Abstract
AIM: To investigate the effect of alcohol on the metabolic syndrome (MS) and fatty liver in Japanese men and women.
METHODS: A cross-sectional study was conducted in a medical health checkup program at a general hospital. This study involved 18 571 Japanese men and women, 18-88 years of age, with a mean body mass index of 22.6 kg/m2. A standardized questionnaire was administered. The total amount of alcohol consumed per week was calculated, and categorized into four grades. Fatty liver was examined by ultrasound modified criteria of the revised National Cholesterol Education Program Adult Treatment Panel III and the new International Diabetes Federation.
RESULTS: The prevalence of fatty liver decreased in men and women with light to moderate alcohol consumption, whereas the prevalence of MS was not so changed. The prevalence of fatty liver of any grade in men was lower than that in those with no or minimal alcohol consumption. In women with light to moderate alcohol consumption, prevalence of fatty liver was lower than that in women with no or minimal alcohol consumption. By logistic regression analysis, the odds ratio (OR) for MS in women with light alcohol consumption was decreased to < 1.0, but this change was not clear in men. The OR for fatty liver was clearly < 1.0 in men with any level of alcohol consumption and in women with light to moderate consumption.
CONCLUSION: Light to moderate alcohol consumption has a favorable effect for fatty liver, but not for MS in Japanese men and women.
Keywords: Alcoholic hepatitis, Epidemiology, Fatty liver, Metabolic syndrome, Alcohol consumption
INTRODUCTION
The metabolic syndrome (MS) is defined by abdominal obesity, hypertension, elevated fasting blood glucose, and dyslipidemia[1]. Importantly, MS is a risk factor for the development of type 2 diabetes mellitus and coronary artery disease, and is associated with an increased risk of cerebrovascular disease and all-cause mortality[2]. The favorable effect of alcohol intake enhances insulin sensitivity, increases high-density lipoprotein-cholesterol (HDL-C), and contributes to a lower risk of type 2 diabetes mellitus[3-6] and cardiovascular disease[7-10]. Some reports have shown that the prevalence of MS is associated with alcohol consumption, irrespective of the amount consumed[11-13]. However, several studies have reported beneficial effects of alcohol consumption on MS[14-16]. Moreover, a study in Korean adults has indicated that light alcohol consumption is associated with a reduced prevalence of MS, whereas substantial alcohol intake leads to a dose-dependent increase in the risk of MS[17]. The effect of alcohol on MS in the general population has been inconsistent in the literature.
Fatty liver is closely associated with MS, and is considered the hepatic manifestation of MS[18]. Findings on the relation between alcohol consumption and fatty liver have also been inconsistent in the literature. Although alcohol consumption certainly may be a cause of fatty liver in some cases[19,20], it potentially plays a protective role against fatty deposition in the liver[21-25].
Therefore recent studies have implied the possibility that the effect of alcohol is different between fatty liver and MS, although fatty liver is closely associated with MS. However, the discrepancy of alcohol effect among the previous studies may be due to differences in the following cofactors: ethnicity, age, body mass index (BMI), drug usage, and lifestyle, such as alcohol consumption, smoking, and exercise. However, no large epidemiological study has investigated the effect of alcohol on fatty liver and MS at the same time.
We performed a cross-sectional study to investigate the effect of alcohol on fatty liver and MS at the same time. In this study, we separated the subjects according to the grade of alcohol consumption and compared the prevalence of fatty liver and MS in each grade. We focused on the discrepancy in the association between alcohol and MS and between alcohol and fatty liver. Additionally, we checked the impact of prevalence of MS without fatty liver, or fatty liver without MS.
MATERIALS AND METHODS
Study design
We performed a cross-sectional study of participants of a medical health checkup program, including abdominal ultrasonography. The study was approved by the ethics committee of Murakami Memorial Hospital, Gifu, Japan. The program was conducted in the Medical Health Checkup Center at Murakami Memorial Hospital. The purpose of the medical health checkup program was to promote public health through early detection of chronic diseases and the evaluation of their underlying risk factors. Known as a “human dock”, medical services of this kind are very popular in Japan.
Study population
All of the subjects participating in such health checkup programs at Murakami Memorial Hospital between January 2004 and December 2009 were invited to join this study. Participants who tested positive for hepatitis B antigen or hepatitis C antibody and those who reported a history of known liver disease, including viral, genetic, autoimmune, and drug-induced liver disease, were excluded from the study[26]. We invited 22 119 participants in the health checkup program to enroll in the study. Of these, a total of 19 016 Japanese participants (11 295 men and 7721 women) were enrolled after giving informed consent. We excluded 123 participants (92 men and 31 women) with hepatitis C virus, 312 (214 men and 98 women) with hepatitis B virus, and nine (7 men and 2 women) who were diagnosed with other liver diseases. As a result, this study consisted ultimately of 18 571 participants (10 982 men and 7589 women). The mean age was 46.5 years (SD: 9.9; range: 18-88 years), and the mean BMI was 22.6 kg/m2 (SD: 3.3; range: 14.0-58.3 kg/m2).
Data collection
The health checkup programs that were used for the collection of data included the following tests: eye examinations, urinalysis, blood-cell counts, blood chemistry, electrocardiography, chest radiography, barium examination of the upper gastrointestinal tract, and abdominal ultrasonography. The medical history and lifestyle factors of all participants, including physical activity and habits pertaining to smoking and alcohol consumption, were surveyed by a standardized self-administered questionnaire. When the participants had difficulty completing the questionnaire, trained nurses provided assistance. We undertook blood and urine examinations with MODULAR ANALYTICS (Hitachi High-Technologies Corp. Ltd., Tokyo, Japan).
Standardized questionnaire for lifestyle factors
A standardized questionnaire was administered to all participants by the same trained team of interviewers. Habits regarding alcohol consumption were evaluated by asking the participants about the amount and type of alcoholic beverages consumed per week during the past month, then estimating the mean ethanol intake per week. The validity of information related to alcohol consumption was confirmed previously[27]. The total amount of alcohol consumed per week was calculated in grams, and then categorized into the following four grades: non or minimal alcohol consumption, < 40 g/wk; light alcohol consumption, 40-140 g/wk; moderate alcohol consumption, 140-280 g/wk; and excess alcohol consumption, > 280 g/wk[22,24]. Smoking status was also categorized into three groups (never smoker, ex-smoker, and current smoker). On the questionnaire, participants reported the type, duration and frequency of their participation in sports or recreational activities[28]. When participants performed any kind of sports at least once a week regularly, we categorized them as regular exercisers[29].
Definition of fatty liver
The diagnosis of fatty liver was based on the results of abdominal ultrasonography, which was done by trained technicians with Aloka SSD-650CL (Aloka Co., Ltd., Tokyo, Japan). All ultrasonographic images were stored as photocopies. Gastroenterologists reviewed the photocopies and made the diagnosis of fatty liver without reference to any of the participant’s other individual data. Of four known criteria (hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring), the participants were required to have hepatorenal contrast and liver brightness to be given a diagnosis of fatty liver[30].
MS
There are several differing criteria for the MS worldwide[1,31-34]. In this study, we used the following two definitions: (1) the revised National Cholesterol Education Program Adult Treatment Panel III (rATP III) definition[34]; and (2) the new International Diabetes Federation (IDF) definition[32].
