Abstract
AIM: To assess human epidermal growth factor receptor-2 (HER2)-status in gastric cancer and matched lymph node metastases by immunohistochemistry (IHC) and chromogenic in situ hybridization (CISH).
METHODS: 120 cases of primary gastric carcinomas and 45 matched lymph node metastases from patients with full clinicopathological features were mounted onto multiple-punch and single-punch tissue microarrays, respectively, and examined for HER2 overexpression and gene amplification by IHC and CISH.
RESULTS: Twenty-four tumors (20%) expressed HER2 immunohistochemically. An IHC score of ≥ 2+ was observed in 20 tumors (16.6%). HER2 amplification was detected by CISH in 19 tumors (15.8%) and in their matched lymph node metastases. A high concordance rate was found between HER2 positivity (as detected by IHC) and HER2 gene amplification (as detected by CISH), since 19 of the 20 IHC positive cases were amplified (95%). All amplified cases had 2+ or 3+ IHC results. Amplification was associated with intestinal phenotype (P < 0.05). No association with grading, staging or survival was found.
CONCLUSION: In gastric cancer, HER2 amplification is the main mechanism for HER2 protein overexpression and is preserved in lymph node metastases.
Keywords: Human epidermal growth factor receptor-2, Immunohistochemistry, Chromogenic in situ hybridization, Gastric cancer
INTRODUCTION
Alterations of the human epidermal growth factor receptor-2 (HER2) gene are implicated in the development and progression of many tumors[1-4]. In breast cancer, HER2 amplification has been found in about 20% of cases and was linked to poor prognosis[5,6]. Breast cancer patients with HER2 amplification have been effectively treated with the monoclonal antibody trastuzumab, a HER2 inhibitor[7-10]. Recently, a number of studies have suggested a response to trastuzumab therapy for other cancers with HER2 amplification, including germ cell, endometrium and salivary duct carcinoma[11-13].
In gastric cancer, HER2 amplification has been found in 7% to 27% of tumors[14-19]. Reports of trastuzumab therapy in metastatic gastric cancer showed complete tumor regression and disappearance of the metastases in two cases[20,21]. A phase III randomized study (Trastuzumab for HER2-positive metastatic gastric cancer) in patients with inoperable, metastasizing and/or recurring gastric cancer with HER2 overexpression or HER2 gene amplification, documented that 47.3% of the patients who received trastuzumab, along with their chemotherapy, showed a significant regression of the primary tumor and/or the metastases. Moreover, trastuzumab caused a prolongation of the median survival time by 2.4 mo in all patients[22]. Based on these reports, gastric cancer patients with HER2 overexpression and/or amplification could be good candidates for trastuzumab therapy.
HER2 testing can be performed either by immunohistochemical evaluation of protein expression or by evaluating the gene copy number by in situ hybridization, most commonly using fluorescence in situ hybridization (FISH). However, while immunohistochemistry (IHC) is a relatively inexpensive, easy to perform method for most pathology laboratories, FISH is technically demanding, expensive and requires special equipment[23-25]. An alternative method, chromogenic in situ hybridization (CISH), is a combination of in situ hybridization with a detection system using a chromogen similar to IHC. Slides are visible under a light microscope and show correlation with morphology. A number of studies compared HER2 testing with IHC, FISH and CISH in breast carcinoma and have shown good correlation between CISH and FISH results[25-30].
We evaluated HER2 overexpression and gene amplification by IHC and CISH, respectively, in 120 cases of gastric carcinoma patients and 45 matched lymph node metastases mounted onto multiple-punch and single-punch tissue microarrays respectively. Our data suggests that, in gastric cancer, HER2 amplification is the main mechanism for HER2 protein overexpression and is preserved in lymph node metastases.
MATERIALS AND METHODS
Patients
The current study involved 120 non-consecutive patients with gastric carcinoma, surgically treated at the 3rd and 4th Departments of Surgery, University of Athens, between 2004 and 2007. Histomorphological data were reviewed from the corresponding hematoxylin and eosin stained slides. Clinical data were obtained from corresponding reports. Clinicopathological information included: gender, age, tumor diameter, histological subtype, tumor location, pT stage, pN stage, pM stage, vascular and lymphatic invasion, survival time, and information on post-operative therapy. Characteristics of patients are summarized in Table 1.
