Abstract
Background
Previous studies show that prazosin, an α1-adrenergic receptor antagonist, decreases alcohol drinking in animal models of alcohol use and dependence (Walker et al., 2008, Rasmussen et al., 2009) and in alcohol-dependent men (Simpson et al., 2009). This study extended these findings by using a paradigm that allows for separate assessment of prazosin on motivation to seek versus consume alcohol or sucrose in selectively bred rats.
Methods
Alcohol-preferring P rats were trained to complete an operant response that resulted in access to either 2% sucrose or 10% alcohol. A 4-week Seeking Test Phase examined responding in single, weekly extinction sessions when no reinforcer could be obtained. A 4-week Drinking Test Phase consisted of rats lever-pressing to “pay” a specified amount up front to gain access to unlimited alcohol (or sucrose) for a 20-minute period. On Seeking and Drinking test days, prazosin (0.0, 0.5, 1.0, 1.5 mg/kg) was administered (IP) 30 min prior to behavioral sessions.
Results
Rats were self-administering an average of 0.9 (±0.09) g/kg alcohol on vehicle test day, and had pharmacologically relevant BECs. Prazosin significantly decreased alcohol-seeking at all doses tested. The highest dose of prazosin also increased the latency to first response for alcohol and decreased alcohol intake. While sucrose-seeking and intake were similarly affected by prazosin, the high dose of prazosin did not increase response latency.
Conclusions
These findings are consistent with and extend previous research and suggest that prazosin decreases motivation to initiate and engage in alcohol consumption. The specificity of prazosin in attenuating the initiation of alcohol- but not sucrose-seeking suggests that this effect is not due to prazosin-induced motor-impairment or malaise. Together with previous findings, these data suggest that prazosin may be an effective pharmacotherapy, with specific application in people that drink excessively or have a genetic predisposition to alcohol abuse.
Keywords: Prazosin, Alcohol, P rats, Sipper tube, Noradrenergic
INTRODUCTION
Alcohol is the most commonly used brain depressant affecting as many as 90% of adults in the United States with approximately 30-60% of those individuals having an adverse alcohol-related event at some point in their lives (American Psychiatric Association [APA], 1994). Currently, there are only three major drugs that are FDA approved to treat alcohol abuse (naltrexone, acamprosate, and disulfiram) and all three have limitations with regard to their efficacy and use (Garbutt et al., 1999; Kranzler & Van Kirk, 2001).
Prazosin (“Minipress”) was originally marketed as an antihypertensive drug by Pfizer Pharmaceuticals (Constantine et al, 1973). It is an α1-adrenergic antagonist that reduces central adrenergic activity by blocking noradrenaline binding to postsynaptic receptors, and is unique amongst α1 antagonists in that it is active at central nervous system sites when administered peripherally (Menkes et al., 1981). Since prazosin works as a centrally active α1-adrenergic antagonist, Raskind and colleagues (2003) hypothesized that prazosin should counteract excessive noradrenergic activity that occurs in patents with post-traumatic stress disorder (PTSD) and, therefore, should reduce symptoms of PTSD. Clinical studies provided evidence that prazosin decreased stressful symptoms related to PTSD, specifically distressing nightmares and night awakenings (Raskind et al., 2003). A convergence of factors then led to the examination of prazosin specifically for treatment of alcohol abuse: chronic ethanol exposure and withdrawal in rats caused changes in physiology and behavior that suggested profound alterations in the noradrenergic system related to drinking and relapse (e.g., Rasmussen et al., 2001; 2006), there is a high comorbidity rate of substance abuse and PTSD (Brady et al., 2000 for review), and PTSD patients receiving prazosin reported decreased motivation to drink alcohol (Raskind, unpublished observations). These observations resulted in preclinical studies revealing that prazosin blocks dependence-induced increases in operant responding for alcohol in Wistar rats, with higher doses necessary to be effective in nondependent animals (Walker et al., 2008). In addition, prazosin decreases alcohol drinking in alcohol-preferring P rats in a 2-hour, 2-bottle choice (alcohol versus water) paradigm (Rasmussen et al., 2009). Clinical investigation confirmed that prazosin treatment in non-PTSD alcohol-dependent individuals resulted in fewer drinking days per week and fewer drinks per week during the final 3 weeks of a 6-week study (Simpson et al., 2009).
