Abstract
AIM: To evaluate the correlation between the level of 18F-fluoro-2-deoxyglucose (18F-FDG) uptake and glucose transporter 1 (GLUT1) expression in colorectal adenocarcinoma (CRA).
METHODS: Forty four patients with resected CRA and preoperative 18F-FDG positron emission tomography - computed tomography data were investigated in this study. Comparison of maximum standardized uptake value (SUVmax) of the lesion was made with GLUT1 expression by immunohistochemistry and various clinicopathologic factors including tumor volume, invasion depth, gross finding, and lymph node metastasis.
RESULTS: SUVmax was 14.45 ± 7.0 in negative GLUT1 expression cases, 15.51 ± 5.7 in weak GLUT1 expression cases, and 16.52 ± 6.8 in strong GLUT1 expression cases, and there was no correlation between between GLUT1 expression and SUVmax. SUVmax was significantly correlated with tumor volume (P < 0.001). However, there was no significant differences in SUVmax and GLUT1 expression among other clinicopathologic factors.
CONCLUSION: GLUT1 expression does not correlates significantly with 18F-FDG uptake in CRA. 18F-FDG uptake was increased with tumor volume, which is statistically significant.
Keywords: 18F-fluoro-2-deoxyglucose, Glucose transporter 1, Colorectal cancer
INTRODUCTION
Cancer cell growth is an energy-related process supported by increased glucose metabolism[1]. This uptake is mediated by glucose transporter (GLUT) proteins, which are membrane proteins responsible for the transport of glucose across cellular membranes. A family of seven glucose transporters have been cloned[2]. Among these, GLUT1 is the best-known basic, high-affinity glucose transporter, which is restricted to erythrocytes and blood-tissue barriers such as the blood-brain and blood-nerve barriers, in most normal tissues[3,4]. It has long been recognized that cancer cells have increased rates of glucose metabolism compared with normal cells[5]. Increased GLUT1 expression has been described in many cancers, including breast, lung, kidney, urinary bladder, stomach, colorectum, endometrium, thyroid, head and neck, liver, ovary, salivary gland, and prostate cancer[6] due to a high metabolic rate and fast growth environment, but, generally absent in benign epithelial tissues. The expression of GLUT1 thus would appear to be a potential marker for malignant transformation, and the degree of tumor GLUT1 expression might correlate with biologic behavior in individual patients[7].
Positron emission tomography (PET) using 18F-fluoro-2-deoxyglucose (FDG) is a rapidly developing functional-imaging modality that has shown great promise in the fields of primary, recurrent and metastatic tumor detection, planning and monitoring therapy[8-12]. The cellular mechanism underlying the increased 18F-FDG accumulation in malignant tumors is associated with a higher rate of phosphorylation and diminished rate of dephosphorylation of intracellular phosphorylated glucose, a higher rate of glucose transport across the cell membrane, and higher activity of hexokinase[13]. There have been several studies about possible associations of GLUT1 expression with other clinicopathologic parameters and 18F-FDG PET findings in several cancers, such as carcinoma of lung, pancreas, and breast[1]. However, to the best of our knowledge, it has not been elucidated in colorectal adenocarcinoma (CRA). Therefore, we conducted a prospective study to determine the association between GLUT1 expression and the maximum standardized uptake values (SUVmax) obtained from 18F-FDG PET scans. The relationship between GLUT1 and SUVs with other clinicopathologic factors was also evaluated. Additionally, we evaluated a difference in GLUT1 expression between adenoma and carcinoma in the colorectum.