Table 1.
Characteristics of patients with gastric cancer
| Clinicopathological feature | Frequency n (%) | |
| Patient age at diagnosis (yr) | Mean 69.6, min-max 27-96 | |
| Tumor diameter (cm) | Mean 4.6, min-max 1.3-12 | |
| Gender | Male | 84 (70) |
| Female | 36 (30) | |
| Histological type | Intestinal | 80 (66.66) |
| Diffuse | 24 (20) | |
| Mixed | 16 (13.33) | |
| Tumor location | Cardia | 37 (30.8) |
| Corpus | 39 (32.5) | |
| Antrum | 44 (36.66) | |
| pT stage | pT1 | 15 (12.5) |
| pT2 | 65 (54.16) | |
| pT3-4 | 40 (33.33) | |
| pN stage | pN0 | 36 (30) |
| pN1 | 43 (35.8) | |
| pN2+3 | 41 (34.2) | |
| pM stage | pM0 | 105 (87.5) |
| pM1 | 15 (12.5) | |
| Tumor grade | G1-2 | 42 (35) |
| G3 | 78 (65) | |
| Venous invasion | Present | 37 (30.8) |
| Absent | 83 (69.2) | |
| Lymphatic invasion | Present | 84 (70) |
| Absent | 36 (30) | |
| Adjuvant therapy | None | 32 (26.6) |
| Treated | 88 (73.4) | |
| Chemotherapy | 55 (62.5) | |
| Chemo/Radiotherapy | 33 (37.5) | |
| 5-year survival (%) | (95% CI) | 38.9 (25-52) |
Specimen characteristics
Paraffin-embedded tissue blocks of primary tumors and matched positive lymph nodes were retrieved from the Department of Pathology, University of Athens. The use of this material was approved by the local Ethics committee. Two tissue microarrays (TMAs) were constructed. The first included punches from primary tumors. In order to exclude bias due to possible tumor heterogeneity, each patient had multiple tumor punches taken from formalin-fixed, paraffin-embedded blocks using a tissue cylinder with a diameter of 1 mm, which were subsequently transferred into one recipient paraffin block (3 cm × 2.5 cm) using a semiautomated tissue arrayer. Each patient had on average 5.1 tissue punches included on this array, including at least 4 tumor punches. The second TMA included single punches from matched metastatic lymph nodes in 45 patients.
Assay methods
IHC: Five µm TMA sections were dewaxed and rehydrated in distilled water. Endogenous peroxidase was blocked using 0.5% H2O2. To determine the HER2 expression immunohistochemically, the HercepTestTM (Dako, Glostrup, Denmark) was used according to the manufacturer`s protocol. Following pressure cooker-mediated antigen retrieval sections were incubated with the prediluted primary antibody. Control samples included normal gastric mucosa and breast cancer tissue. Immunostaining was scored by an experienced gastrointestinal pathologist following a 4-step score (0, 1+, 2+, 3+), according to the consensus panel recommendations on HER2 scoring for gastric cancer[31].
CISH: HER2 CISH was performed using a CISH HER2 probe and Immunodetection Kit (ZytoDot2C SPEC HER2/CEN 17 Probe Kit). TMA sections were deparaffinized and incubated for 5 min in 3% H2O2, followed by Heat Pretreatment Solution EDTA in a covered staining jar standing in a boiling water bath at 98 °C for 15 min. After washing in distilled water, Pepsin Solution (ES1) was applied and slides were incubated for 5 min at room temperature in a humidity chamber. Sections were then washed in distilled water, dehydrated in increasing ethanol, and air dried. ZytoDot2C SPEC HER2/CEN 17 Probe was applied and sections were covered with a coverslip sealed with a layer of hot glue. Samples were then denaturated at 80 °C for 5 min, transferred to a humidity chamber and left to hybridize overnight at 37 °C. On day 2, immunodetection was performed according to the manufacturer’s instructions and sections were counterstained with Hematoxylin and mounted.