The alcohol-preferring (P) rat line was developed as a model of excessive voluntary alcohol drinking. P rats consume more than 5 g/kg/day of alcohol and achieve pharmacologically relevant blood ethanol concentrations through voluntary drinking (Li et al., 1987). Additionally, P rats meet all of the criteria for an animal model of alcoholism and exhibit many alcohol drinking patterns seen in humans genetically predisposed toward developing alcoholism such as binge drinking, relapse drinking, and adolescent drinking (for review see Froehlich, 2010). Interestingly, evidence also suggests that animal models genetically bred for high alcohol consumption may exhibit a down-regulation of norepinephrine transporters in the locus coeruleus (Murphy et al., 2002), a brain region involved in the stress response and a major site of CNS norepinephrine synthesis.
Prazosin, the α1-adrenergic receptor antagonist, has been shown to decrease alcohol self-administration in rats in two different behavioral paradigms. The animal models previously utilized focused on either an exclusively consummatory response (i.e., home cage drinking; Rasmussen et al., 2009) or on a combined seeking/drinking response (i.e., lever-press required for access to each 0.1ml of the reinforcer solution; Walker et al., 2008). In the present study, we further examined the effects of acute prazosin administration on separate measures of alcohol-seeking and drinking at binge-like levels, in alcohol-preferring P rats. In this paradigm, the alcohol (or sucrose) seeking response (i.e., lever-presses) is procedurally separated from consummatory responding (i.e., drinking). We have previously shown these measures to be sensitive to drugs that specifically affect reinforcer-seeking versus drinking (Czachowski et al. 2001a; Czachowski et al, 2001b; Czachowski et al., 2002).
MATERIALS and METHODS
Subjects
Fifteen male alcohol-naïve alcohol-preferring (P) rats from the sixty-eighth generation of selective breeding were used in this study and randomly divided into sucrose and alcohol-reinforced groups (n=8 and 7/group, respectively). Body weights ranged from 165 to 220g on arrival and averaged 473g at completion of training immediately prior to treatment. Ad libitum access to food and water was maintained except as noted below. The rats were housed individually on a 12-h light/dark cycle (6:00 A.M. to 6:00 P.M.), and animal care was in accordance with NIH guidelines (Guide for the Care and Use of Laboratory Animals; NIH Guide 1996) and approved by the Institutional Animal Care and Use Committee.
Apparatus
Daily sessions were conducted in modular chambers (Med-Associates; St. Albans, VT, USA; 30 × 30 × 24.5 cm) equipped with a houselight, a retractable lever, and a retractable graduated cylinder tube with rubber stopper and a stainless steel spout with double ball bearings to prevent leakage. The lever was located on the wall opposite to the sipper tube drinking bottle. All chambers were housed in sound-attenuated enclosures equipped with exhaust fans that masked external noise. Electrical inputs and outputs of each chamber were controlled using Med-Associates software (Med-Associates).
Drugs
Alcohol (ethanol) solutions were prepared volume/volume in water using 95% ethanol. For sucrose/alcohol solutions and sucrose solutions, the sucrose solution was prepared weight/volume and used as a solute. Prazosin hydrochloride (Sigma-Aldrich, Inc., St. Louis, MO) was dissolved in sterile water at 2ml/kg BW. Prazosin was injected intraperitoneally (IP) in doses of 0.0, 0.5, 1.0, or 1.5 mg/kg BW, 30 minutes prior to operant sessions. Prazosin was given 30 minutes prior to the start of the operant session based on an onset of action between 5-40 minutes (Menkes et al., 1981).
Training and alcohol initiation
Upon arrival, subjects were weighed and handled for a minimum of 3 days. Daily sessions were conducted 5 days/week at the same time each day during the lights-on cycle. Subjects were initially trained to press the lever on a fixed-ratio (FR) 1 schedule that resulted in 15 sec of access to the sipper tube with 10% sucrose in 30-minute sessions. Subjects were water-restricted for the initial two to five sessions only, after which food and water were available ad libitum in the home cage. Over a 3-week period, the training/sucrose-fading (Samson, 1986) procedures involved the following: increasing the FR from 1-4, decreasing the sucrose concentration to 2% and in the alcohol groups introducing alcohol and increasing the concentration from 2% to 10% while fading the sucrose entirely (final solutions: Sucrose group, 2% sucrose; Alcohol group, 10% ethanol). The procedural separation between seeking (lever pressing) and drinking was then instituted: the FR schedule was discontinued, and following the completion of a single response requirement, access to the sipper tube was provided for 20 uninterrupted minutes. Over two weeks, the response requirement was increased from four to 10, and then the response requirement of 10 was maintained for two additional weeks.