MATERIALS AND METHODS
Patients
Among patients who had FDG-PET examination and underwent curative surgery for CRA at Chosun University Hospital from January 2007 to December 2010, the present study was conducted on a non-consecutive series of 44 patients where paraffin embedded tissues were relatively well preserved and complete medical records were present. Patients who underwent preoperative chemoradiotherapy and emergency surgery, and patients who had evidence of hereditary non-polyposis colorectal cancer or familial adenomatous polyposis were excluded from the study. The various clinicopathological parameters of the patients were confirmed by reviewing the patient medical records and pathology files. The relationship between clinicopathological parameters for the patients and the immunohistochemical findings with survival was investigated for all 44 patients. Additionally, there were 27 adenomatous cases, including 15 cases of tubular adenoma (TA), 7 villous adenomas (VA), and 5 tubulo-villous adenomas (TVA).
Histopathological analysis
Microscopic examination: each tumor was re-evaluated by retrospective analysis of the medical records and the tissue slide files of the Department of Pathology. Age, gender, tumor size, histological subtypes and the degree of differentiation, the depth of tumor invasion, the status of lymph node metastases and the presence of a distant metastasis were assessed. Stage was defined according to the TNM staging system of the American Joint Committee on Cancer[17]. The examined tissues were fixed in 10% neutral formalin, and the prepared paraffin embedded tissues were sectioned 4-5 μm in thickness. Hematoxylin and eosin staining was performed, and the sections were examined under a light microscope. A representative area suitable for the study purpose was selected, and slides were prepared for immunohistochemical analysis.
Immunohistochemical staining
All of the specimens in this study were tested using a gout polyclonal antibody against GLUT1 (Abcam) according to the manufacturer’’s protocol. Immunolocalization was performed using the mouse ImmunoCruz Staining System: sc-2050 (Santa Cruz Biotechnology), according to the manufacturer’s protocol. The staining process was performed according to a standard protocol. Briefly, the 4 μm sections that were obtained after formalin fixation and paraffin embedding were deparaffinized in xylene and were then rehydrated with distilled water through a graded series of ethanol solutions. The sections were then placed in a glass jar with 10 mmol/L citrate buffer (pH 6.0) and were irradiated in a microwave oven for 15 min. The sections were allowed to cool in the jar at room temperature for 20 min. The slides were then rinsed with Tris buffered saline (TBS). A blocking reagent was added for 10 min after quenching the endogenous peroxidase activity in 0.3% hydrogen peroxide for 10 min. The slides were then washed as described previously, and the slides were subsequently subjected to the primary antibody reaction. Immunohistochemistry was performed on the Nexes ES (Ventana, Tucson, AZ). Slides were incubated with the antibodies for 32 min. The Ventana basic DAB detection kit (catalog No. 760-001) was the secondary detection method. This includes biotinylated immunoglobulin secondary antibody, containing affinity purified goat-antimouse IgG and IgM (b200 lg/mL) and goat-antirabbit IgG (b200 lg/mL) in phosphate buffer with preservative. Incubation was for 8 min. This was followed by conjugated streptavidin horseradish peroxidase. Slides were counterstained with hematoxylin (Ventana catalog No. 760-2021).
Analysis and interpretation of staining
GLUT1 immunostaining was quantified by grading the proportion of cells that were GLUT1 positive. Cells showing strong and distinctive membranous immunoreactivity for GLUT1 were considered positive. Cytoplasmic staining, including a supra nuclear dot pattern or nuclear staining, was regarded as negative[6]. The degree of GLUT1 immunostaining of a specimen was graded according to the proportion of GLUT1-positive cells in it (weakly positive, < 10%; moderately positive, 10%-50%; strongly positive, > 50%)[7].
Statistical analysis
The mean with standard deviation (SD) was calculated for the longest tumor diameter and SUVmax. Mann-Whitney U or Kruskal-Wallis test was used to assess differences in the levels of SUVmax and in the staining scores of GLUT1 between the groups. Correlations between SUVmax and GLUT1 expressions and between SUVmax and tumor diameter were analyzed by Spearman’s rank test. A value of P < 0.05 was considered as statistically significant. The SPSS statistics 17.0 program (SPSS, Korea) was used for statistical evaluation.