Statistical analysis
χ2 tests and contingency tables were used to analyze the relationship between IHC and CISH, and categorical parameters. Overall survival was estimated by the Kaplan-Meier method and evaluated by log-rank testing. All analysis were carried out using SAS (V9, The SAS Institute, NC, United States).
RESULTS
HER2 immunohistochemistry
HER2 protein expression was observed in 24 of the 120 gastric carcinomas (20%). In more detail, one of the 24 diffuse type carcinomas (4.16%) and 23 of the 96 intestinal and mixed type carcinomas (23.95%) showed HER2 protein expression. Immunostaining was always membrane bound and showed basolateral predominance (Figure 1A and B). Immunostaining in mixed type carcinomas was restricted in the intestinal type component. Quantitative analysis of the immunostaining, according to the consensus panel recommendations on HER2 scoring for gastric cancer, resulted in fourteen 3+ cases (11.66%), six 2+ cases (5%) and four 1+ cases (3.33%)[31] (Table 2). IHC was interpretable in 652 of the 660 spots (98.8%). Reasons for non-interpretable results were missing tissue spots or absence of tumor tissue.
Figure 1.
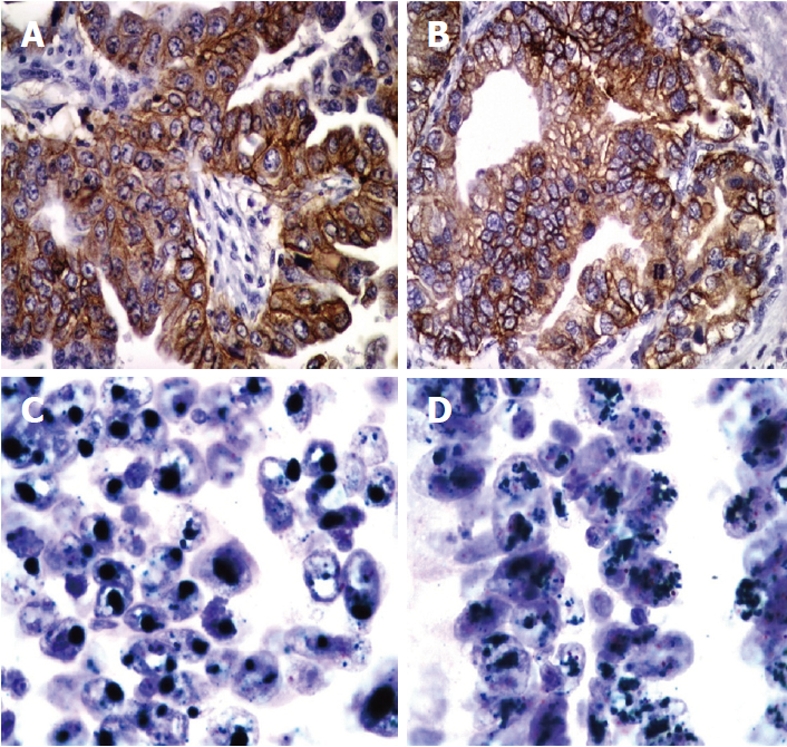
Examples of human epidermal growth factor receptor-2 immunohistochemical expression and amplification in primary and metastatic gastric cancer. Immunohistochemistry shows strong membranous staining of human epidermal growth factor receptor-2 (HER2) in intestinal type gastric cancer (A) and (B) (× 400). Chromogenic in situ hybridization assay shows amplification of HER2 in primary gastric cancer (C) and the corresponding lymph node metastasis (D). Clustered green signals represent the amplified HER2 gene, while red signals represent centromere 17. Cell nuclei are counterstained with hematoxylin (× 1000).
Table 2.