Treatment schedule and test sessions
A four-week Drinking Test phase was then initiated in which all animals received one IP injection of one of four doses of prazosin (0.0, 0.5, 1.0, or 1.5 mg/kg) in a balanced/random design on one day each week (Thursdays; the other 4 days were “normal”, non-injection days with a response requirement of 10). IP injections were given 30 minutes prior to the start of the operant sessions, and the response requirement was reduced to one so that animals were more likely to obtain access to the reinforcer. At the end of the Drinking Test phase, three weeks of no treatment were conducted and the response requirement was gradually increased from ten to twenty.
A four-week Seeking Test phase was then conducted using the same drug treatments as in the Drinking Test. Once a week on Thursdays, each injection preceded a single non-reinforced extinction session. Extinction sessions consisted of 20 minutes of access to the lever, but no reinforcer was provided (bottles were still placed in the retracted holders to control for possible scent cues). Animals were also injected on Tuesdays with vehicle (1ml/kg BW) preceding a reinforced session to control for the possibility that injections would come to predict an extinction session. The other three days of the week were injection-free reinforced sessions with a response requirement of twenty.
Blood ethanol concentration (BEC) determination
Following all prazosin treatments and immediately following the final 20 minutes of alcohol access, blood samples were collected (100 μl) into heparinized capillary tubes from a nick to the tip of the tail while the rats were restrained briefly for a maximum of 2-4 minutes. Samples were stored on ice during collection and then immediately centrifuged, and a 5 μl sample of plasma was analyzed using the AM1 Analyzer (Analox Instruments, Lunenburg, MA, USA). Ethanol concentration was determined with an amperometric oxygen electrode that measures oxygen consumption during the enzymatic oxidation of alcohol to acetaldehyde.
Data analyses and statistics
Total intakes of sucrose and alcohol were determined from the change in fluid volume in the graduated cylinder sipper tube (mls), and g/kg intake was calculated from the intake volume and daily body weight measures. Total lever presses, latency to lever press (sec), as well as cumulative records of responding were recorded for each session. Data were analyzed using two-way within-subject repeated measures analysis of variance (RM ANOVA; reinforcer and dose as the main variables) and post hoc comparisons were performed using Student-Newman-Keuls. In addition, t-tests were used to compare the alcohol and sucrose groups for all responses in the vehicle condition to further assess “baseline” responding. Pearson correlation was used to determine the relationship between alcohol intake (g/kg) and BEC. All analyses were conducted using the SigmaStat 3.5 program (Systat Software, Inc., Chicago, IL) with significance accepted at p < 0.05. Data are presented as mean ± SE.
RESULTS
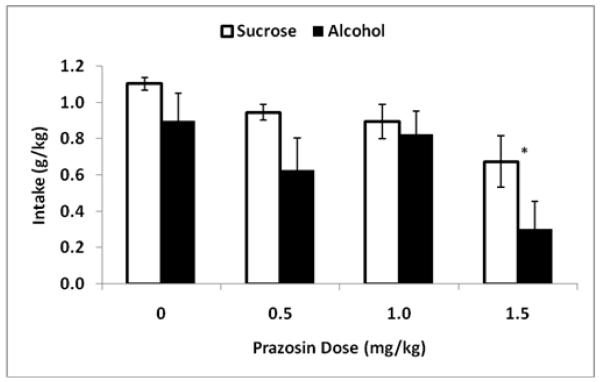
For the Drinking Test Phase only, one rat was dropped from the analyses (alcohol group) for failure to respond on the vehicle injection day (i.e. failure to provide a reliable control response) due to difficulty with the injection procedure (broken toe nail). During the Drinking Test Phase, analysis of reinforcer intake (g/kg) following prazosin treatment (Fig. 1) showed that there was a main effect of dose [F(3,36) = 7.9, p ≤ 0.001] with post hoc analyses indicating that only the high dose of prazosin (1.5 mg/kg) decreased intake relative to all other treatments. There was also a main effect of reinforcer [F(1,36) = 9.9, p ≤ 0.01] with greater intake of sucrose overall, but no interaction between reinforcer and dose. T-test confirmed no difference at vehicle treatment between the alcohol and sucrose groups [t(12) = 1.6, p = 0.13]. Analysis of reinforcer intake volume (mls) revealed the same general pattern of effects. There was greater sucrose intake overall [F(1,36) = 216.2, p ≤ 0.001], with an effect of prazosin treatment [F(3,36) = 4.9, p ≤ 0.01], indicating that only the high dose of prazosin decreased intake relative to vehicle, with no interaction between reinforcer and dose. Volume of intake was the only measure that differed between the groups following vehicle [t(12) = 21.3, p ≤ 0.001], with sucrose intake greater than alcohol intake (mls).