RESULTS
The clinical characteristics of the patients are summarized in Table 1. The average age at the time of surgery was 65.73 years and the ratio of male to female participants was 24:20 (54.5%:45.5%). Mean tumor size was 18.92 cm, and mean SUVmax value was 15.47. In normal epithelium, specific GLUT1 expression was not observed. As expected, erythrocyte membranes were strongly GLUT1 positive. In adenoma cases, GLUT1 expression was absent in 23 cases (85.1%) and weakly positive in 4 cases, which were one VA and 3 TVAs. The positive rate of GLUT1 expression was significantly different (P = 0.008) among the TA, VA, and TVA. Of 44 cases of CRA, 91% had specific GLUT1 immunostaining in the plasma membrane. The extent of expression varied greatly. Of immunopositive cases, 13 cases (29.5%) showed weak staining (< 10% of tumor cells), 12 cases (27.3%) moderate staining (10%-50% of tumor cells), and 15 cases (34.1%) strong expression (> 50% of tumor cells), which were significantly different from adenomatous cases (P < 0.001). In cancer tissue, GLUT1 is usually strongly positive in the center of the necrotic and infiltrative areas (Figure 1). Concerning correlation between GLUT1 expression and SUVmax in PET, the mean SUVmax was 14.45 ± 7.0 in negative GLUT1 expression cases, 15.51 ± 5.7 in weak GLUT1 expression cases, and 16.52 ± 6.8 in strong GLUT1 expression cases, and there was no significant correlation between GLUT1 expression and SUVmax. SUVmax was significantly correlated with tumor volume (P = 0.002). However, GLUT1 expression did not correlate with tumor size. There was no significant difference in SUVmax and GLUT1 expression among other clinicopathologic factors including invasion depth, lymph node metastasis and gross type (Table 2).
Table 1.
Summary of clinicopathologic factors of adenocarcinoma
| Characteristics | n (%) |
| Age (yr) | |
| ≤ 50 | 7 (15.9) |
| 51~59 | 7 (15.9) |
| 60~69 | 10 (22.7) |
| ≥ 70 | 20 (45.5) |
| Sex | |
| Male | 24 (54.5) |
| Female | 20 (45.5) |
| Pathologic tumor classification (pT) | |
| pT1 | 2 (4.5) |
| pT2 | 5 (11.4) |
| pT3 | 35 (79.6) |
| pT4 | 2 (4.5) |
| Pathologic lymph node classification | |
| pN0 | 26 (59.1) |
| pN1 | 17 (38.6) |
| pN2 | 1 (2.3) |
| Metastasis classification (M) | |
| M0 | 42 (95.5) |
| M1 | 2 (4.5) |
| Gross type | |
| I (polypoid) | 8 (18.2) |
| II (ulcerative) | 17 (38.6) |
| III (infiltrative) | 19 (43.2) |
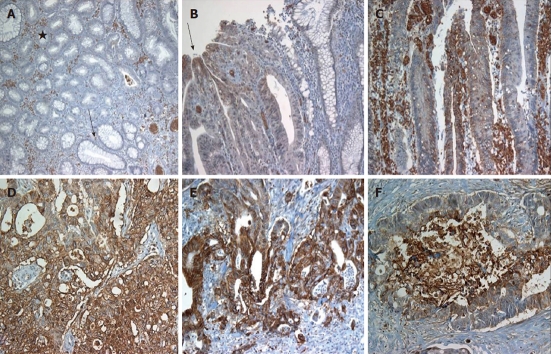
Figure 1.
Glucose transporter 1 expression in normal colonic epithelium, adenomas and adenocarcinomas. A: No glucose transporter 1 (GLUT1) expression in tubular adenoma (star) and normal epithelium (arrow), while immunostaining in erythrocytes; B and C: GLUT1 immunostaining in the villous adenoma (B, arrow: Expression at the tip of villous frond); D: Colorectal adenocarcinoma with strong GLUT1 expression; E and F: More strong expression at the infiltrative border (E) and necrotic center (F).