Lauren phenotype, human epidermal growth factor receptor-2 immunohistochemistry and chromogenic in situ hybridization in gastric carcinoma
| Diffuse(n = 24) | Mixed(n = 16) | Intestinal(n = 80) | ||
| HER2 IHC | 0 | 23 | 14 | 59 |
| 1+ | 0 | 0 | 4 | |
| 2+ | 1 | 0 | 5 | |
| 3+ | 0 | 2 | 12 | |
| HER2 CISH | Non amplified | 24 | 14 | 63 |
| Amplified | 0 | 2 | 17 | |
HER2: Human epidermal growth factor receptor-2; IHC: Immunohistochemistry; CISH: Chromogenic in situ hybridization.
HER2 CISH
Tissue spots were scanned for possible intratumoral heterogeneity by using a 10× objective lens. CISH hybridization signals of the HER2 gene appeared as dark green-colored dot-shaped signals. The chromosome 17 centromeric regions appeared as bright red-colored dot-shaped signals. Areas of necrosis and overlapping nuclei were avoided. Signal enumeration was performed using the 40× objective lens of a light microscope. HER2 amplification was observed in 19 of the 120 primary gastric carcinomas (15.8%). Amplified cases showed intratumoral heterogeneity with areas of low amplification where HER2 signals appeared as multiple dots or small clusters, and areas of high amplification with presence of large, green HER2 gene signal clusters (Figure 1C and D). All amplified cases had 2+ or 3+ IHC results (Table 2). HER2 amplification showed significant association with histologic tumor type. Seventeen (21.25%) of the 80 intestinal and two (12.5%) of the 16 mixed type cancers, but 0 (0%) of the diffuse type cancers, were amplified (P < 0.002, Table 2). No association between HER2 gene amplification and tumor grade, size, stage or localization was found.
HER2 alterations in lymph node metastases
Comparative analysis of primary tumors and corresponding lymph node metastases, performed in 45 cases, showed a high concordance in the presence of HER2 overexpression or amplification (P < 0.0001, Table 3).
Table 3.
Comparison of human epidermal growth factor receptor-2 immunohistochemistry and chromogenic in situ hybridization in primary gastric carcinomas and matched lymph node metastases
| HER2 IHC | HER2 CISH | |||||
| PT | LNM | Concordance | PT | LNM | Concordance | |
| Positive | 7 | 6 | 85.7% | 6 | 6 | 100% |
| Negative | 38 | 39 | 97.4% | 39 | 39 | 100% |
HER2: Human epidermal growth factor receptor-2; IHC: Immunohistochemistry; CISH: Chromogenic in situ hybridization; PT: Primary gastric cancer; LNM: Lymph node metastases.
Clinicopathological characteristics
Increasing tumor grade and stage were associated with reduced patient survival (P < 0.0001 each). No correlation was observed between patient survival and HER2 overexpression or amplification, even after including postoperative therapy of the patients and location of the tumors in the analysis (Figure 2).
Figure 2.
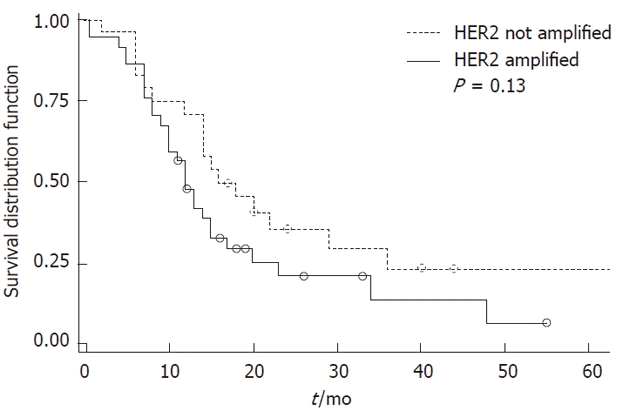
Kaplan-Meier curve for disease-specific survival and human epidermal growth factor receptor-2 amplification in gastric carcinomas. HER2: Human epidermal growth factor receptor-2.