Figure 1.
Mean (±SE) total reinforcer intake (g/kg over 20 min) on Drinking Test days for the sucrose-reinforced (white) and alcohol-reinforced (black) groups over the four prazosin treatment conditions (0, 0.5, 1.0 and 1.5 mg/kg; IP; −30min). Asterisk indicates the main effect of dose; difference from all other doses.
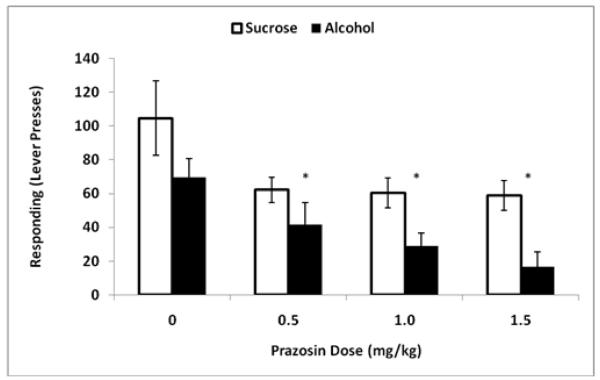
During the Seeking Test Phase, alcohol and sucrose-seeking were assessed using extinction lever-press responding (Fig. 2). Analysis of responding showed that there was a main effect of dose [F(3,39) = 8.3, p ≤ 0.001] with post hoc analyses indicating that all prazosin doses (0.5, 1.0, 1.5 mg/kg) decreased responding relative to vehicle. There was also a main effect of reinforcer [F(1,39) = 12.4, p ≤ 0.01], with greater sucrose lever-press responding overall, but no interaction between reinforcer and dose. There was no difference in responding between the groups at vehicle treatment [t(13) = 1.4, p = 0.17].
Figure 2.
Mean (±SE) reinforcer-seeking (lever-presses in 20 min) on the Seeking Test days for the sucrose-reinforced (white) and alcohol-reinforced (black) groups over the four prazosin treatment conditions (0, 0.5, 1.0 and 1.5 mg/kg; IP; −30min). Asterisks indicate the main effect of dose; difference from vehicle.
In the Seeking Test phase, all subjects made at least one response following vehicle treatment, and t-test confirmed no difference in start latency between the alcohol and sucrose groups [t(13) = 1.8, p = 0.26]. However, administration of the high dose of prazosin resulted in two animals in the alcohol group making zero lever-press responses while all sucrose subjects responded at all doses. Therefore, for an initial analysis of latency to lever press (Table 1), the two subjects in the alcohol group that failed to respond were assigned a latency of 1200 seconds (the session time). This approach yielded a main effect of reinforcer [F(1,39) = 8.2, p ≤ 0.05], with alcohol latencies longer than sucrose latencies, and a main effect of dose [F(3,39)= 15.1, p ≤ 0.01], with the high dose of prazosin differing from all other treatments. The interaction between dose and reinforcer [F(3,39) = 4.6, p ≤ 0.01] indicated that there were no effects of prazosin on latency to lever-press in sucrose-reinforced animals. Within the alcohol group, the high dose of prazosin produced longer start latencies as compared to all other treatments within that group, and there were longer latencies within the high dose for alcohol vs. sucrose reinforcement with no differences between alcohol and sucrose at the other doses or following vehicle treatment. Alternatively, when the two subjects in the alcohol group that failed to respond on the lever were excluded from the analyses (rather than assigning the 1200 value), the pattern of results was identical (reinforcer: [F(1,39) = 35.1, p ≤ 0.01]; dose: [F(3,39) = 4.5, p ≤ 0.01]; interaction: [F(3,39 = 3.1, p ≤ 0.05]), again indicating that the high dose of prazosin specifically increased latency to lever-press in the alcohol group only. Finally, since the latency data were skewed, nonparametric analyses using Mann-Whitney Rank Sum test at each dose confirmed that latencies were greater in the alcohol group than the sucrose group at the high dose; U = 2.0, p ≤ 0.01 and at the middle dose; U = 10.0, p ≤ 0.05, with no differences following the low dose of prazosin or vehicle.