Table 2.
Relation between glucose transporter 1 expression/maximum standardized uptake values and clinicopathologic parameters
| Clinicopathologic factors | n | GLUT1 expression1 | SUVmax | ||||
| 0 | 1 | 2 | P value | Medium P value | |||
| T stage | |||||||
| T1 | 2 | 2 | 0 | 0 | 0.282 | 6 | 0.108 |
| T2 | 5 | 1 | 2 | 2 | 15.1 | ||
| T3 | 35 | 12 | 10 | 13 | 24.22 | ||
| T4 | 2 | 2 | 0 | 0 | 17.5 | ||
| N stage | |||||||
| N0 | 26 | 10 | 7 | 9 | 20.3 | ||
| N1 | 17 | 6 | 5 | 6 | 0.795 | 25.09 | 0.346 |
| N2 | 1 | 1 | 0 | 0 | 12 | ||
| Gross type | |||||||
| I (polypoid) | 8 | 3 | 4 | 1 | 19.94 | ||
| II (ulcerative) | 17 | 7 | 3 | 7 | 0.473 | 24.94 | 0.496 |
| III (infiltrative) | 19 | 7 | 5 | 7 | 20.39 | ||
| Tumor size (median) | 19.59 | 28.71 | 20.83 | 0.14 | 0.0022 | ||
GLUT1 expression;
Statistically significant, P < 0.05. 0: Negative or weak; 1: Moderate; 2: Strong expression. GLUT: Glucose transporter; SUVmax: Maximum standardized uptake values.
DISCUSSION
Among Gluts, Glut-1 and Glut-3 have been proven to show overexpression in both messenger RNA and protein in a variety of cancer cells[14-18]. Therefore, Glut-1 and Glut-3 may play an important role in glucose uptake by these cancers and could be useful biomarkers for malignant transformation[1]. We herein demonstrate that GLUT1 protein expression is a marker for malignant transformation in CRA. For CRA, an initial report showed increased expression of GLUT1 mRNA compared with normal colon[19], and GLUT1 immunostaining was subsequently demonstrated in seven of nine colorectal carcinomas[20]. A recent study of 53 colon carcinomas demonstrated the presence of GLUT1 immunostaining in 83%, and a higher degree of GLUT1 expression correlated with the presence of lymph node metastases[21]. The greater degree of GLUT1 expression in these tumors most likely reflects a greater enhancement of glycolytic metabolism in the more malignant tumors. It has recently been reported that GLUT1 (and/or GLUT3 expression) correlates with poor prognosis and tumor aggressiveness in carcinomas of the lung and bladder, and in squamous cell carcinoma of the head and neck[22-24]. Although the present study did not show these results, these data suggest the possibility that tumors with absent GLUT1 staining might express another GLUT iso-form such as GLUT3, which also might be associated with poor prognosis[22].
In the present study, the normal and most adenomatous colorectal mucosa did not express GLUT1 protein. In benign colorectal neoplasms, GLUT1 expression was absent in TA, and in VA and TVA, there was only rare focal staining at the tips of villous fronds. These results are consistent with a recent report that some VAs have very limited focal GLUT1 expression[21]. GLUT1 expression in VA is consistent with the concept that GLUT1 is a marker of neoplastic progression in the colon, because it is this subtype of colonic adenoma that is believed to have the greatest potential for malignant transformation[7].
In cancer tissue, GLUT1 is usually strongly positive in the luminal border and center of the necrotic and infiltrative areas. Rapid proliferation relative to vascular support exposes tumor cells to persistent hypoxic conditions with potential necrotic or apoptotic effects[6]. Malignant cells, however, can undergo genetic and adaptive changes that allow them to avoid oxygen deprivation-induced death. One of these changes is an increased uptake of glucose and other sugars compared with normal cells[25]. In normal human small intestinal villi, the tips of villi may be a site of relative hypoxia[26]. Because hypoxia is known to stimulate glycolysis and GLUT1 expression[27], the localization of GLUT1 immunostaining to this site in VAs also might reflect an adaptation to enhanced local glycolytic demand[7].