According to the rATP III definition[1], subjects who had three or more of the following criteria were identified as having MS: (1) triglycerides ≥ 150 mg/100 mL; (2) HDL-C < 40 mg/100 mL for men, and < 50 mg/100 mL for women; (3) elevated blood pressure (systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg); (4) fasting glucose ≥ 100 mg/100 mL instead of ≥ 110 mg/100 mL; or (5) abdominal-obesity-modified waist circumference cutoffs (≥ 90 cm for men and ≥ 80 cm for women) were used instead of the waist circumference cutoffs (≥ 102 cm for men and ≥ 88 cm for women) proposed in the existing definition.
According to the new IDF definition, Japanese people were defined as having MS if the subjects had abdominal obesity (waist circumference cutoffs ≥ 90 cm for men and ≥ 80 cm for women) plus two or more of the following risk factors: (1) elevated triglyceride level ≥ 150 mg/100 mL or on treatment; (2) low HDL–C < 40 mg/100 mL for men and < 50 mg/100 mL for women or on treatment; (3) elevated blood pressure (systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg); and (4) high fasting glucose ≥ 100 mg/100 mL[32].
Sample size
Because preliminary studies indicated that the number in the excess alcohol consumption group was small (n = 22 and 24), we invited as many subjects as possible. Practically, we collected data for waist circumference from 2004. Then, we set the study period from 2004 to 2009.
Statistical analysis
The R version 2.4.1 (available from http://www.r-project.org/) was used for statistical analyses. Data was expressed as mean (SD). Two groups of subjects were compared by χ2 test. The significance of differences between non or minimal alcohol consumption and the others was determined by two-tailed, multiple χ2 tests with Bonferroni correction (P < 0.016 for three comparisons in four groups). The linear association of alcohol consumption with several parameters associated with MS was evaluated by Spearman’s rank correlation, and a P value < 0.05 was accepted as significant. We assessed the odds ratio (OR) of the alcohol consumption grade for MS and fatty liver using a multivariate logistic model while controlling for potential covariates. In a multivariate logistic model, we selected age, use of drugs that potentially affect MS, and lifestyle, such as alcohol consumption, regular exercise, and smoking as the potential covariates. The adjusted OR and 95% CIs were calculated.
RESULTS
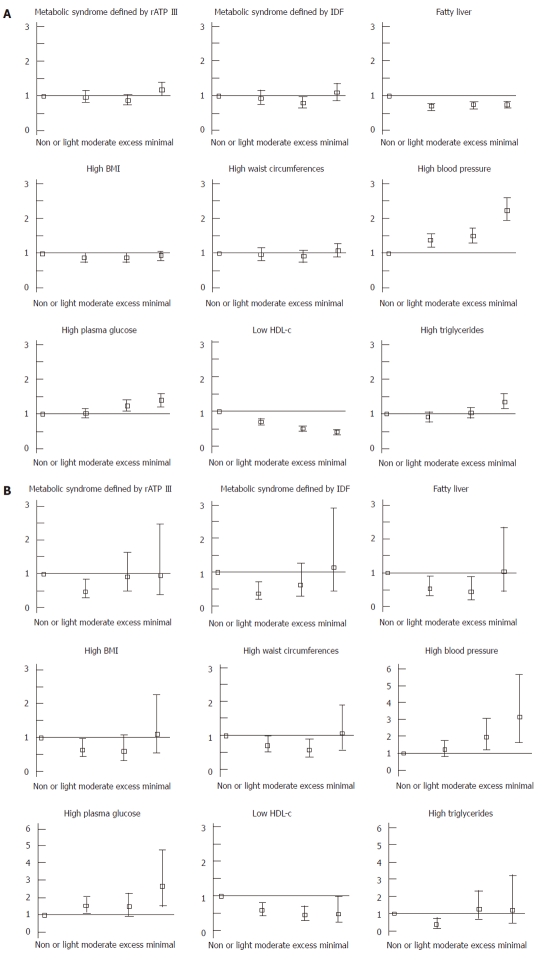
We investigated the OR of alcohol consumption for MS defined by rATP III and fatty liver using a logistic regression model (Figure 1). The OR for MS was decreased to < 1.0 in women with light alcohol consumption, but it was not clear in men. The OR for fatty liver was clearly < 1.0 in men with any level of alcohol consumption and in women with light to moderate consumption. In men and women, the ORs for high blood pressure and high fasting plasma glucose were increased as the level of alcohol consumption increased. Conversely, the OR for low HDL-C was decreased to < 1.0 in men and women. However, the OR for high triglycerides was increased to > 1.0 in men with excess alcohol consumption, and decreased to < 1.0 in women with light consumption. Moreover, the OR for high waist circumference was not significant, and was the same as the OR for high BMI. The actual adjusted ORs are shown in Table 1.
Figure 1.
Adjusted odds ratio of the level of alcohol consumption for metabolic syndrome and fatty liver in men (A) and women (B). We assessed the odds ratio (OR) of the level of alcohol consumption for metabolic syndrome (MS) and fatty liver using a multivariate logistic model while controlling for potential covariates. In a multivariate logistic model, we selected age, usage of drugs that potentially affected MS, and lifestyle factors such as wine consumption, regular exercise, and smoking status, as the potential covariates. Bars mean the adjusted OR and 95% CIs. HDL-c: High-density lipoprotein-cholesterol; rATP III: Revised National Cholesterol Education Program Adult Treatment Panel III; IDF: International Diabetes Federation; BMI: Body mass index.
Table 1.