Table 1.
Latency to lever-press by treatment.
Mean (±SE) latency to first lever press (seconds) on the Seeking Test days for the sucrose- and alcohol-reinforced groups over the four prazosin treatment conditions (0, 0.5, 1.0 and 1.5 mg/kg; IP; −30min). At the high dose, two animals in the alcohol group failed to respond and were therefore either assigned a latency of 1200 seconds (the total session time) or were excluded from the analyses. All subjects in the sucrose group responded at all doses. Asterisks indicate significant difference from all other conditions within the alcohol group and from the sucrose group, by both parametric and nonparametric analyses. # indicates significant difference between alcohol and sucrose groups at this dose (Mann-Whitney U).
| Latency to Lever Press (sec) | ||||
|---|---|---|---|---|
| Sucrose | Alcohol | |||
| Prazosin Dose (mg/kg) | Mean | ± SE | Mean | ± SE |
| 0 | 13.53 | 6.84 | 28.77 | 12.66 |
| 0.5 | 17.05 | 2.94 | 51.33 | 14.73 |
| 1 | 23.09 | 9.37 | 65.77 # | 13.7 |
| 1.5 (all subjects – 1200 for no response) |
27.23 | 8.23 | 457.53 * | 212.94 |
| 1.5 (only subjects that responded) |
160.54 * | 66.67 | ||
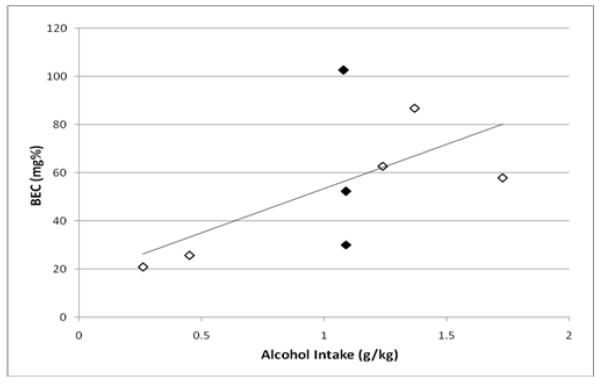
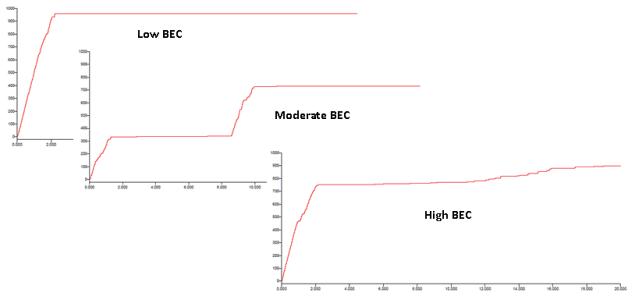
On the final day of operant sessions, the average alcohol intake was 1.04 g/kg (±0.07) which yielded BECs of 54.9 (±10.3), as measured at the end of the 20 minute self-administration period. Interestingly, there was a moderate but non-significant correlation between the two measures (r2 = 0.60, p = 0.12) (Fig 3a). To clarify the somewhat disparate relationship between alcohol intake and BEC, the drinking patterns over the 20-minute access were further examined for the three subjects (Fig 3b) with identical g/kg intake but disparate BECs. These data indicated that the subject with the low BEC consumed all 1.09 g/kg alcohol during the first two minutes of the alcohol access period (959 total licks), and therefore this rat’s blood sample was collected at 18 minutes post-consumption. The subject with the moderate BEC engaged in two large drinking bouts; the first during the first 1.5 minutes of access and the second about eight minutes later, with all drinking terminating by minute ten (total of 728 licks) which was ten minutes prior to the sample collection. Finally, the subject with the high BEC also consumed the majority of the total in the first two minutes (~750 out of a total of 896 licks), but then continued a slow and steady pattern of licking through the final minutes of the session, and therefore this rat’s sample collection was coincident with the end of self-administration. Therefore, the patterns of intake and lack of correlation between intake and BEC suggest that the actual peak BECs for many subjects were likely higher than those measured at the 20-minute timepoint.