DISCUSSION
The HER2 protein is a frequently analyzed gene product, especially in breast cancer. Recent studies have examined HER2 expression in other tumor types, including gastric cancer[18,32,33]. Immunohistochemical HER2 expression and protein positivity (≥ 2+) was observed in 20% and 14.58%, respectively, of the 120 gastric carcinomas in our study. These results are in keeping with previous reports demonstrating similar frequencies of HER2 overexpression in gastric cancer[18,32,33]. HER2 positivity was observed in 23% of the cases in the study by Yano et al[18] and in 22.6% of the cases in the study by Kim et al[33]. More recently, a TMA study of 166 gastric carcinomas by Marx et al[32] found a HER2 positivity rate of 17% and a strong correlation between IHC and HER2 gene amplification detected by FISH. In our cases, comparable to others, HER2 overexpression and/or amplification were almost exclusively found in gastric cancers of the intestinal type. This finding supports the presence of different molecular characteristics between the main histologic tumor types that seem to develop through different molecular pathways.
No correlation between HER2 overexpression and/or amplification and tumor localization could be demonstrated in our study. This might be contradictory to previous studies where a high frequency of HER2 expression was reported in cardia carcinomas[34]. However, adenocarcinomas of the gastroesophageal junction, many of which are Barrett carcinomas, are known to have a high rate of HER2 amplification and cannot always be differentiated from cardia carcinomas. This may lead to an artificial increase in the rate of HER2 expression reported in cardia carcinomas.
A high concordance rate was found between HER2 positivity, as detected by IHC, and HER2 gene amplification, as detected by CISH, since 19 of the 20 IHC positive cases (i.e., ≥ 2+) were amplified (95%). Many of them (73.6%) had high HER2 gene amplification. However, one HER2 2+ case, detected in a diffuse carcinoma, was not found to be amplified, which may be attributed to a technical error of IHC associated with formalin fixation of the tissue. Alternatively, other mechanisms of HER2 protein overexpression can contribute to inconsistencies between IHC and ISH results.
In the present study we found a high concordance rate of HER2 status between primary tumors and their corresponding lymph node metastases. This finding is in keeping with previous published results, where HER2 amplification status was found to be almost identical in the primary gastric carcinomas and their corresponding lymph node metastases[32] and provides further evidence for the role of HER2 amplification in gastric cancer.
Our findings support gastric cancer HER2 amplification as being the main mechanism that leads to HER2 protein overexpression. Similar findings have previously been reported for esophageal cancer[35]. Moreover, Tapia et al[19], in a large-scale TMA study, examined more than 4000 samples from 120 different tumors and could not find any tumors with HER2 overexpression in the absence of gene amplification.
HER2 testing has developed over a number of years, and many retrospective studies using formalin-fixed, paraffin-embedded material and different methodologies have provided inconsistent results[36,37]. Correlation between technical methods can be used to obtain a high concordance rate and to better define the assays with the best ability to identify patient groups that would benefit from a targeted therapy. Although in breast cancer HER2 IHC is an acceptable method of predicting HER2 status, previous studies have shown marked variation in different diagnostic laboratories regarding the interpretation of positive staining[25]. On the other hand, FISH is technically demanding, expensive and does not produce a permanent archival slide. In our study, CISH testing showed a good correlation to IHC, and therefore seems to represent a reliable methodology for HER2 testing. Moreover, the slides are visible under a light microscope and show good correlation with tumor morphology.
In this study, we used the tissue microarray technique using multiple tissue punches per case to account for possible heterogeneity in terms of protein expression or gene amplification in the primary tumor. Each patient had an average of 4 tumor punches taken. This is particularly important for HER2 testing in gastric cancer since considerable heterogeneity concerning gene amplification has been reported in many studies[38,39]. Such heterogeneity was also noted in our study, since in many amplified cases, areas with low and high amplification were found within the same tumor. Multiple sampling thus helped to minimize possible biases in evaluation, as suggested by Goethals et al[40], who recommended that at least four punches of primary tumor are required to account for possible heterogeneity. However, a single tissue punch was sampled in the case of lymph nodes since the issue of heterogeneity may be substantially less important. Our study also benefits from complete clinicopathological and follow-up characterization of patients. In contrast to previous studies[34,41] we could not demonstrate any association between HER2 positivity and clinical outcome.