The adjusted odds ratio (95% CI) for the metabolic syndrome and fatty liver
| Men odds ratio (95% CI) | P value | Women odds ratio (95% CI) | P value | |
| For MetS defined by rATP III | ||||
| The grade of alcohol consumption | ||||
| Light | 0.98 (0.83-1.15) | 0.77 | 0.48 (0.27-0.82) | 0.008 |
| Moderate | 0.88 (0.75-1.04) | 0.14 | 0.9 (0.5-1.65) | 0.74 |
| Excess | 1.18 (1.01-1.39) | 0.043 | 0.96 (0.37-2.48) | 0.93 |
| Age | 1.01 (1-1.02) | < 0.001 | 1.08 (1.05-1.1) | < 0.001 |
| The usage of drugs | 4.95 (4.3-5.69) | < 0.001 | 7.46 (4.96-11.24) | < 0.001 |
| Wine consumers | 1.13 (0.67-1.9) | 0.66 | 0.52 (0.24-1.15) | 0.11 |
| Regular exercisers | 0.6 (0.51-0.71) | < 0.001 | 0.6 (0.38-0.94) | 0.027 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.21 (1.03-1.41) | 0.017 | 0.89 (0.48-1.65) | 0.72 |
| Current smoker/non smoker | 1.13 (0.97-1.33) | 0.12 | 1.07 (0.58-1.98) | 0.83 |
| For MetS defined by IDF | ||||
| The grade of alcohol consumption | 0.93 (0.75-1.14) | 0.46 | 0.36 (0.19-0.69) | < 0.001 |
| Light | 0.78 (0.63-0.97) | 0.029 | 0.61 (0.3-1.25) | 0.18 |
| Moderate | 1.08 (0.87-1.33) | 0.48 | 1.14 (0.45-2.9) | 0.78 |
| Excess | 1 (0.99-1.01) | 0.66 | 1.08 (1.05-1.1) | < 0.001 |
| Age | 3.64 (3.05-4.34) | < 0.001 | 4.85 (3.09-7.63) | < 0.001 |
| The usage of drugs | 0.78 (0.37-1.65) | 0.52 | 0.6 (0.26-1.35) | 0.22 |
| Wine consumers | 0.64 (0.51-0.79) | < 0.001 | 0.55 (0.34-0.92) | 0.021 |
| Regular exercisers | ||||
| Smoking states | 1.3 (1.06-1.59) | 0.01 | 1.03 (0.54-1.97) | 0.93 |
| Ex smoker/non smoker | 1.09 (0.89-1.35) | 0.39 | 1.07 (0.54-2.1) | 0.85 |
| Current smoker/non smoker | ||||
| For fatty liver | ||||
| The grade of alcohol consumption | ||||
| Light | 0.69 (0.6-0.79) | < 0.001 | 0.54 (0.34-0.88) | 0.012 |
| Moderate | 0.72 (0.63-0.83) | < 0.001 | 0.43 (0.21-0.88) | 0.021 |
| Excess | 0.74 (0.64-0.85) | < 0.001 | 1.02 (0.44-2.35) | 0.97 |
| Age | 1 (0.99-1) | 0.21 | 1.06 (1.04-1.08) | < 0.001 |
| The usage of drugs | 2.09 (1.83-2.38) | < 0.001 | 2.17 (1.4-3.38) | < 0.001 |
| Wine consumers | 0.85 (0.53-1.35) | 0.48 | 0.59 (0.3-1.15) | 0.12 |
| Regular exercisers | 0.67 (0.59-0.77) | < 0.001 | 0.76 (0.52-1.13) | 0.17 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.24 (1.09-1.41) | < 0.001 | 0.38 (0.18-0.79) | 0.01 |
| Current smoker/non smoker | 0.92 (0.81-1.05) | 0.21 | 1 (0.57-1.74) | 1 |
| For high BMI | ||||
| The grade of alcohol consumption | ||||
| Light | 0.86 (0.75-0.99) | 0.034 | 0.64 (0.43-0.97) | 0.036 |
| Moderate | 0.85 (0.73-0.98) | 0.026 | 0.59 (0.33-1.06) | 0.077 |
| Excess | 0.9 (0.78-1.05) | 0.18 | 1.1 (0.53-2.31) | 0.8 |
| Age | 0.99 (0.98-0.99) | < 0.001 | 1.04 (1.02-1.06) | < 0.001 |
| The usage of drugs | 2.19 (1.91-2.51) | < 0.001 | 1.77 (1.15-2.74) | 0.01 |
| Wine consumers | 0.79 (0.48-1.29) | 0.34 | 0.68 (0.39-1.21) | 0.19 |
| Regular exercisers | 0.93 (0.81-1.06) | 0.29 | 0.69 (0.48-0.99) | 0.047 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.19 (1.04-1.36) | 0.013 | 0.56 (0.32-0.97) | 0.037 |
| Current smoker/non smoker | 0.99 (0.87-1.14) | 0.92 | 0.95 (0.58-1.57) | 0.85 |
| For high waist circumferences | ||||
| The grade of alcohol consumption | ||||
| Light | 0.96 (0.81-1.13) | 0.61 | 0.71 (0.51-0.97) | 0.03 |
| Moderate | 0.89 (0.75-1.06) | 0.21 | 0.57 (0.36-0.9) | 0.016 |
| Excess | 1.06 (0.89-1.27) | 0.5 | 1.06 (0.58-1.93) | 0.85 |
| Age | 1 (0.99-1.01) | 0.85 | 1.07 (1.06-1.09) | < 0.001 |
| The usage of drugs | 2.35 (2.01-2.74) | < 0.001 | 1.64 (1.14-2.36) | 0.007 |
| Wine consumers | 0.63 (0.32-1.23) | 0.18 | 0.68 (0.43-1.07) | 0.095 |
| Regular exercisers | 0.71 (0.6-0.84) | < 0.001 | 0.69 (0.52-0.92) | 0.012 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.26 (1.07-1.49) | 0.005 | 0.93 (0.64-1.35) | 0.69 |
| Current smoker/non smoker | 1.04 (0.88-1.23) | 0.67 | 0.91 (0.6-1.37) | 0.64 |
| For high blood pressure | ||||
| The grade of alcohol consumption | ||||
| Light | 1.33 (1.16-1.53) | < 0.001 | 1.22 (0.85-1.75) | 0.27 |
| Moderate | 1.47 (1.27-1.7) | < 0.001 | 1.97 (1.26-3.07) | < 0.001 |
| Excess | 2.24 (1.93-2.59) | < 0.001 | 3.13 (1.71-5.72) | < 0.001 |
| Age | 1.04 (1.03-1.05) | < 0.001 | 1.07 (1.06-1.09) | < 0.001 |
| The usage of drugs | 6.16 (5.33-7.13) | < 0.001 | 16.09 (10.87-23.83) | < 0.001 |
| Wine consumers | 1.09 (0.68-1.73) | 0.73 | 0.72 (0.42-1.23) | 0.23 |
| Regular exercisers | 0.81 (0.71-0.93) | < 0.001 | 0.8 (0.57-1.12) | 0.19 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.01 (0.88-1.15) | 0.93 | 0.78 (0.5-1.23) | 0.28 |
| Current smoker/non smoker | 0.67 (0.59-0.77) | < 0.001 | 0.73 (0.45-1.18) | 0.2 |
| For high plasma glucose | ||||
| The grade of alcohol consumption | ||||
| Light | 1 (0.88-1.13) | 0.94 | 1.52 (1.11-2.08) | 0.009 |
| Moderate | 1.23 (1.08-1.4) | < 0.001 | 1.46 (0.95-2.25) | 0.085 |
| Excess | 1.38 (1.2-1.58) | < 0.001 | 2.66 (1.49-4.76) | < 0.001 |
| Age | 1.03 (1.03-1.04) | < 0.001 | 1.07 (1.06-1.09) | < 0.001 |
| The usage of drugs | 2.05 (1.79-2.33) | < 0.001 | 1.88 (1.29-2.72) | < 0.001 |
| Wine consumers | 1.78 (1.17-2.72) | 0.007 | 0.56 (0.33-0.96) | 0.034 |
| Regular exercisers | 0.76 (0.68-0.86) | < 0.001 | 0.92 (0.68-1.24) | 0.58 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.22 (1.08-1.38) | < 0.001 | 0.68 (0.44-1.05) | 0.083 |
| Current smoker/non smoker | 0.94 (0.83-1.06) | 0.31 | 0.67 (0.42-1.06) | 0.087 |
| For low HDL-c | ||||
| The grade of alcohol consumption | ||||
| Light | 0.7 (0.61-0.8) | < 0.001 | 0.58 (0.42-0.79) | < 0.001 |
| Moderate | 0.49 (0.42-0.57) | < 0.001 | 0.43 (0.27-0.69) | < 0.001 |
| Excess | 0.41 (0.35-0.48) | < 0.001 | 0.48 (0.24-0.96) | 0.039 |
| Age | 1.01 (1-1.01) | 0.082 | 1.01 (1-1.02) | 0.055 |
| The usage of drugs | 3.4 (2.96-3.9) | < 0.001 | 4.24 (2.97-6.03) | < 0.001 |
| Wine consumers | 0.79 (0.47-1.32) | 0.37 | 0.9 (0.6-1.35) | 0.61 |
| Regular exercisers | 0.71 (0.62-0.82) | < 0.001 | 0.68 (0.52-0.91) | 0.009 |
| Smoking states | ||||
| Ex smoker/non smoker | 1.06 (0.92-1.22) | 0.45 | 0.64 (0.43-0.95) | 0.027 |
| Current smoker/non smoker | 1.65 (1.44-1.9) | < 0.001 | 1.47 (1.03-2.11) | 0.036 |
| For high triglycerides | ||||
| The grade of alcohol consumption | ||||
| Light | 0.89 (0.76-1.03) | 0.11 | 0.37 (0.19-0.74) | 0.005 |
| Moderate | 1.01 (0.87-1.18) | 0.88 | 1.27 (0.68-2.35) | 0.45 |
| Excess | 1.34 (1.16-1.56) | < 0.001 | 1.2 (0.45-3.2) | 0.72 |
| Age | 1 (0.99-1) | 0.19 | 1.06 (1.04-1.09) | < 0.001 |
| The usage of drugs | 3.06 (2.67-3.51) | < 0.001 | 11.13 (7.07-17.53) | < 0.001 |
| Wine consumers | 0.83 (0.49-1.4) | 0.48 | 0.92 (0.43-1.99) | 0.83 |
| Regular exercisers | 0.68 (0.59-0.79) | < 0.001 | 0.73 (0.44-1.21) | 0.22 |
| Smoking states | ||||
| Ex smoker/Non smoker | 1.26 (1.09-1.46) | < 0.001 | 0.99 (0.49-2.01) | 0.98 |
| Current smoker/Non smoker | 1.44 (1.24-1.66) | < 0.001 | 1.91 (1.02-3.55) | 0.042 |
BMI: Body mass index; HDL-c: High density lipoprotein cholesterol; rATP III: Revised National Cholesterol Education Program Adult Treatment Panel III; IDF: International Diabetes Federation.
