Abstract
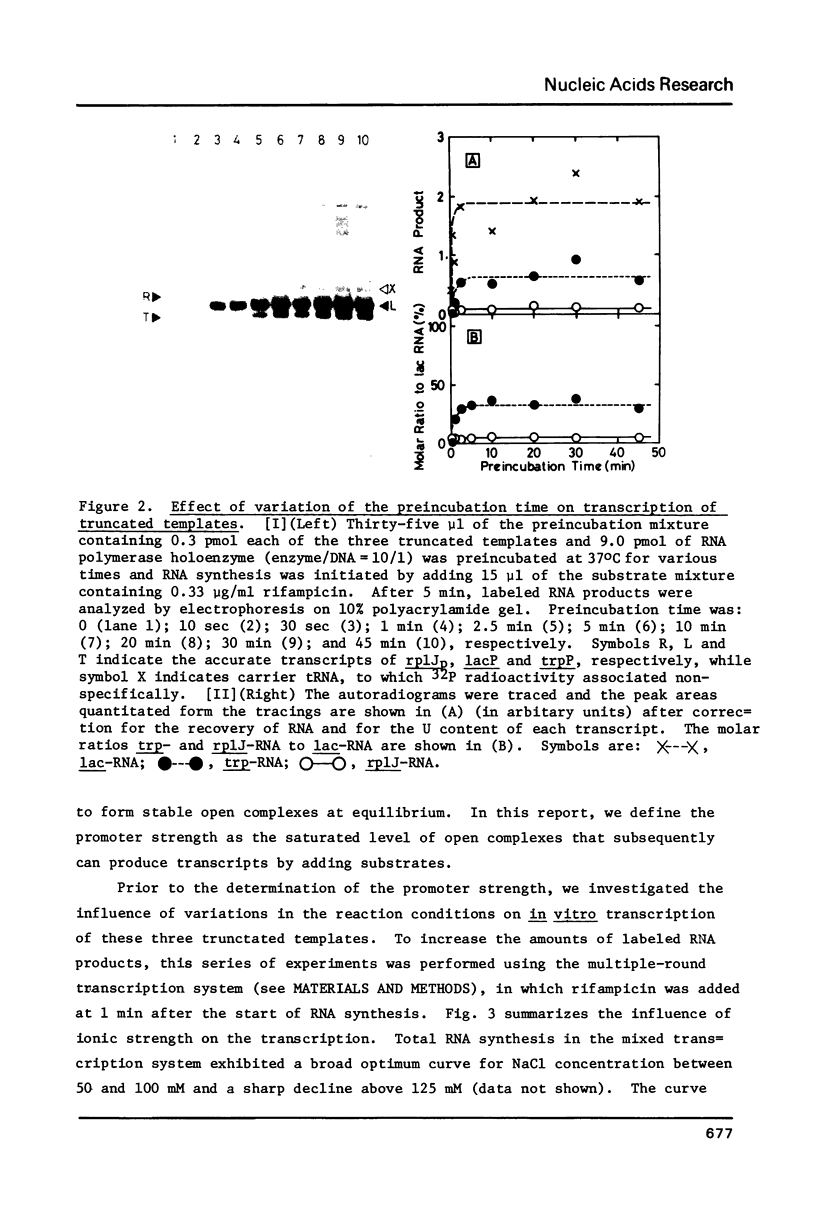
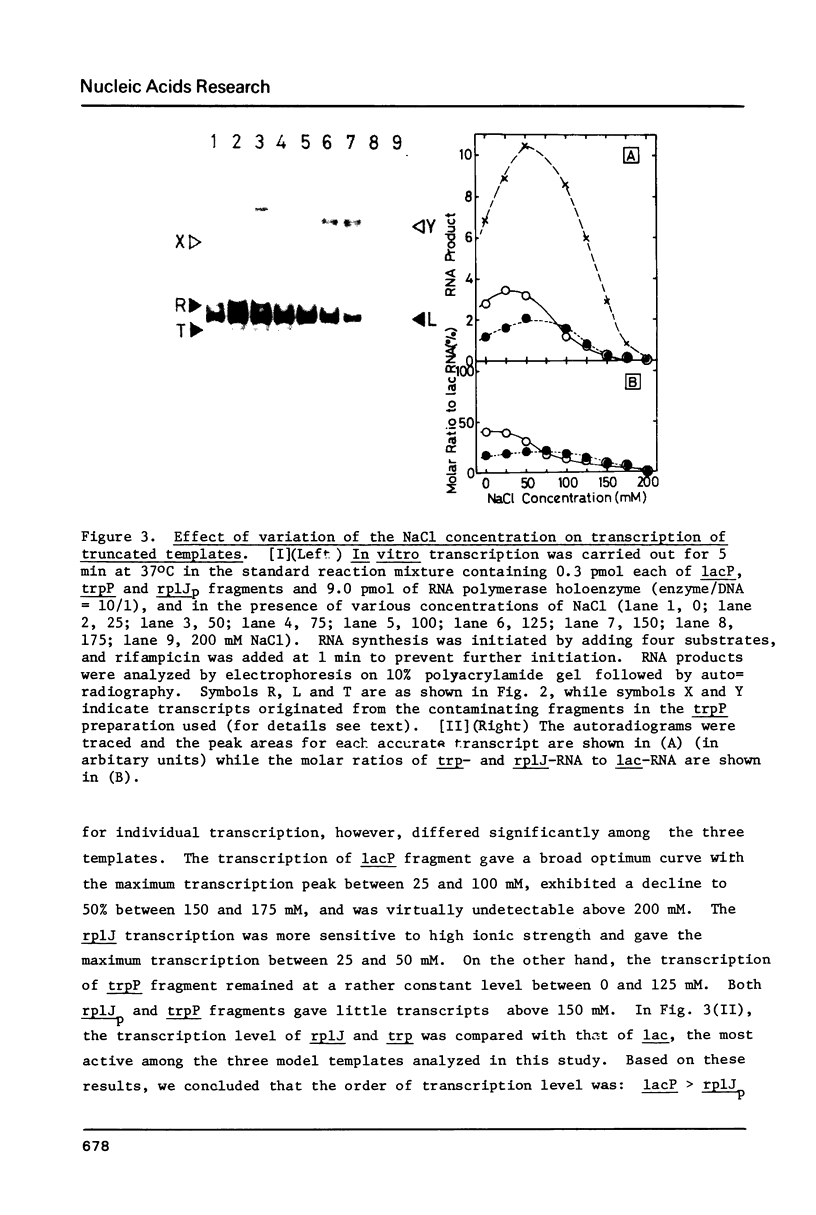
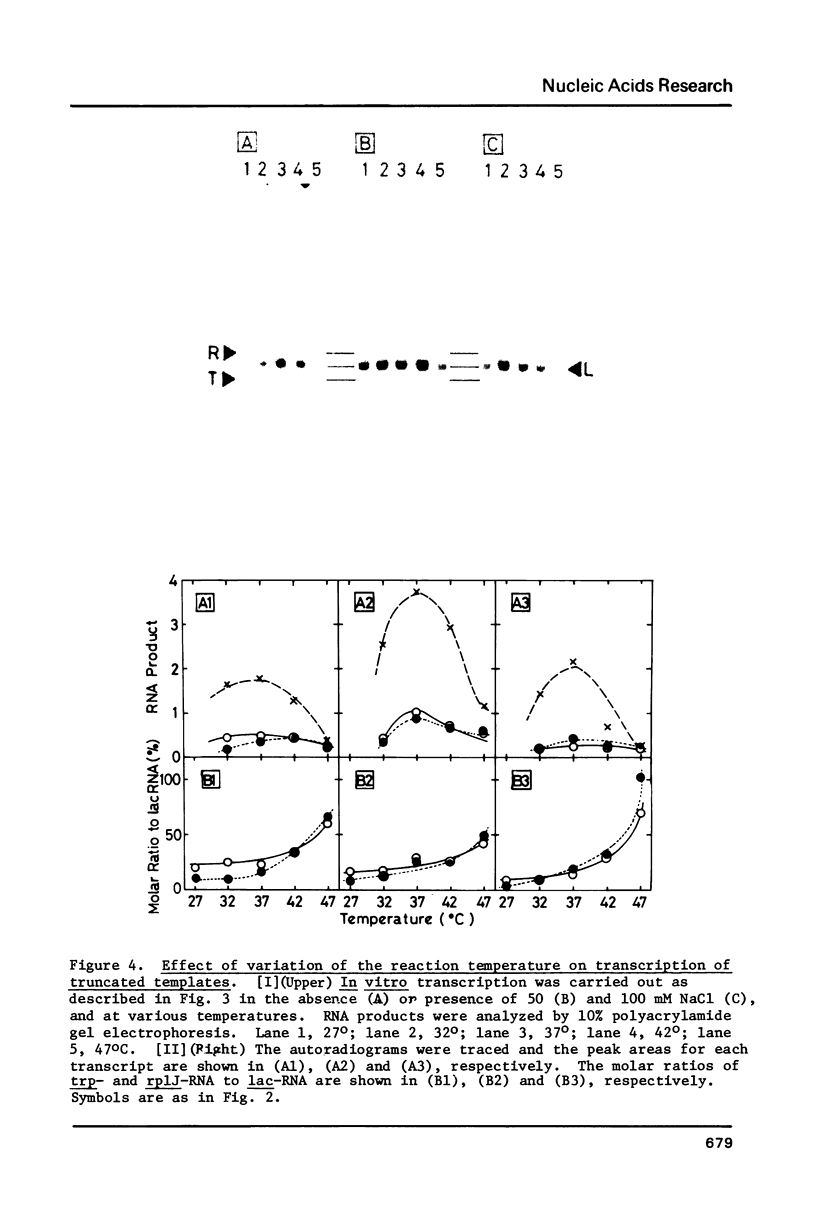
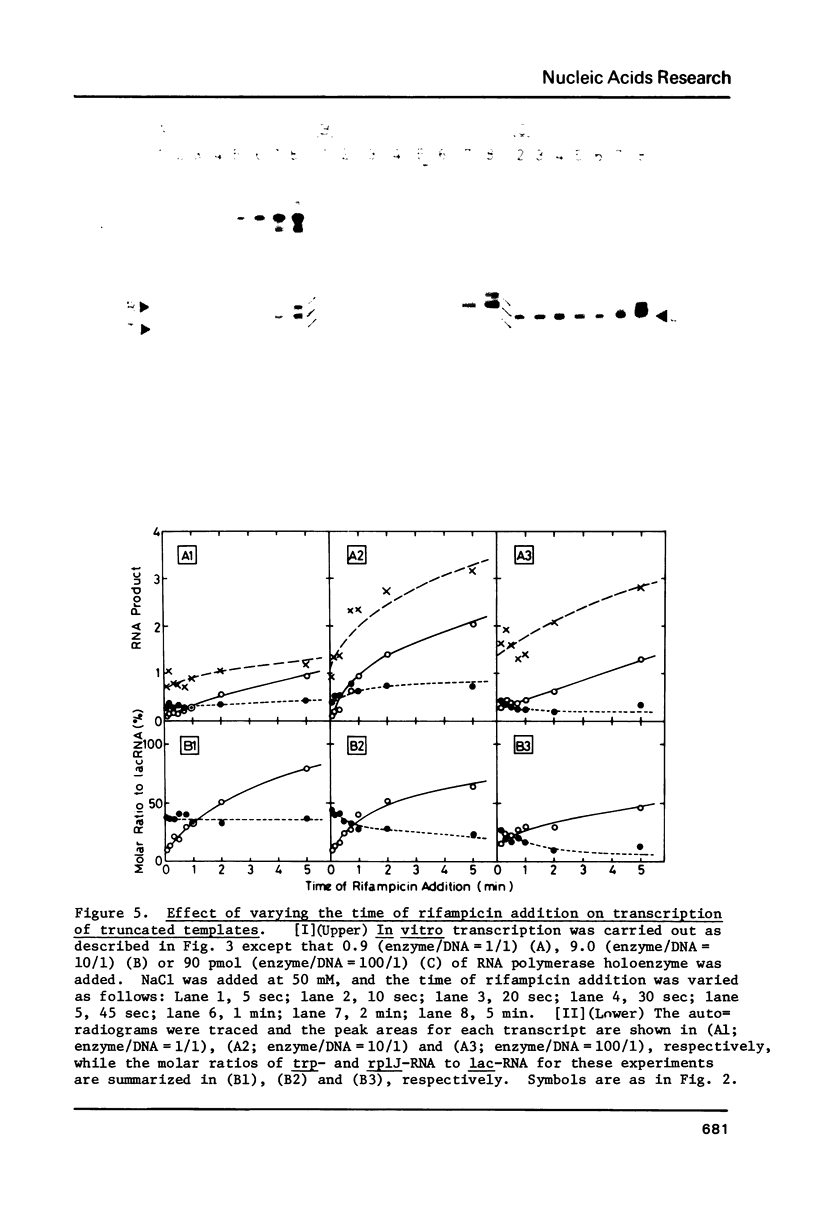
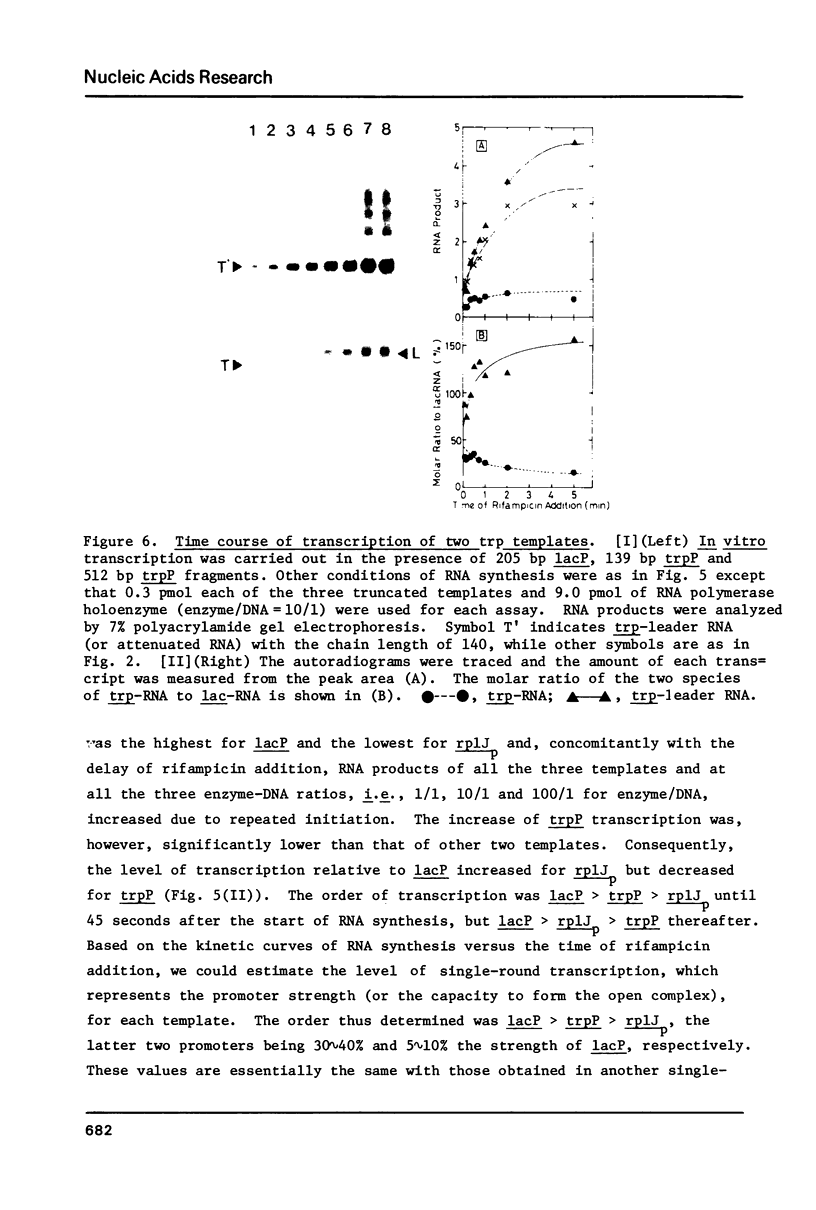
In vitro transcription was performed in a single reaction mixture, which contained three species of truncated E. coli DNA template, each carrying one specific promoter, lacP (UV5), trpP or rplJp, and the transcripts of distinct sizes were analyzed by electrophoresis on the same gel. Using this "mixed transcription" system, the order of the promoter strength, i.e., the capacity to form stable open complex, was determined in the single-round transcription under the standard conditions (50 mM NaCl and 37 degrees C) to be lacP greater than trpP greater than rplJp, the latter two promoters being 30--40% and 5--10% the