diagnosis were not (Model 1; Table 3). Even though underlying diagnosis was not significantly associated overall with revision risk, a diagnosis of tumor or osteoarthritis were each associated with 2–2.5 fold higher hazard of revision, compared to rheumatoid arthritis. In model that also included BMI (model 2: age, gender, diagnosis, BMI), both BMI and age were significant risk factors for revision risk (Model 2; Table 3). In model that also adjusted for ASA (model 3: age, gender, diagnosis, ASA), age was significantly associated with risk of revision (Model 3; Table 3).
Table 3.
Multivariable-adjusted models for the endpoint of revision following hemi-arthroplasty (Humeral Head Replacement) using Cox proportional Hazards regression analysis
| Model 1 (age, gender, diagnosis) | Model 2 (Model 1 + BMI) | Model 3 (Model 1 + ASA) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value | |
|
| ||||||
| Female | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| Male | 1.19 (0.80, 1.75) | 0.39 | 1.62 (0.96, 2.75) | 0.07 | 1.55 (0.88, 2.73) | 0.13 |
| Age at surgery | 0.97 (0.96, 0.98) | <0.001 | 0.97 (0.96, 0.99) | <0.001 | 0.97 (0.95, 0.99) | 0.002 |
| Diagnosis | 0.07 | 0.05 | 0.22 | |||
| Rheumatoid arthritis | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| Trauma | 1.67 (0.92, 3.03) | 0.09 | 1.47 (0.58, 3.73) | 0.42 | 2.29 (0.75, 7.05) | 0.15 |
| Tumor | 2.53 (1.19, 5.41) | 0.02 | 2.30 (0.79, 6.68) | 0.13 | 3.19 (0.90, 11.37) | 0.07 |
| Osteoarthritis | 2.25 (1.14, 4.45) | 0.02 | 2.02 (0.75, 5.42) | 0.16 | 3.28 (1.00, 10.74) | 0.05 |
| Rotator cuff | 1.37 (0.48, 3.92) | 0.55 | 1.35 (0.40, 4.60) | 0.63 | 2.04 (0.51, 8.24) | 0.32 |
| Other | 1.80 (0.81, 4.00) | 0.15 | 1.71 (0.49, 5.97) | 0.40 | 2.34 (0.58, 9.43) | 0.23 |
| Body Mass Index (BMI) in kg/ma | 1.04 (1.01, 1.08) | 0.02 | ||||
| ASA (1, 2)b | 1.0 (ref) | |||||
| ASA (3, 4) | 0.62 (0.35, 1.13) | 0.12 | ||||
Model 1 adjusted for the three variables significant in univariate models and available for the entire 33-year period, i.e., age, gender, and diagnosis
available from 9/1/1987 to present
available from 11/1/1988 to present; for 9 patients with TSA and 7 patients with hemiarthroplasty, ASA class was missing
Significant hazard ratios and p-values are in bold
Discussion
In this study, that included 1,359 patients with 1,431 shoulder HHRs performed over 33-year period, we found that 85% were revision-free at 20-year follow-up. Although in univariate analyses several variables were associated with higher risk of revision, including male gender, older age, higher BMI, higher ASA class and underlying diagnosis, in multivariable analyses we found only younger age and higher BMI were significantly associated with higher risk of revision. To our knowledge, this is the longest follow-up study to date assessing the risk of revision in patients with shoulder hemi-arthroplasty. Several findings from our study merit further discussion.
We provide 5-, 10- and 20-year revision-free survival for patients who have undergone HHR. These data add significantly to the literature by providing the longest follow-up to date after HHR and allow comparisons to the previously published 15-year revision-free survival rates from the Norwegian register 5 and a small series published 12 years ago 13. Our 5-, 10- and 20-year revision-free survival of 94%, 90% and 85%, are similar to the recently published revision-free rates of 6% at 5-years and 8% at 10-years 5, and lower than those reported in a smaller subsample of the 1976–1985 HHR cohort a decade ago 13. This information fills an important knowledge gap and suggests that revision-free survival may have improved over time. These revision-free survival rates in patients with shoulder hemiarthroplasty are slightly lower than those reported after knee and hip replacement surgeries 1; 10.