Table 2 indicates the basic characteristics of men and women in the four grades. And liner association between alcohol consumption and several factors associated with MetS were evaluated by Spearman’s rank correlation (Table 3). BMI and waist circumferences were lowest in light consumption (Table 2), but Spearman’s rank correlation coefficients were not significant (Table 3). Systolic blood pressure, diastolic blood pressure, and fasting plasma glucose were increased as the alcohol consumption increased (Tables 2 and 3). On the other hand, the associations of consumption with diastolic blood pressure, and that with fasting plasma glucose were not statistically significant in women, while systolic blood pressure was also increased as the consumption increased in women. In men and women, low-density lipoprotein (LDL) cholesterol, non HDL cholesterol, and LDL cholesterol/HDL cholesterol ratio were decreased and HDL cholesterol were increased as the consumption increased (Tables 2 and 3). Triglycerides were lower in light consumption and were higher in moderate and excess than those in non or minimal (Table 2). Over all, the trend of the associations between alcohol consumption and MetS were not so changed between men and women.
Table 2.
The correlation between components for the metabolic syndrome, liver associated enzymes and alcohol consumptions in participants free from drugs
| Men | Women | |||||||
| Non or minimal | Light | Moderate | Excess | Non or minimal | Light | Moderate | Excess | |
| No. of subjects | 6154 | 1734 | 1616 | 1478 | 6893 | 406 | 207 | 84 |
| Age, yr | 46.3 (10) | 47.5 (9.4) | 49.3 (9.4) | 49.4 (8.9) | 45.1 (9.9) | 45.2 (8.8) | 45.5 (8.5) | 45 (9.3) |
| Aspartate aminotransferase, IU/L | 20.2 (8.7) | 19.6 (7.1) | 20.8 (10.2) | 23.2 (11.6) | 17.2 (9) | 17.1 (4.9) | 17.5 (5.8) | 19 (6.2) |
| Alanine aminotransferase, IU/L | 26.1 (16.9) | 23.7 (13.3) | 23.8 (14.1) | 25.5 (14.7) | 15.9 (13.1) | 15.2 (6.7) | 15.6 (6.7) | 16 (7) |
| Gamma-glutamyltransferase, IU/L | 24.3 (21.9) | 28.9 (24.6) | 36 (34.5) | 48.8 (51) | 13.8 (9.5) | 15.8 (12.1) | 17.1 (10.6) | 22.6 (21.8) |
| BMI, kg/m2 | 23.5 (3.3) | 23.2 (2.9) | 23.3 (2.7) | 23.4 (2.9) | 21.4 (3.2) | 20.9 (2.7) | 20.9 (3.1) | 21.3 (3.2) |
| Waist circumference, cm | 81.8 (8.6) | 81.5 (7.7) | 82.2 (7.5) | 82.9 (7.7) | 72.3 (8.8) | 71.5 (7.9) | 71.5 (8.6) | 73.9 (9.4) |
| Systolic blood pressure, mmHg | 120.1 (15.6) | 121.7 (15.8) | 123.9 (15.8) | 127.4 (16.1) | 111.2 (16.2) | 111.2 (15.6) | 114.3 (19.4) | 116.7 (17) |
| Diastolic blood pressure, mmHg | 75.9 (10.3) | 77.2 (10.2) | 79 (10.4) | 81.3 (10.1) | 69.1 (10.3) | 69.6 (10.6) | 72.2 (11.9) | 72.9 (10.9) |
| Fasting plasma glucose, , mg/dL | 100.5 (19.3) | 100 (16.4) | 102.4 (19.9) | 103.5 (19.1) | 91.3 (12.7) | 91.8 (10.1) | 91.5 (9.8) | 96.1 (18.7) |
| HDL-c, mg/dL | 46 (11.4) | 49.6 (12.5) | 52 (13.4) | 53.3 (14.2) | 59.9 (13.3) | 65.7 (14.1) | 69 (14.9) | 68.6 (14) |
| LDL-c, mg/dL | 128 (30) | 122.8 (29.6) | 121.5 (29.5) | 116.6 (31.6) | 118.1 (31.1) | 108.4 (29.6) | 105.6 (27.7) | 102 (26.5) |
| nonHDL-c, mg/dL | 155.4 (33.9) | 150.1 (33.4) | 150.6 (33) | 149.2 (34.7) | 139.3 (34.8) | 129 (32.2) | 127.3 (31.8) | 124.4 (28.1) |
| LDL-c/HDL-c ratio | 3 (1) | 2.6 (0.9) | 2.5 (0.9) | 2.4 (0.9) | 2.1 (0.8) | 1.7 (0.7) | 1.6 (0.6) | 1.6 (0.6) |
| Triglycerides, mg/dL | 109.5 (83) | 108.6 (100.2) | 117.7 (107.7) | 135.5 (116.7) | 64.6 (44.5) | 60 (31.9) | 69 (53.4) | 67.2 (42.2) |
Among 18 571 participants (10 982 men and 7589 women), we separate men and women into the following four grades: non or minimal alcohol consumption, < 40 g/wk; light alcohol consumption, 40-140 g/wk; moderate alcohol consumption, 140-280 g/wk; and excess alcohol consumption. Data was expressed as mean (SD). BMI: Body mass index; HDL-c: High density lipoprotein cholesterol; LDL-c: Low density lipoprotein cholesterol.