Our study provides evidence supporting gastric cancer HER2 amplification as being the main mechanism for HER2 protein overexpression and that HER2 amplification is preserved in the lymph node metastases. Therefore, gastric cancer patients with HER2 overexpression and/or amplification seem to be good candidates for anti-HER2 therapy. Moreover, CISH is a reliable and inexpensive method that can be used for HER2 testing of gastric cancer.
COMMENTS
Background
Alterations of the human epidermal growth factor receptor-2 (HER2) gene are implicated in the development and progression of many tumors. Breast cancer patients with HER2 amplification have been effectively treated with the monoclonal antibody trastuzumab, a HER2 inhibitor. Recently, a number of studies have suggested a response to trastuzumab therapy for other cancers with HER2 amplification, including gastric cancer, where HER2 amplification has been found in 7% to 27% of the tumors. Reports of trastuzumab therapy in metastatic gastric cancer showed complete tumor regression and disappearance of the metastases in two cases. Based on these reports, gastric cancer patients with HER2 overexpression and/or amplification could be good candidates for trastuzumab therapy.
Research frontiers
The HER2 protein is a frequently analyzed gene product, especially in breast cancer. Recent studies have examined HER2 expression in other tumor types, including gastric cancer. The frequency and significance of HER-2/neu amplification in gastric carcinoma are investigated. In this study, immunohistochemical HER2 expression and protein positivity (≥ 2+) was observed in 20% and 14.58%, respectively, of the 120 gastric carcinomas.
Innovations and breakthroughs
The authors evaluated HER2 overexpression and gene amplification by immunohistochemistry and chromogenic in situ hybridization (CISH) respectively, in 120 cases of gastric carcinoma patients and 45 matched lymph node metastases mounted onto multiple-punch and single-punch tissue microarrays respectively. The data suggest that, in gastric cancer, HER2 amplification is the main mechanism for HER2 protein overexpression and is preserved in the lymph node metastases.
Applications
This study provides evidence supporting gastric cancer HER2 amplification as being the main mechanism for HER2 protein overexpression and that HER2 amplification is preserved in the lymph node metastases. Therefore, gastric cancer patients with HER2 overexpression and/or amplification seem to be good candidates for anti-HER2 therapy. Moreover, CISH is a reliable and inexpensive method that can be used for HER2 testing of gastric cancer.
Peer review
The authors studied HER2/neu expression and gene amplification in a cohort of Greek patients with gastric cancer. The manuscript includes 2 aspects. Firstly, they describe the expression/amplification of HER2-neu in the study group. Secondly, they highlight the potential applicability of CISH as a routine method. Data are mostly well documented and conclusions drawn are appropriate.
Footnotes
Supported by The University of Athens
Peer reviewer: Dr. Thomas Wex, PD, Clinic of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Leipziger Str. 44, Magdeburg 39120, Germany
S- Editor Sun H L- Editor Rutherford A E- Editor Li JY
References