An important novel contribution of this study is the identification of higher BMI as a significant risk factor for higher revision rate in patients with HHR. To our knowledge, this has not been reported previously in HHR patients. Obesity or higher BMI is typically thought as a risk factor for lower extremity arthroplasty outcomes due to biomechanical effects on knee and/or hip joints. The higher rate of revision in more obese patients may be due to systemic and/or biomechanical effects of obesity. Obesity is associated with greater production of pro-inflammatory cytokines (interleukin (IL)-6, IL-1, IL-8, IL-18, TNFalpha), adipokines and neuropeptides, such as substance P and nerve growth factor 6. These substances can promote synovial inflammation, cartilage degradation, and bone matrix remodeling, which can lead to more osteolysis and earlier revision. Metabolic syndrome-associated comorbidities, such as diabetes, etc., may also negatively impact implant survival. This finding is very intriguing in conjunction with the recent finding of more unsatisfactory results in morbidly obese patients with BMI >40 undergoing primary shoulder arthroplasty 7 and needs further study. Further studies are needed to explore the biological rationale for this finding. Weight reduction prior to HHR and/or better patient counseling in patients with obesity may be indicated if these findings are confirmed. Studies with adequate sample size are needed; underpowered studies with few revisions and/or short follow-up are unlikely to confirm or refute these findings due to type II error, i.e., missing a real effect due to a small sample size and lack of power.
Our finding of association of younger age with higher risk of revision after HHR, i.e., older age being protective, confirms a similar finding from multivariable-adjusted analyses from the Norwegian registry 5, and extends this finding to the U.S. population undergoing HHR. In addition to the variables included in the earlier Norwegian study (age, gender, underlying diagnosis), we included additional important modifiable factors, such as BMI, Deyo-Charlson comorbidity index, ASA class, and implant fixation (cemented versus not). Thus, our study provides more robust analyses that are less likely to be confounded by unmeasured important factors compared to the earlier study. Several potential reasons may be responsible for higher revision in younger patients, including a higher demands in younger patients leading to higher wear rates, refusal of surgery by older patients with chronically dislocated shoulders and tuberosity fractures due to personal choice, and presence of higher comorbidity in older patients making them higher risk for revision surgeries, which would be otherwise indicated.
Underlying diagnosis had non-significant trend towards association with revision risk overall, after adjustment in multivariable models. In the main model that adjusted for age, gender and diagnosis, the underlying diagnosis was not significantly associated with revision risk overall. However, diagnoses of tumor or osteoarthritis were each associated with significantly higher hazard of revision, compared to rheumatoid arthritis. We noted a similar trend in the model that additionally adjusted for BMI. This indicated that underlying diagnosis may be associated with revision risk; however, this risk is minimal once other factors (age, gender, BMI, etc.) are accounted for.
Our study has several limitations. Due to cohort study design, residual confounding is possible, since we did not adjust for every potential variable. Despite the prospective intensive follow-up in Total Joint Registry, some patients may have had revisions at other medical care centers; therefore, our revision rates are likely conservative estimates. This implies that similar to other prospective studies, our follow-up is not 100%. Future studies in community settings should examine if these results can be replicated. The focus of this study was revision rates and patient factors as risk factors, which limited us from studying other important factors such as implant designs and other important outcomes such as patient satisfaction and function. The strengths of this study include a large sample size, a long follow-up period, availability of data on important potential predictors and robust estimates that varied little with adjustment for potential confounding factors.
Conclusion
In conclusion, this study provides the revision-free survival rate for HHR up to 20-year follow-up. The revision-free survival at 20-year follow-up after HHR was good at 85%. Higher BMI and younger age were significant risk factors for revision surgery, while other patient factors such as gender, comorbidity, ASA class and underlying diagnoses were not associated. Studies are needed to explore the reasons for higher revision rates in obese patients undergoing HHR and to examine the effect of weight reduction program on post-HHR outcomes, including revision rates.
Acknowledgments
Grant support: This material is the result of work supported with National Institute of Health (NIH) Clinical Translational Science Award 1 KL2 RR024151-01 (Mayo Clinic Center for Clinical and Translational Research) and the resources and the use of facilities at the Birmingham VA Medical Center, Alabama, USA.
other contributors: We thank Scott Harmsen, MS and Cathy Schleck, BS for performing statistical analyses and Youlonda Lochler for providing the study cohort.
acknowledgement of funding sources: This material is the result of work supported with National Institute of Health (NIH) Clinical Translational Science Award 1 KL2 RR024151-01 (Mayo Clinic Center for Clinical and Translational Research) and the resources and the use of facilities at the Birmingham VA Medical Center, Alabama, USA.
statement of role of funding source in publication, etc.: The study sponsors had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and, in the decision to submit the manuscript for publication.