Table 3.
The linear association between the amount of alcohol consumption and several parameters associated with the metabolic syndrome
| Men | Women | |||
| ρ | P value | ρ | P value | |
| Age, yr | 0.11 | < 0.001 | -0.09 | < 0.001 |
| Aspartate aminotransferase, IU/L | 0.09 | < 0.001 | 0 | 0.77 |
| Alanine aminotransferase, IU/L | -0.02 | 0.08 | -0.02 | 0.05 |
| Gamma-glutamyltransferase, IU/L | 0.34 | < 0.001 | 0.08 | < 0.001 |
| BMI, kg/m2 | -0.01 | 0.16 | -0.02 | 0.1 |
| Waist circumference, cm | 0.04 | < 0.001 | -0.01 | 0.32 |
| Systolic blood pressure, mmHg | 0.14 | < 0.001 | -0.04 | < 0.001 |
| Diastolic blood pressure, mmHg | 0.17 | < 0.001 | -0.02 | 0.18 |
| Fasting plasma glucose, mg/dL | 0.08 | < 0.001 | -0.01 | 0.28 |
| HDL-c, mg/dL | 0.23 | < 0.001 | 0.13 | < 0.001 |
| LDL-c, mg/dL | -0.13 | < 0.001 | -0.13 | < 0.001 |
| nonHDL-c, mg/dL | -0.08 | < 0.001 | -0.13 | < 0.001 |
| LDL-c/HDL-c ratio | -0.25 | < 0.001 | -0.17 | < 0.001 |
| Triglycerides, mg/dL | 0.06 | < 0.001 | -0.06 | < 0.001 |
The liner association between alcohol consumption and several factors associated with the metabolic syndrome were evaluated by Spearman’s rank correlation in 10 982 men and 7589 women, respectively, and a P value of < 0.05 was accepted as a significant level. BMI: Body mass index; HDL-c: High density lipoprotein cholesterol; LDL-c: Low density lipoprotein cholesterol.
This observed inverse association between alcohol consumption and fatty liver might be due to changed habits of alcohol use after previous detection of fatty liver. We analyzed the study population according to previous data. Among 10 981 men, 6547 were new participants and 4434 were repeat participants. Among 7573 women, 5138 were new participants and 2435 were repeat participants. The alcohol consumption of repeat participants with fatty liver was the same as that of new participants with fatty liver (Table 4). Next, we analyzed repeat participants and separated them into four groups according to previous and present fatty liver. We assessed the changed habit of alcohol consumption. At first, the change in alcohol consumption was small in each group (Table 5). Moreover, the level of change was no different among the four groups of men and women.
Table 4.
Alcohol consumptions of new and repeat participants
| Men | Women | |||||
| n | Alcohol consumption (g/wk) | P value | n | Alcohol consumption (g/wk) | P value | |
| New participants without fatty liver | 4417 | 113.02 (161.03) | < 0.0012 | 4662 | 21.37 (67.57) | 0.0922 |
| New participants with fatty liver | 2130 | 96.14 (157.95) | 0.71 | 476 | 13.99 (68.47) | 0.8411 |
| Repeat participants without fatty liver | 2986 | 125.79 (157.24) | < 0.0013 | 2154 | 21.48 (58.33) | 0.8533 |
| Repeat participants with fatty liver | 1448 | 102.02 (155.3) | 281 | 18.09 (75.15) | ||
New participants with fatty liver vs repeat participants with fatty liver;
New participants with fatty liver vs new participants without fatty liver;
Repeat participants with fatty liver vs repeat participants without fatty liver.
Table 5.
Changed alcohol consumption habits among four groups
| Previous fatty liver | Present fatty liver | Men | Women | ||
| n | Change of alcohol consumption (g/wk) | n | Change of alcohol consumption (g/wk) | ||
| Negative | Negative | 2784 | –1.69 (109.95) | 2112 | –5.38 (48.13) |
| Negative | Positive | 269 | 4.07 (116.14) | 75 | –20.69 (142.71) |
| Positive | Negative | 202 | 6.07 (104.09) | 42 | –20.9 (56.96) |
| Positive | Positive | 1179 | –4.45 (100.32) | 206 | –5.56 (31.87) |
Change in alcohol consumption (g/wk) was calculated by present alcohol consumption minus previous alcohol consumption, and expressed as mean (SD). We performed a Tukey test to investigate the statistical significance of the difference between two groups. No significant difference was identified.
We calculated the number of subjects with both MS and fatty liver, those who had only MS, and those who had only fatty liver (Table 6). Unexpectedly, more than half of the participants with fatty liver were not diagnosed with MS, even if the definition was changed. 42.5% or 25.5% of men with fatty liver were diagnosed with MS defined by rATP III or IDF, respectively. Conversely, 66.0% or 78.8% of men with MS defined by rATP III or IDF were diagnosed with fatty liver. The result was similar in women: 43.7% or 38.6% of women with fatty liver were diagnosed with MS defined by rATP III or IDF, respectively. Conversely, 49.1% or 55.9% of women with MS defined by rATP III or IDF were diagnosed with fatty liver. The prevalence of fatty liver among men with MS decreased along with level of alcohol consumption and prevalence of fatty liver among women with MS decreased in those with light or moderate consumption (Table 6).
Table 6.