Two possible mechanisms may explain the activation of GLUT1 gene expression in CRA and other malignancies[7]. First, increased glycolysis and concomitant GLUT1 expression may be a constitutive feature of the malignant phenotype in many cancers. This is consistent with observations that transformation of cultured cells with ras or src oncogenes induces increased glucose uptake and GLUT1 expression[28,29]. Second, local hypoxia in the tumor microenvironment may result in an adaptive increase in glycolytic metabolism and GLUT1 expression[7]. The latter mechanism is also demonstrated in the present study; GLUT1 tended to be expressed stronger at the luminal border and center of tumor nests, increasing with distance from stromal blood vessels.
Higher levels of GLUT1 expression in neoplastic tissue reflect an increased glycolytic metabolism[30]. In previous studies of CRA, a high level of GLUT1 expression was significantly associated with the presence of lymph node metastases[21] and poorer prognosis[7]. These studies suggested that the expression of GLUT1 could be a marker for malignant potential. In our study, the analysis of the association between GLUT1 expression and other clinicopathologic parameters did not show any significant correlation. These results differ from those of previously published data for other tumors[21,31,32], but they are compatible with the results of Avril et al[33] for breast cancer. In a study by Haber et al[7], the proportion of GLUT1 staining did not correlate with Dukes’ stage of the CRA, but Sakashita et al[30] demonstrated that in T1 and T2 stage CRA, GLUT1 expression correlated with Duke stage. The discrepancy between the two studies could have been caused by differences in the clinical characteristics of the subjects enrolled. Haber’s study included only 6 Dukes’ A cases and all other cases were more advanced, while Sakashita’s study analysed only T1 and T2 stage cases. So, Sakashita et al[30] speculated as follows: in early-stage carcinomas GLUT1 positivity is low, but correlates with the depth of the lesion. In contrast, in the more advanced stages, the tumor cells already show high GLUT expression, and no further increase of GLUT1 expression occurs, even when the cancer invades more deeply.
Cancer cells have higher rates of glucose metabolism than normal cells. Malignant tissues typically demonstrated higher 18F-FDG uptake than benign lesions and normal tissue[34]. PET-CT using 18F-FDG has been known to be a useful tool for several malignant tumors. Several immunohistochemical studies have demonstrated overexpression of GLUT1 in human malignancy and a correlation between GLUT1 expression and neoplastic progression[22]. The overexpression of GLUT1 in human cancers has been reported to be closely related to 18F-FDG uptake on PET-CT[18]. Another report, however, showed no relation between GLUT1 expression and 18F-FDG uptake on PET[33], and there is a controversial report that did not demonstrate a statistically significant correlation between GLUT1 expression and FDG uptake[35].
In present study 18F-FDG uptake related to tumor size, whereas GLUT1 frequency did not. Brown et al[36] had mentioned that 18F-FDG uptake and GLUT1 expression appeared to be associated with tumor size, but our data did not support their findings. Tumor size is one of the most important factors affecting the SUVmax[37]. The 18F-FDG uptake might be influenced by the total amount of glucose uptake into the tumor. Therefore, the larger a carcinoma is, the higher is the 18F-FDG uptake by the carcinoma shown on the PET scan. It is well known that SUVmax has a lower than “real” value when the tumor size is < 20 mm because of the limited resolution of current PET scanners[37,38]. In contrast, GLUT1 staining is examined through a microscope, and GLUT1 frequency is determined microscopically. Therefore, GLUT1 frequency shows microscopic activity of glucose uptake into the tumor and is influenced by cell type, cellularity, and pathological structure[39]. At the result, GLUT1 frequency would not be related to tumor size. GLUT1 expression could become strongly positive even in small carcinomas with high cellular density or metabolic activity.