strength of lacP, respectively. After the multiple-round transcription, however, the level of trp transcription was the lowest due to low cyclic-reaction rate but became the highest when another trp fragment containing the natural terminator was used as the template. The order of the transcription level also varied depending on the ionic strength and the reaction temperature and, as a result, lacP was no more the strongest under high salt concentration and at high temperature.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bremer H. Chain growth rate and length of enzymatically synthesized RNA molecules. Mol Gen Genet. 1967;99(4):362–371. doi: 10.1007/BF00330911. [DOI] [PubMed] [Google Scholar]
- Crouch R. J., Hall B. D., Hager G. Control of gene transcription in T-even bacteriophages: alterations in RNA polymerase accompanying phage infection. Nature. 1969 Aug 2;223(5205):476–479. doi: 10.1038/223476a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Enami M., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. XII. Noncoordinate synthesis of core enzyme subunits after suppression of cell growth. Mol Gen Genet. 1982;185(3):373–378. doi: 10.1007/BF00334127. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Iwakura Y., Ishihama A. Heterogeneity of RNA polymerase in Escherichia coli. I. A new holoenzyme containing a new sigma factor. J Mol Biol. 1974 Mar;83(3):353–367. doi: 10.1016/0022-2836(74)90284-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez N., Wiggs J., Chamberlin M. J. A simple procedure for resolution of Escherichia coli RNA polymerase holoenzyme from core polymerase. Arch Biochem Biophys. 