Positive prevalence of parameters for four levels of alcohol consumption n (%)
| Non or minimal | Light | Moderate | Excess | Non or minimal vs light | Light vs moderate | Moderate vs excess | |
| Men | |||||||
| Fatty liver | 2248 (36.5) | 457 (26.4) | 449 (27.8) | 424 (28.7) | < 0.001 | < 0.001 | < 0.001 |
| MS defined by rATP III | 1282 (20.8) | 331 (19.1) | 317 (19.6) | 373 (25.2) | 0.12 | 0.33 | < 0.001 |
| MS defined by IDF | 668 (10.9) | 165 (9.5) | 143 (8.8) | 182 (12.3) | 0.12 | 0.04 | 0.15 |
| Fatty liver among men with MS defined by rATP III | 923 (72) | 204 (61.6) | 188 (59.3) | 206 (55.2) | < 0.001 | < 0.001 | < 0.001 |
| Fatty liver among men with MS defined by IDF | 568 (85) | 118 (71.5) | 100 (69.9) | 127 (69.8) | < 0.001 | < 0.001 | < 0.001 |
| MS defined by rATP III among men with fatty liver | 923 (41.1) | 204 (44.6) | 188 (41.9) | 206 (48.6) | 0.17 | 0.79 | 0.005 |
| MS defined by IDF among men with fatty liver | 568 (25.3) | 118 (25.8) | 100 (22.3) | 127 (30) | 0.85 | 0.22 | 0.058 |
| Components of MS | |||||||
| High waist circumference | 968 (15.7) | 257 (14.8) | 236 (14.6) | 257 (17.4) | 0.38 | 0.32 | 0.15 |
| High blood pressure | 1766 (28.7) | 591 (34.1) | 626 (38.7) | 707 (47.8) | < 0.001 | < 0.001 | < 0.001 |
| High fasting plasma glucose | 2332 (37.9) | 681 (39.3) | 749 (46.3) | 729 (49.3) | 0.31 | < 0.001 | < 0.001 |
| Low HDL-C | 2133 (34.7) | 425 (24.5) | 333 (20.6) | 284 (19.2) | < 0.001 | < 0.001 | < 0.001 |
| High triglycerides | 1385 (22.5) | 360 (20.8) | 394 (24.4) | 459 (31.1) | 0.13 | 0.14 | < 0.001 |
| High BMI (> 25 kg/m2) | 1731 (28.1) | 417 (24) | 388 (24) | 379 (25.6) | < 0.001 | < 0.001 | 0.07 |
| Smoking status | |||||||
| Current smoker | 2012 (32.7) | 602 (34.7) | 657 (40.7) | 740 (50.1) | 0.12 | < 0.001 | < 0.001 |
| Never smoker | 2220 (36.1) | 446 (25.7) | 308 (19.1) | 180 (12.2) | < 0.001 | < 0.001 | < 0.001 |
| Ex smoker | 3933 (63.9) | 1288 (74.3) | 1308 (80.9) | 1298 (87.8) | < 0.001 | < 0.001 | < 0.001 |
| Usage of drugs | 938 (15.2) | 249 (14.4) | 307 (19) | 326 (22.1) | 0.38 | 0.00 | 0.00 |
| Regular exerciser | 1096 (17.9) | 361 (20.9) | 326 (20.3) | 272 (18.5) | 0.01 | 0.04 | 0.64 |
| Wine consumer | 45 (0.7) | 18 (1) | 17 (1.1) | 14 (0.9) | 0.26 | 0.30 | 0.52 |
| Women | |||||||
| Fatty liver | 719 (10.5) | 22 (5.4) | 9 (4.3) | 7 (8.3) | < 0.001 | 0.01 | 0.65 |
| MS defined by rATP III | 632 (9.2) | 19 (4.7) | 17 (8.2) | 6 (7.1) | < 0.001 | 0.73 | 0.65 |
| MS defined by IDF | 494 (7.2) | 12 (3) | 10 (4.8) | 6 (7.1) | < 0.001 | 0.25 | 0.84 |
| Fatty liver among women with MS defined by rATP III | 322 (50.9) | 4 (21.1) | 3 (17.6) | 2 (33.3) | 0.020 | 0.013 | 0.65 |
| Fatty liver among women with MS defined by IDF | 284 (57.5) | 3 (25) | 3 (30) | 2 (33.3) | 0.051 | 0.16 | 0.44 |
| MS defined by rATP III among women with fatty liver | 322 (44.8) | 4 (18.2) | 3 (33.3) | 2 (28.6) | 0.024 | 0.73 | 0.63 |
| MS defined by IDF among women with fatty liver | 284 (39.5) | 3 (13.6) | 3 (33.3) | 2 (28.6) | 0.026 | 0.97 | 0.84 |
| Components of MS | |||||||
| High waist circumference | 1257 (18.2) | 61 (15) | 26 (12.6) | 16 (19) | 0.12 | 0.05 | 0.96 |
| High blood pressure | 1026 (14.9) | 65 (16) | 45 (21.7) | 22 (26.2) | 0.58 | 0.01 | 0.01 |
| High fasting plasma glucose | 906 (13.1) | 70 (17.2) | 33 (15.9) | 19 (22.6) | 0.02 | 0.29 | 0.02 |
| Low HDL-C | 1772 (25.7) | 58 (14.3) | 25 (12.1) | 10 (11.9) | < 0.001 | < 0.001 | 0.01 |
| High triglycerides | 507 (7.4) | 12 (3) | 18 (8.7) | 6 (7.1) | 0.00 | 0.56 | 0.89 |
| High BMI (> 25 kg/m2) | 792 (11.5) | 30 (7.4) | 14 (6.8) | 9 (10.7) | 0.01 | 0.05 | 0.96 |
| Smoking status | |||||||
| Current smoker | 372 (5.4) | 50 (12.3) | 57 (27.5) | 25 (29.8) | < 0.001 | < 0.001 | < 0.001 |
| Never smoker | 6086 (88.3) | 291 (71.7) | 106 (51.2) | 37 (44) | < 0.001 | < 0.001 | < 0.001 |
| Ex smoker | 804 (11.7) | 115 (28.3) | 101 (48.8) | 47 (56) | < 0.001 | < 0.001 | < 0.001 |
| Usage of drugs | 575 (8.3) | 36 (8.9) | 22 (10.6) | 6 (7.2) | 0.78 | 0.30 | 0.87 |
| Regular exerciser | 1179 (17.2) | 82 (20.3) | 34 (16.7) | 19 (22.6) | 0.13 | 0.91 | 0.25 |
| Wine consumer | 140 (2) | 30 (7.4) | 17 (8.2) | 11 (13.1) | < 0.001 | < 0.001 | < 0.001 |
This table indicates the positive prevalence of parameters for four levels of alcohol consumption: non or minimal, < 40 g/wk; light, 40–140 g/wk; moderate, 140–280 g/wk; and excess, > 280 g/wk. Two groups of subjects were compared by using the unpaired t test and χ2 test. The significance of differences between two side-by-side groups among the four groups was determined by two-tailed, multiple χ2 tests with Bonferroni correction (P < 0.016 for three comparisons in four groups). Smoking status was also categorized into three groups; never smoker, ex smoker and current smoker. Regular exercisers were defined as participants who performed any kind of sports at least once a week. Usage of drugs was defined as participants who receive any drugs that potentially affected metabolic syndrome (MS). HDL-c: High-density lipoprotein-cholesterol; rATP III: Revised National Cholesterol Education Program Adult Treatment Panel III; IDF: International Diabetes Federation; BMI: Body mass index.
DISCUSSION
As far as we know, the present study is the first to investigate the association of alcohol with ultrasonography-proven fatty liver and MS at the same time in a general population. Our study clearly indicated that the effect of alcohol was different between fatty liver and MS, although they are correlated closely. The effect of alcohol consumption on MS was not consistent, because it differed among components of MS. Alcohol consumption was associated with lower risk for HDL-C, but was associated with higher risk for high blood pressure and high fasting plasma glucose. Waist circumferences were not affected by level of alcohol consumption. Moreover, these results were similar when we used the Japan Society for The Study of Obesity definition for MS[33]. Our study clearly indicated that light to moderate alcohol consumption was associated with lower risk of fatty liver. Moreover, any level of alcohol consumption could have a protective effect on fatty liver in men. Our study was cross-sectional, therefore, the findings might be due to changed alcohol consumption after previous detection of fatty liver. However, our sub-analysis indicated that changes in alcohol consumption were small and were not due to previous detection of fatty liver.
Previous studies have indicated that the presence of fatty liver is a strong predictor of MS[36], and fatty liver correlates with all the components of MS[37]. Among populations with no or light alcohol consumption, liver fat content in participants with MS is significantly increased up to fourfold higher than those without MS[37], and the incidence of fatty liver has been shown to be increased fourfold in men and 11-fold in women with MS[27]. Although fatty liver is considered to be a hepatic manifestation of MS, more than half of Japanese men and women with fatty liver were not diagnosed with MS.