- 1.García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, García-Muñiz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234–241. doi: 10.1245/aso.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro F, Salomon DS. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6:243–257. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- 3.Rabindran SK. Antitumor activity of HER-2 inhibitors. Cancer Lett. 2005;227:9–23. doi: 10.1016/j.canlet.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Salto-Tellez M, Do E, Putti TC, Koay ES. Evaluation of HER-2/neu oncogene status in breast tumors on tissue microarrays. Hum Pathol. 2003;34:362–368. doi: 10.1053/hupa.2003.60. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy D, Slamon DJ, Cobleigh M, Arnold A, Saleh M, Mortimer JE, Murphy M, Stewart SJ. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22:1063–1070. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 8.Leyland-Jones B. Trastuzumab therapy for the metastatic patient: does the primary match? Ann Oncol. 2002;13:993–994. doi: 10.1093/annonc/mdf295. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26:78–83. [PubMed] [Google Scholar]
- 10.Tuma RS. Trastuzumab trials steal show at ASCO meeting. J Natl Cancer Inst. 2005;97:870–871. doi: 10.1093/jnci/97.12.870. [DOI] [PubMed] [Google Scholar]
- 11.Kollmannsberger C, Pressler H, Mayer F, Kanz L, Bokemeyer C. Cisplatin-refractory, HER2/neu-expressing germ-cell cancer: induction of remission by the monoclonal antibody Trastuzumab. Ann Oncol. 1999;10:1393–1394. doi: 10.1023/a:1008365216323. [DOI] [PubMed] [Google Scholar]
- 12.Jewell E, Secord AA, Brotherton T, Berchuck A. Use of trastuzumab in the treatment of metastatic endometrial cancer. Int J Gynecol Cancer. 2006;16:1370–1373. doi: 10.1111/j.1525-1438.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 13.Prat A, Parera M, Reyes V, Peralta S, Cedrés S, Andreu J, Huguet P, del Campo JM. Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck. 2008;30:680–683. doi: 10.1002/hed.20714. [DOI] [PubMed] [Google Scholar]
- 14.Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A. Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer. 2002;98:833–837. doi: 10.1002/ijc.10257. [DOI] [PubMed] [Google Scholar]
- 15.Risio M, De Rosa G, Sarotto I, Casorzo L, Capussotti L, Torchio B, Aglietta M, Chiecchio L. HER2 testing in gastric cancer: molecular morphology and storage time-related changes in archival samples. Int J Oncol. 2003;23:1381–1387. [PubMed] [Google Scholar]
- 16.Im SA, Lee KE, Nam E, Kim DY, Lee JH, Han HS, Seoh JY, Park HY, Cho MS, Han WS, et al. Potential prognostic significance of p185(HER2) overexpression with loss of PTEN expression in gastric carcinomas. Tumori. 2005;91:513–521. doi: 10.1177/030089160509100612. [DOI] [PubMed] [Google Scholar]
- 17.Kanta SY, Yamane T, Dobashi Y, Mitsui F, Kono K, Ooi A. Topoisomerase IIalpha gene amplification in gastric carcinomas: correlation with the HER2 gene. An immunohistochemical, immunoblotting, and multicolor fluorescence in situ hybridization study. Hum Pathol. 2006;37:1333–1343. doi: 10.1016/j.humpath.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 19.Tapia C, Glatz K, Novotny H, Lugli A, Horcic M, Seemayer CA, Tornillo L, Terracciano L, Spichtin H, Mirlacher M, et al. Close association between HER-2 amplification and overexpression in human tumors of non-breast origin. Mod Pathol. 2007;20:192–198. doi: 10.1038/modpathol.3800729. [DOI] [PubMed] [Google Scholar]
- 20.Rebischung C, Barnoud R, Stéfani L, Faucheron JL, Mousseau M. The effectiveness of trastuzumab (Herceptin) combined with chemotherapy for gastric carcinoma with overexpression of the c-erbB-2 protein. Gastric Cancer. 2005;8:249–252. doi: 10.1007/s10120-005-0342-7. [DOI] [PubMed] [Google Scholar]
- 21.Inui T, Asakawa A, Morita Y, Mizuno S, Natori T, Kawaguchi A, Murakami M, Hishikawa Y, Inui A. HER-2 overexpression and targeted treatment by trastuzumab in a very old patient with gastric cancer. J Intern Med. 2006;260:484–487. doi: 10.1111/j.1365-2796.2006.01715.x. [DOI] [PubMed] [Google Scholar]
- 22.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett JM, Going JJ, Mallon EA, Watters AD, Reeves JR, Stanton P, Richmond J, Donald B, Ferrier R, Cooke TG. Evaluating HER2 amplification and overexpression in breast cancer. J Pathol. 2001;195:422–428. doi: 10.1002/path.971. [DOI] [PubMed] [Google Scholar]