1977 Aug;182(2):404–408. doi: 10.1016/0003-9861(77)90521-5. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kawakami K., Saitoh T., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. IX. Growth-dependent variations in the synthesis rate, content and distribution of RNA polymerase. Mol Gen Genet. 1979 Jul 13;174(2):107–116. doi: 10.1007/BF00268348. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Isolation and properties of the transcription complex of Escherichia coli RNA polymerase. Biochim Biophys Acta. 1975 Aug 6;402(1):88–104. doi: 10.1016/0005-2787(75)90373-1. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Nüsslein C., Schaller H. Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem. 1977 Mar 15;74(1):107–113. doi: 10.1111/j.1432-1033.1977.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. D. Kinetic investigation of the mechanism of RNA polymerase binding to mutant lac promoters. J Biol Chem. 1980 Nov 10;255(21):10423–10430. [PubMed] [Google Scholar]
- Tacon W., Carey N., Emtage S. The construction and characterisation of plasmid vectors suitable for the expression of all DNA phases under the control of the E. coli tryptophan promoter. Mol Gen Genet. 1980 Feb;177(3):427–438. doi: 10.1007/BF00271481. [DOI] [PubMed] [Google Scholar]
- Travers A. RNA polymerase specificity and the control of growth. Nature. 1976 Oct 21;263(5579):641–646. doi: 10.1038/263641a0. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Yanofsky C. Pausing of RNA polymerase during in vitro transcription of the tryptophan operon leader region. Biochemistry. 1981 Jun 23;20(13):3738–3744. doi: 10.1021/bi00516a011. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]