Interpretations
Alcohol consumption is a lifestyle factor, and its effects on health range from beneficial to detrimental. The dose-response relationship between alcohol and all-cause mortality follows a J- or U-shaped curve, which points to lower all-cause mortality among those with light to moderate alcohol consumption compared to excess consumption[38]. This effect is thought to be due mainly to a reduction in cardiovascular disease[7]. This reduction in cardiovascular disease has been attributed to the beneficial impact of alcohol on plasma lipid levels, hemostatic factors[8], and insulin sensitivity[3,6]. Some studies have suggested that as much as half of the cardiovascular benefit attributable to alcohol consumption may be because it increases HDL-C level[7-10]. We found that HDL-C was increased as the quantity of alcohol consumption increased, which was consistent with previous reports[7-10]. On the other hand, alcohol consumption contributes to elevated blood pressure[39,40]. Then, we also found that blood pressure was increased as the quantity of alcohol consumption increased.
In fact, studies about the association between alcohol consumption and obesity have not been consistent. Waist-to-hip ratio increases as the quantity of alcohol consumption increases[41,42], and waist circumference increases with excess alcohol consumption (> 40 g/d)[43]. Alcohol consumption of 30 g/d or more significantly increases BMI and the risk of weight gain[44]. In contrast, another study has reported that light-to-moderate alcohol consumption reduces waist circumference[16]. Moreover, some studies have found no significant association between alcohol consumption and obesity[45,46]. We also found no significant association between alcohol consumption and high waist circumference and BMI (BMI > 25 kg/m2).
Alcohol consumption may increase triglyceride concentrations[8]. Triglycerides have been reported to be higher in individuals with excess alcohol consumption, but lower in those with light to moderate consumption[8]. Similar results were found in our study, which indicated that the OR for high triglyceride levels was increased to > 1.0 in men with excess alcohol consumption, and was decreased to < 1.0 in women with light consumption. The favorable effect of alcohol consumption contributing to enhance insulin sensitivity has been reported[3-6]. However, another study has provided different evidence that insulin resistance is related to alcohol consumption in a U-shaped manner[47]. In our study, the OR for high fasting plasma glucose was increased as the level of alcohol consumption increased.
Overall, the present study proved that the effect of alcohol consumption for MS was not significant in the Japanese general population. In fact, studies regarding the association between alcohol consumption and MS have not been consistent[11-17], because the relationship heavily depends on the individual components of MS. Moreover, previous studies have claimed that the type of alcohol is important for the association of alcohol consumption and MS[23,48,49]. Modest wine consumption has been reported to be associated with reduced all-cause mortality[48,49] and fatty liver[23]. In our study, however, the association between wine consumption and MS and fatty liver was not significant. Similarly, Djousse et al[15] have reported that the association between alcohol consumption and MS is unrelated to the type of alcoholic beverage.
Findings on the relation between alcohol consumption and fatty liver have also been inconsistent[19-25]. Fat deposition in the liver has been shown to be primarily due to an increased influx of fatty acids to the liver; most likely as a result of the increased lipolysis associated with obesity and insulin resistance, and as a result of increased hepatic de novo lipogenesis[50]. Reduced fatty acid oxidation and mitochondrial dysfunction and decreased export of fat further contribute to the accumulation of liver fat[51,52]. Alcohol-dehydrogenase-mediated ethanol metabolism generates a reduced form of nicotinamide adenine dinucleotide, which promotes steatosis by stimulating the synthesis of fatty acids and opposing their oxidation[53]. The hepatic lipogenic pathway is activated after the consumption of 24 g/d ethanol[53]. Sterol regulatory element binding protein 1c (SREBP1c)[54] and peroxisome proliferator activated receptor (PPAR)α[55], are altered with alcohol consumption. The involvement of AMP-activated protein kinase activity in the action of ethanol on the liver has been demonstrated in experimental models of ethanol-induced steatosis[56].
SREBP1c is upregulated, which potentially results in increased conversion of glucose to fatty acids and triglycerides in experimental models of obesity[52]. PPARα is a nuclear receptor that is important in fatty acid uptake and oxidation, and has been shown to be underexpressed in experimental models of non-alcoholic steatosis[57]. In addition, the administration of adiponectin reverses non-alcoholic steatosis in experimental models[57,58].
Thus, several pivotal factors in the pathogenesis of fatty liver may be common in both alcoholic and non-alcoholic subjects. Therefore, the possible mechanism by which alcohol has a protective effect against fatty deposition in the liver remains unclear. Our study also provides clear evidence that light to moderate alcohol consumption has a favorable effect on fatty liver.
Some limitations of our study should be noted. First, although ultrasonography has been validated for detecting fatty liver, it may give an incorrect diagnosis compared to liver biopsy[30]. Second, self-reported information regarding alcohol intake is frequently subject to underreporting, and misreporting could be a source of bias. However, the self-reported information regarding alcohol intake in our study was validated previously[27]. Third, the generalizability of our study to non-Japanese populations is uncertain.
In conclusion, the effect of alcohol consumption was different between MS and fatty liver. The relationship between alcohol consumption and MS depends on the individual components of MS given the inconsistency of the association between alcohol consumption and MS. Light to moderate alcohol consumption has a favorable effect on fatty liver in Japanese men and women. Moreover, any level of alcohol consumption may have a protective effect against fatty liver in men. Unexpectedly, more than half of Japanese men and women with fatty liver were not diagnosed with MS, although fatty liver is considered to be a hepatic manifestation of MS. However, our previous studies have implied that the risk of fatty liver for cardiovascular disease is independent of MS[59]. Thus, fatty liver without MS is an important disease in the general population.
A future longitudinal study is needed to clarify that alcohol consumption has true hepatoprotective effects. Furthermore, the protective mechanism of alcohol against fatty deposition in the liver remains unclear. Thus, basic research is also needed to clarify the mechanisms that underlie modest alcohol consumption and fatty liver.
ACKNOWLEDGMENTS
We thank all of the staff members in the medical health checkup center at Murakami Memorial Hospital. The authors declare no conflict of interest associated with the manuscript.
COMMENTS
Background
The effects of alcohol on the metabolic syndrome (MS) have been inconsistent in previous studies. Fatty liver is closely associated with MS, and is considered to be the hepatic manifestation of MS. Findings on the relation between alcohol consumption and fatty liver have also been inconsistent.
Research frontiers
The favorable effect of alcohol intake enhances insulin sensitivity, and increases high-density lipoprotein-cholesterol, which contributes to a lower risk of type 2 diabetes mellitus, and cardiovascular disease. Moreover, alcohol consumption plays a protective role against fatty deposition in the liver, although alcohol consumption certainly could be a cause of fatty liver in some cases.
Innovations and breakthroughs
The authors investigated the association of alcohol consumption with ultrasonography-proven fatty liver and the MS at the same time in a general population, and clearly indicated that light to moderate alcohol consumption has favorable and unfavorable effects for components of MS, but has a protective effect for fatty liver.
Applications
Light to moderate alcohol consumption has a favorable effect on fatty liver in Japanese men and women. Moreover, any level of alcohol consumption may have a protective effect in men. A future longitudinal study is needed to clarify that alcohol consumption has true hepatoprotective effects. Furthermore, the mechanism of alcohol protection against fatty deposition in the liver remains unclear. Thus, basic research is also needed to clarify the mechanisms that underlie the effect of modest alcohol consumption on fatty liver.