Figure 3a.
Blood ethanol concentration (mg%) as a function of alcohol intake (g/kg) assessed from the final operant session following completion of all prazosin treatments. Blood samples were collected immediately at the end of the 20 minutes of sipper tube access. The filled symbols represent three subjects with identical alcohol intake but disparate BEC measurements.
Figure 3b.
The cumulative records of lick responding over the 20 minutes of sipper tube access for the three individual rats highlighted in Figure 3a that consumed the same amount of total alcohol (g/kg). The subject with the lowest BEC consumed the entire 1.09 g/kg in the first two minutes of the session, the subject with a moderate BEC exhibited two separate binges and stopped licking by ten minutes into the session, and the subject with the high BEC had one large binge in the first two minutes of access but continued to lick through the final minute of the session.
DISCUSSION
The purpose of the present study was to evaluate the role of the α1-adrenergic receptor antagonist, prazosin, on distinct alcohol-seeking and drinking behaviors in P rats consuming binge-like amounts of alcohol. At all doses tested (0.5-1.5 mg/kg), prazosin decreased appetitive (seeking) responding, and the high dose decreased alcohol drinking. These findings are consistent with and extend those of Walker et al. (2008) where a high dose of prazosin (2.0 mg/kg) was necessary to observe decreases in a combined seeking/drinking response (i.e., fixed ratio responding for “sips” of alcohol) in nondependent, nonselected Wistar rats. Notably, the nondependent rats in that study had relatively low baseline responding and intake. In alcohol-dependent Wistar rats in the same experiment, baseline responding was twice as high as in nondependent animals and prazosin decreased responding/intake at a lower dose (1.5 mg/kg), which was the same dose as was required to decrease drinking in the present study. An examination of a purely consummatory response (i.e., free-access drinking) in P rats showed that acute injection of prazosin at moderate doses (1.0-2.0mg/kg) decreased alcohol intake from a baseline of approximately 2 g/kg/hour (Rasmussen et al., 2009), with the 0.5 dose becoming effective only after three daily treatments. The present findings, therefore, clarify that a purely appetitive response is more sensitive to prazosin than either a pure consummatory response or a mixed appetitive/consummatory response and also further suggest that either a history of alcohol dependence or a genetic background predisposing alcohol preference may increase susceptibility to prazosin’s suppressive effect.
In general, prazosin decreased sucrose-seeking and drinking responses at the same doses that decreased alcohol-reinforced responding. However, there were some indications that the effects of prazosin were selective for alcohol reinforcement. The highest dose of prazosin decreased alcohol-seeking and drinking by 76% and 67%, respectively, while decreasing sucrose-seeking and drinking by only 44% and 39%, respectively. Moreover, the highest dose of prazosin also increased the latency to initiate a seeking response in the alcohol-reinforced group and resulted in subjects failing to emit any seeking response, which was not observed in the sucrose group. The fact that sucrose-seeking behaviors were unaffected by the high dose of prazosin suggests that the effects seen in the alcohol group were not attributable to prazosin-induced motor-impairment or malaise. Generalized effects of prazosin on arousal cannot be completely ruled out, however the specificity of prazosin’s effects on alcohol versus sucrose response initiation do not support this mechanism of action. It should also be noted that these seeking response measures were taken during extinction sessions, when no alcohol was available, and therefore the effects of prazosin on ethanol seeking were not due to an interaction between alcohol and prazosin. However, since these animals had been drinking pharmacologically relevant amounts of alcohol five days per week for 15 weeks at the start of the Seeking Phase testing, these findings may suggest that prazosin is preferentially effective as a result of some neuroadaptation following chronic binge-like alcohol intake.