In conclusion, in contrast to other malignant tumor such as lung cancer[39], squamous cell carcinoma of the cervix[1] and head and neck cancer[40], and cholangiocarcinoma[41], GLUT1 expression did not correlate significantly with 18F-FDG uptake and other clinicopathologic parameters in CRA, which suggests that overexpression of GLUT1 cannot fully explain the biologic behavior of CRA. The 18F-FDG uptake was significantly correlated with tumor size only. We identified that GLUT1 is usually strongly positive in the center of the necrotic and infiltrative areas in colorectal cancer. Although overexpression of GLUT1 is very important for 18F-FDG uptake in cancer cells, further investigations should evaluate the contributions of other factors concerning tumor hypoxia and glucose metabolism.
COMMENTS
Background
Cancer cell growth is an energy-related process supported by increased glucose metabolism. This uptake is mediated by glucose transporter (GLUT) proteins, which are membrane proteins responsible for the transport of glucose across cellular membranes. Positron emission tomography (PET) using 18F-fluoro-2-deoxyglucose (FDG) is a rapidly developing functional-imaging modality that has shown great promise in the fields of primary, recurrent and metastatic tumor detection, planning and monitoring therapy. Therefore, the authors conducted a prospective study to determine the association between GLUT1 expression and the maximum standardized uptake values (SUVmax) obtained from 18F-FDG PET scans. The relationship between GLUT1 and SUVs with other clinicopathologic factors was also evaluated. Additionally, the authors evaluated the difference in GLUT1 expression between adenoma and carcinoma in the colorectum.
Research frontiers
This article may present information to the colorectal oncologist for further study about glucose metabolism of colorectal adenocarcinoma (CRA), and to the colorectal oncologist the usefulness of PET CT in evaluating colorectal cancer patients.
Innovations and breakthroughs
In contrast to other malignant tumors such as lung cancer, squamous cell carcinoma of cervix and head and neck, and cholangiocarcinoma, GLUT1 expression did not correlate significantly with 18F-FDG uptake and other clinocopathologic parameters in CRA, which suggests that overexpression of GLUT1 cannot fully explain the biologic behavior of CRA. The 18F-FDG uptake was significantly correlated with tumor size only. The authors identified that GLUT1 is usually strongly positive in the center of the necrotic and infiltrative areas in colorectal cancer. Although overexpression of GLUT1 is very important for 18F-FDG uptake in cancer cells, further investigations should evaluate the contributions of other factors concerning tumor hypoxia and glucose metabolism.
Peer review
Overall the manuscript is reasonably well written and provides additional information on GLUT1 expression and FDG uptake.
Footnotes
Supported by National Research Foundation of Korea Grant funded by the Ministry of Education, Science and Technology through the Research Center for Resistant Cells, No. R13-2003-009
Peer reviewers: Dr. Kevin J Spring, PhD, Conjoint Gastroenterology Laboratory, The Queensland Institute of Medical Research, the Bancroft Centre, rm H07, PO Royal Brisbane Hospital, Herston QLD 4029, Australia; Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City 999075, Cuba
S- Editor Sun H L- Editor O’Neill M E- Editor Zhang DN
References
- 1.Yen TC, See LC, Lai CH, Tsai CS, Chao A, Hsueh S, Hong JH, Chang TC, Ng KK. Standardized uptake value in para-aortic lymph nodes is a significant prognostic factor in patients with primary advanced squamous cervical cancer. Eur J Nucl Med Mol Imaging. 2008;35:493–501. doi: 10.1007/s00259-007-0612-1. [DOI] [PubMed] [Google Scholar]