Footnotes
Financial Conflict: There are no financial conflicts related directly to this study. J.A.S. has received speaker honoraria from Abbott; research and travel grants from Takeda, Savient, Wyeth and Amgen; and consultant fees from URL pharmaceuticals, Novartis and Savient. J.S. has received royalties from Aircast and Biomet, consultant fees from Tornier and owns stock in Tornier. R.C. has received royalties from Smith and Nephew.
IRB approval: This study was approved by the Mayo Clinic Institutional Review Board and all investigations were conducted in conformity with ethical principles of research.
Author contributions: JAS- Study and protocol design, IRB application, review of data analysis and interpretation, manuscript preparation and revision
JS- Review of study and protocol design, review of data analysis and interpretation, critical revision of manuscript
RC- Review of study and protocol design, review of data analysis and interpretation, critical revision of manuscript
Conflict of Interest
There are no financial conflicts related to this work. J.A.S. has received speaker honoraria from Abbott; research and travel grants from Allergan, Takeda, Savient, Wyeth and Amgen; and consultant fees from Savient, URL pharmaceuticals and Novartis. J.S. has received royalties from Aircast and Biomet, consultant fees from Tornier and owns stock in Tornier. Dr. Cofield has received royalties from Smith and Nephew.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coyte PC, Hawker G, Croxford R, Wright JG. Rates of revision knee replacement in Ontario, Canada. J Bone Joint Surg Am. 1999;81:773–82. doi: 10.2106/00004623-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 3.Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178:261–6. doi: 10.1001/jama.1961.03040420001001. [DOI] [PubMed] [Google Scholar]
- 4.Edwards TB, Kadakia NR, Boulahia A, Kempf JF, Boileau P, Nemoz C, et al. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg. 2003;12:207–13. doi: 10.1016/s1058-2746(02)86804-5. 10.1016/S1058-2746(02)86804-5. [DOI] [PubMed] [Google Scholar]
- 5.Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Risk factors for revision after shoulder arthroplasty: 1,825 shoulder arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop. 2009;80:83–91. doi: 10.1080/17453670902805098. 10.1080/17453670902805098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannone F, Lapadula G. Obesity and inflammation--targets for OA therapy. Curr Drug Targets. 2010;11:586–98. doi: 10.2174/138945010791011857. 10.2174/138945010791011857. [DOI] [PubMed] [Google Scholar]
- 7.Linberg CJ, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in morbidly obese patients. J Shoulder Elbow Surg. 2009;18:903–6. doi: 10.1016/j.jse.2009.02.006. S1058-2746(09)00121-9. 10.1016/j.jse.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Neer CS., 2nd Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56:1–13. [PubMed] [Google Scholar]
- 9.Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2007;16:396–402. doi: 10.1016/j.jse.2006.10.017. 1016/j.jse.2006.10.017 10. [DOI] [PubMed] [Google Scholar]
- 10.Sibanda N, Copley LP, Lewsey JD, Borroff M, Gregg P, MacGregor AJ, et al. Revision rates after primary hip and knee replacement in England between 2003 and 2006. PLoS Med. 2008;5:e179. doi: 10.1371/journal.pmed.0050179. 10.1371/journal.pmed.0050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh JA, Sperling J, Buchbinder R, McMaken K. Surgery for shoulder osteoarthritis. Cochrane Database Syst Rev. 2010;10:CD008089. doi: 10.1002/14651858.CD008089.pub2. 10.1002/14651858.CD008089.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88:508–13. doi: 10.2106/JBJS.E.00132. 10.2106/JBJS.E.00132. [DOI] [PubMed] [Google Scholar]
- 13.Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80:464–73. doi: 10.2106/00004623-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 14.van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35:426–34. doi: 10.1080/03009740600759720. [DOI] [PubMed] [Google Scholar]
- 15.Weaver F, Hynes D, Hopkinson W, Wixson R, Khuri S, Daley J, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18:693–708. doi: 10.1016/s0883-5403(03)00259-6. [DOI] [PubMed] [Google Scholar]