The present study suggests that the noradrenergic system plays a key role in maintaining alcohol seeking and drinking in animals selectively bred for alcohol preference. Similarly, noradrenergic signaling is important for the maintenance of the reinforcing properties of nicotine and heroin and prazosin has been shown to affect nicotine and heroin self-administration (Greenwell et al., 2009; Forget et al., 2010; Bruijnzeel et al., 2010). Prazosin dose-dependently decreased the self-administration of nicotine, as well as reinstatement of extinguished nicotine seeking induced by a nicotine prime or nicotine cues, in Long Evans rats (Forget et al., 2010). Prazosin also dose-dependently decreased the elevations in brain reward thresholds associated with nicotine withdrawal in Wistar rats (Bruijnzeel et al., 2010) and dose-dependently reduced IV heroin self-administration in Wistar rats (Greenwell et al., 2009). With regard to the present findings, depletion of brain levels of norepinephrine results in a decrease of alcohol self-administration in Sprague-Dawley rats (Davis et al., 1978), consistent with the central actions of prazosin. Finally and most recently, prazosin has been found to block yohimbine (a prototypical α2-adrenergic antagonist)-induced reinstatement of food and alcohol-seeking as well as footshock-induced reinstatement of alcohol-seeking in rats (Le et al., 2011).
In summary, acute administration of prazosin, an α1-adrenergic receptor antagonist, was able to attenuate reinforcer-seeking and drinking and decreased the motivation to initiate alcohol seeking in a binge-drinking model. The operant paradigm utilized, that procedurally separates seeking from drinking responses, revealed that prazosin decreased reinforcer seeking at lower doses than were required to affect consumption. The results suggest that prazosin may be an effective pharmacotherapeutic agent for treating alcohol use disorders, and possibly a treatment that targets the motivation to initiate episodes of excessive alcohol consumption.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism R01AA018604 (JCF & DDR); P60AA007611 (JCF & CLC); R01AA016101 (CLC); Dept of Veterans Affairs and P20AA017839 (DDR).
REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 7):22–32. [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KFM, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology. 2010;212(4):485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine JW, McShane WK, Scriabine A, Hess HJ. Analysis of the hypotensive action of prazosin. Postgrad Med. 1975:18–35. Spec No. [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcoholism-Clinical and Experimental Research. 2001a;25(10):1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, DeLory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology. 2009;204(2):335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Effects of acamprosate on ethanol-seeking and self-administration in the rat. Alcoholism-Clinical and Experimental Research. 2001b;25(3):344–350. [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28(1):39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- David WM, Smith SG, Werner TE. Noradrenergic role in the self-administration of ethanol. Pharmacol Biochem Behav. 1978;9:369–374. doi: 10.1016/0091-3057(78)90298-8. [DOI] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha(1) Receptors as a Novel Target for the Treatment of Nicotine Addiction. Neuropsychopharmacology. 2010;35(8):1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC. What aspects of human alcohol use disorders can be modeled using selectively bred rat lines? Subst Use Misuse. 2010;45(11):1727–41. doi: 10.3109/10826084.2010.482424. [DOI] [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. Journal of Substance Abuse Treatment. 2009;36(1):S15–S23. [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha(1) adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacology Biochemistry and Behavior. 2009;91(3):295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: A meta-analysis. Alcoholism-Clinical and Experimental Research. 2001;25(9):1335–1341. [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011 Feb; doi: 10.1007/s00213-011-2178-7. e-pub ahead of print; DOI 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol. 1987;(Suppl 1):91–6. [PubMed] [Google Scholar]
- Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by alpha-1-adrenoceptors in brain. Naunyn-Schmiedebergs Archives of Pharmacology. 1981;317(3):273–275. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior Genetics. 2002;32(5):363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. American Journal of Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha(1)-Adrenergic Receptor Antagonist, Prazosin, Reduces Alcohol Drinking in Alcohol-Preferring (P) Rats. Alcoholism-Clinical and Experimental Research. 2009;33(2):264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25(7):999–1005. [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38(3):173–7. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food-sated and water-sated rats. Alcoholism-Clinical and Experimental Research. 1986;10(4):436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: Rodent models. International Review of Neurobiology. 2003;54(54):109–145. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A Pilot Trial of the Alpha-1 Adrenergic Antagonist, Prazosin, for Alcohol Dependence. Alcoholism-Clinical and Experimental Research. 2009;33(2):255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha(1)-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42(2):91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]