- 2.Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990;265:18035–18040. [PubMed] [Google Scholar]
- 4.Froehner SC, Davies A, Baldwin SA, Lienhard GE. The blood-nerve barrier is rich in glucose transporter. J Neurocytol. 1988;17:173–178. doi: 10.1007/BF01674204. [DOI] [PubMed] [Google Scholar]
- 5.Isselbacher KJ. Sugar and amino acid transport by cells in culture--differences between normal and malignant cells. N Engl J Med. 1972;286:929–933. doi: 10.1056/NEJM197204272861707. [DOI] [PubMed] [Google Scholar]
- 6.Sung JY, Kim GY, Lim SJ, Park YK, Kim YW. Expression of the GLUT1 glucose transporter and p53 in carcinomas of the pancreatobiliary tract. Pathol Res Pract. 2010;206:24–29. doi: 10.1016/j.prp.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, Slater G, Weiss A, Burstein DE. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40. doi: 10.1002/(sici)1097-0142(19980701)83:1<34::aid-cncr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, Spaulding MB. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755–760. doi: 10.1148/radiology.206.3.9494497. [DOI] [PubMed] [Google Scholar]
- 9.Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J Nucl Med. 2003;44:224–239. [PubMed] [Google Scholar]
- 10.Findlay M, Young H, Cunningham D, Iveson A, Cronin B, Hickish T, Pratt B, Husband J, Flower M, Ott R. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol. 1996;14:700–708. doi: 10.1200/JCO.1996.14.3.700. [DOI] [PubMed] [Google Scholar]
- 11.Kim TS, Moon WK, Lee DS, Chung JK, Lee MC, Youn YK, Oh SK, Choe KJ, Noh DY. Fluorodeoxyglucose positron emission tomography for detection of recurrent or metastatic breast cancer. World J Surg. 2001;25:829–834. doi: 10.1007/s002680020095. [DOI] [PubMed] [Google Scholar]
- 12.Oriuchi N, Higuchi T, Ishikita T, Miyakubo M, Hanaoka H, Iida Y, Endo K. Present role and future prospects of positron emission tomography in clinical oncology. Cancer Sci. 2006;97:1291–1297. doi: 10.1111/j.1349-7006.2006.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haberkorn U, Ziegler SI, Oberdorfer F, Trojan H, Haag D, Peschke P, Berger MR, Altmann A, van Kaick G. FDG uptake, tumor proliferation and expression of glycolysis associated genes in animal tumor models. Nucl Med Biol. 1994;21:827–834. doi: 10.1016/0969-8051(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 14.Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–387. doi: 10.1200/JCO.2002.20.2.379. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi Y, Saito A, Miyagi Y, Yamanaka S, Marat D, Doi C, Yoshikawa T, Tsuburaya A, Ito T, Satoh S. Suppression of facilitative glucose transporter 1 mRNA can suppress tumor growth. Cancer Lett. 2000;154:175–182. doi: 10.1016/s0304-3835(00)00392-x. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–641. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, Doi R, Hosotani R, Imamura M, Konishi J. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002;43:173–180. [PubMed] [Google Scholar]
- 18.Higashi K, Ueda Y, Sakurai A, Wang XM, Xu L, Murakami M, Seki H, Oguchi M, Taki S, Nambu Y, et al. Correlation of Glut-1 glucose transporter expression with. Eur J Nucl Med. 2000;27:1778–1785. [PubMed] [Google Scholar]
- 19.Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- 20.Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 1996;56:1164–1167. [PubMed] [Google Scholar]
- 21.Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res. 1996;2:1151–1154. [PubMed] [Google Scholar]
- 22.Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Younes M, Juarez D, Lechago LV, Lerner SP. Glut 1 expression in transitional cell carcinoma of the urinary bladder is associated with poor patient survival. Anticancer Res. 2001;21:575–578. [PubMed] [Google Scholar]