Peer review
The strengths of the study include its large sample size and validated questionnaire.
Footnotes
Supported by A grant from the Gifu Medical Association; Young Scientists (B) from Japan Society for the Promotion of Science, No. 23790791, in part
Peer reviewer: Zongli Zheng, MD, PhD, MS, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Nobels vag 12 A, SE-171 77, Stockholm, Sweden
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, Bonora E. Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study) BMJ. 1996;313:1040–1044. doi: 10.1136/bmj.313.7064.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsumura K, Hayashi T, Suematsu C, Endo G, Fujii S, Okada K. Daily alcohol consumption and the risk of type 2 diabetes in Japanese men: the Osaka Health Survey. Diabetes Care. 1999;22:1432–1437. doi: 10.2337/diacare.22.9.1432. [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Blair SN. Alcohol intake and incidence of type 2 diabetes in men. Diabetes Care. 2000;23:18–22. doi: 10.2337/diacare.23.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287:2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 8.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 10.Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186:113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Baik I, Shin C. Prospective study of alcohol consumption and metabolic syndrome. Am J Clin Nutr. 2008;87:1455–1463. doi: 10.1093/ajcn/87.5.1455. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama H, Hiroshi H, Ohgo H, Hibi T, Saito I. Effects of excessive ethanol consumption on the diagnosis of the metabolic syndrome using its clinical diagnostic criteria. Intern Med. 2007;46:1345–1352. doi: 10.2169/internalmedicine.46.6196. [DOI] [PubMed] [Google Scholar]
- 13.Rosell M, De Faire U, Hellénius ML. Low prevalence of the metabolic syndrome in wine drinkers--is it the alcohol beverage or the lifestyle? Eur J Clin Nutr. 2003;57:227–234. doi: 10.1038/sj.ejcn.1601548. [DOI] [PubMed] [Google Scholar]
- 14.Gigleux I, Gagnon J, St-Pierre A, Cantin B, Dagenais GR, Meyer F, Després JP, Lamarche B. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J Nutr. 2006;136:3027–3032. doi: 10.1093/jn/136.12.3027. [DOI] [PubMed] [Google Scholar]
- 15.Djoussé L, Arnett DK, Eckfeldt JH, Province MA, Singer MR, Ellison RC. Alcohol consumption and metabolic syndrome: does the type of beverage matter? Obes Res. 2004;12:1375–1385. doi: 10.1038/oby.2004.174. [DOI] [PubMed] [Google Scholar]
- 16.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Curtis Ellison R. Alcohol consumption and the prevalence of the Metabolic Syndrome in the US.: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2954–2959. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 17.Yoon YS, Oh SW, Baik HW, Park HS, Kim WY. Alcohol consumption and the metabolic syndrome in Korean adults: the 1998 Korean National Health and Nutrition Examination Survey. Am J Clin Nutr. 2004;80:217–224. doi: 10.1093/ajcn/80.1.217. [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 20.Donohue TM. Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13:4974–4978. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Angulo P, St Sauver J, Muto A, Okada T, Lindor K. Light to moderate alcohol consumption is associated with lower frequency of hypertransaminasemia. Am J Gastroenterol. 2007;102:1912–1919. doi: 10.1111/j.1572-0241.2007.01274.x. [DOI] [PubMed] [Google Scholar]
- 23.Dunn W, Xu R, Schwimmer JB. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology. 2008;47:1947–1954. doi: 10.1002/hep.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the Japanese male population. Am J Gastroenterol. 2009;104:2189–2195. doi: 10.1038/ajg.2009.361. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, Fukatsu M, Suzuki S, Yoshida T, Tokudome S, Joh T. Alcohol drinking may not be a major risk factor for fatty liver in Japanese undergoing a health checkup. Dig Dis Sci. 2010;55:176–182. doi: 10.1007/s10620-008-0693-0. [DOI] [PubMed] [Google Scholar]
- 26.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 27.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 29.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–77. doi: 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 30.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 31.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 32.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 33.Definition and the diagnostic standard for metabolic syndrome--Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Nihon Naika Gakkai Zasshi. 2005;94:794–809. [PubMed] [Google Scholar]
- 34.Stone NJ, Saxon D. Approach to treatment of the patient with metabolic syndrome: lifestyle therapy. Am J Cardiol. 2005;96:15E–21E. doi: 10.1016/j.amjcard.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization Western Pacific Region, International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications; 2000. [Google Scholar]
- 36.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 37.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 38.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 39.Marmot MG, Elliott P, Shipley MJ, Dyer AR, Ueshima H, Beevers DG, Stamler R, Kesteloot H, Rose G, Stamler J. Alcohol and blood pressure: the INTERSALT study. BMJ. 1994;308:1263–1267. doi: 10.1136/bmj.308.6939.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–1117. doi: 10.1161/hy1101.093424. [DOI] [PubMed] [Google Scholar]
- 41.Sakurai Y, Umeda T, Shinchi K, Honjo S, Wakabayashi K, Todoroki I, Nishikawa H, Ogawa S, Katsurada M. Relation of total and beverage-specific alcohol intake to body mass index and waist-to-hip ratio: a study of self-defense officials in Japan. Eur J Epidemiol. 1997;13:893–898. doi: 10.1023/a:1007416322031. [DOI] [PubMed] [Google Scholar]
- 42.Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, Messineo D, Sasso GF, Gasbarrini G, Ceccanti M. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol. 2000;95:2323–2327. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee MY, Kim MY, Kim SY, Kim JH, Kim BH, Shin JY, Shin YG, Yun JH, Ryu SY, Lee TY, et al. Association between alcohol intake amount and prevalence of metabolic syndrome in Korean rural male population. Diabetes Res Clin Pract. 2010;88:196–202. doi: 10.1016/j.diabres.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr. 2003;77:1312–1317. doi: 10.1093/ajcn/77.5.1312. [DOI] [PubMed] [Google Scholar]
- 45.Sherwood NE, Jeffery RW, French SA, Hannan PJ, Murray DM. Predictors of weight gain in the Pound of Prevention study. Int J Obes Relat Metab Disord. 2000;24:395–403. doi: 10.1038/sj.ijo.0801169. [DOI] [PubMed] [Google Scholar]
- 46.French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, Baxter JE. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord. 1994;18:145–154. [PubMed] [Google Scholar]
- 47.Magis DC, Jandrain BJ, Scheen AJ. [Alcohol, insulin sensitivity and diabetes] Rev Med Liege. 2003;58:501–507. [PubMed] [Google Scholar]
- 48.Renaud SC, Guéguen R, Siest G, Salamon R. Wine, beer, and mortality in middle-aged men from eastern France. Arch Intern Med. 1999;159:1865–1870. doi: 10.1001/archinte.159.16.1865. [DOI] [PubMed] [Google Scholar]
- 49.Grønbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, Jensen G, Sørensen TI. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloomgarden ZT. Second World Congress on the Insulin Resistance Syndrome: insulin resistance syndrome and nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1518–1523. doi: 10.2337/diacare.28.6.1518. [DOI] [PubMed] [Google Scholar]
- 52.Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423–433. doi: 10.1111/j.1478-3231.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 53.Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr. 1999;70:928–936. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 54.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 55.Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- 56.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 57.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 59.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]