- 24.Diaz NM. Laboratory testing for HER2/neu in breast carcinoma: an evolving strategy to predict response to targeted therapy. Cancer Control. 2001;8:415–418. doi: 10.1177/107327480100800504. [DOI] [PubMed] [Google Scholar]
- 25.Francis GD, Jones MA, Beadle GF, Stein SR. Bright-field in situ hybridization for HER2 gene amplification in breast cancer using tissue microarrays: correlation between chromogenic (CISH) and automated silver-enhanced (SISH) methods with patient outcome. Diagn Mol Pathol. 2009;18:88–95. doi: 10.1097/PDM.0b013e31816f6374. [DOI] [PubMed] [Google Scholar]
- 26.Arnould L, Denoux Y, MacGrogan G, Penault-Llorca F, Fiche M, Treilleux I, Mathieu MC, Vincent-Salomon A, Vilain MO, Couturier J. Agreement between chromogenic in situ hybridisation (CISH) and FISH in the determination of HER2 status in breast cancer. Br J Cancer. 2003;88:1587–1591. doi: 10.1038/sj.bjc.6600943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta D, Middleton LP, Whitaker MJ, Abrams J. Comparison of fluorescence and chromogenic in situ hybridization for detection of HER-2/neu oncogene in breast cancer. Am J Clin Pathol. 2003;119:381–387. doi: 10.1309/p40p2ead42pukdmg. [DOI] [PubMed] [Google Scholar]
- 28.Park K, Kim J, Lim S, Han S, Lee JY. Comparing fluorescence in situ hybridization and chromogenic in situ hybridization methods to determine the HER2/neu status in primary breast carcinoma using tissue microarray. Mod Pathol. 2003;16:937–943. doi: 10.1097/01.MP.0000086487.78558.7D. [DOI] [PubMed] [Google Scholar]
- 29.Bilous M, Morey A, Armes J, Cummings M, Francis G. Chromogenic in situ hybridisation testing for HER2 gene amplification in breast cancer produces highly reproducible results concordant with fluorescence in situ hybridisation and immunohistochemistry. Pathology. 2006;38:120–124. doi: 10.1080/00313020600561518. [DOI] [PubMed] [Google Scholar]
- 30.Penault-Llorca F, Bilous M, Dowsett M, Hanna W, Osamura RY, Rüschoff J, van de Vijver M. Emerging technologies for assessing HER2 amplification. Am J Clin Pathol. 2009;132:539–548. doi: 10.1309/AJCPV2I0HGPMGBSQ. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 32.Marx AH, Tharun L, Muth J, Dancau AM, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Brümmendorf TH, Bokemeyer C, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2009;40:769–777. doi: 10.1016/j.humpath.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim MA, Jung EJ, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol. 2007;38:1386–1393. doi: 10.1016/j.humpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 35.Reichelt U, Duesedau P, Tsourlakis MCh, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120–129. doi: 10.1038/modpathol.3800712. [DOI] [PubMed] [Google Scholar]
- 36.Ferretti G, Felici A, Papaldo P, Fabi A, Cognetti F. HER2/neu role in breast cancer: from a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol. 2007;19:56–62. doi: 10.1097/GCO.0b013e328012980a. [DOI] [PubMed] [Google Scholar]
- 37.Todorović-Raković N, Jovanović D, Nesković-Konstantinović Z, Nikolić-Vukosavljević D. Prognostic value of HER2 gene amplification detected by chromogenic in situ hybridization (CISH) in metastatic breast cancer. Exp Mol Pathol. 2007;82:262–268. doi: 10.1016/j.yexmp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Höhler T, Rüschoff J, Ridwelski K, Moehler M. [HER2 testing and targeted therapy in advanced gastric cancer] Onkologie. 2010;33 Suppl 4:26–30. doi: 10.1159/000308451. [DOI] [PubMed] [Google Scholar]
- 40.Goethals L, Perneel C, Debucquoy A, De Schutter H, Borghys D, Ectors N, Geboes K, McBride WH, Haustermans KM. A new approach to the validation of tissue microarrays. J Pathol. 2006;208:607–614. doi: 10.1002/path.1934. [DOI] [PubMed] [Google Scholar]
- 41.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–568. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]