- 24.Baer S, Casaubon L, Schwartz MR, Marcogliese A, Younes M. Glut3 expression in biopsy specimens of laryngeal carcinoma is associated with poor survival. Laryngoscope. 2002;112:393–396. doi: 10.1097/00005537-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 25.Kang SS, Chun YK, Hur MH, Lee HK, Kim YJ, Hong SR, Lee JH, Lee SG, Park YK. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn J Cancer Res. 2002;93:1123–1128. doi: 10.1111/j.1349-7006.2002.tb01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks DA, Jacobson ED. Mesenteric circulation. 2nd ed. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1987. pp. 1649–1670. [Google Scholar]
- 27.Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. Am J Physiol. 1992;262:C682–C690. doi: 10.1152/ajpcell.1992.262.3.C682. [DOI] [PubMed] [Google Scholar]
- 28.Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 29.Birnbaum MJ, Haspel HC, Rosen OM. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987;235:1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- 30.Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer. 2001;37:204–209. doi: 10.1016/s0959-8049(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 31.Chang S, Lee S, Lee C, Kim JI, Kim Y. Expression of the human erythrocyte glucose transporter in transitional cell carcinoma of the bladder. Urology. 2000;55:448–452. doi: 10.1016/s0090-4295(99)00474-4. [DOI] [PubMed] [Google Scholar]
- 32.Cantuaria G, Magalhaes A, Penalver M, Angioli R, Braunschweiger P, Gomez-Marin O, Kanhoush R, Gomez-Fernandez C, Nadji M. Expression of GLUT-1 glucose transporter in borderline and malignant epithelial tumors of the ovary. Gynecol Oncol. 2000;79:33–37. doi: 10.1006/gyno.2000.5910. [DOI] [PubMed] [Google Scholar]
- 33.Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, Nathrath W, Schwaiger M. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- 34.Kubota K, Matsuzawa T, Fujiwara T, Ito M, Hatazawa J, Ishiwata K, Iwata R, Ido T. Differential diagnosis of lung tumor with positron emission tomography: a prospective study. J Nucl Med. 1990;31:1927–1932. [PubMed] [Google Scholar]
- 35.Marom EM, Aloia TA, Moore MB, Hara M, Herndon JE, Harpole DH, Goodman PC, Patz EF. Correlation of FDG-PET imaging with Glut-1 and Glut-3 expression in early-stage non-small cell lung cancer. Lung Cancer. 2001;33:99–107. doi: 10.1016/s0169-5002(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 36.Brown RS, Leung JY, Kison PV, Zasadny KR, Flint A, Wahl RL. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med. 1999;40:556–565. [PubMed] [Google Scholar]
- 37.Keyes JW. SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–1839. [PubMed] [Google Scholar]
- 38.Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, Yanagihara K, Li M, Tanaka F, Wada H, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia. 2005;7:369–379. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Usuda K, Sagawa M, Aikawa H, Ueno M, Tanaka M, Machida Y, Zhao XT, Ueda Y, Higashi K, Sakuma T. Correlation between glucose transporter-1 expression and 18F-fluoro-2-deoxyglucose uptake on positron emission tomography in lung cancer. Gen Thorac Cardiovasc Surg. 2010;58:405–410. doi: 10.1007/s11748-010-0603-1. [DOI] [PubMed] [Google Scholar]
- 40.Deron P, Vangestel C, Goethals I, De Potter A, Peeters M, Vermeersch H, Van de Wiele C. FDG uptake in primary squamous cell carcinoma of the head and neck. The relationship between overexpression of glucose transporters and hexokinases, tumour proliferation and apoptosis. Nuklearmedizin. 2011;50:15–21. doi: 10.3413/nukmed-0324-10-06. [DOI] [PubMed] [Google Scholar]
- 41.Paudyal B, Oriuchi N, Paudyal P, Higuchi T, Nakajima T, Endo K. Expression of glucose transporters and hexokinase II in cholangiocellular carcinoma compared using [18F]-2-fluro-2-deoxy-D-glucose positron emission tomography. Cancer Sci. 2008;99:260–266. doi: 10.1111/j.1349-7006.2007.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]