Abstract
Neurofibromatosis type 2 (NF2) is an inherited predisposition cancer syndrome characterized by the development of multiple benign tumors in the nervous system including schwannomas, meningiomas, and ependymomas. Using a disease model comprising primary human schwannoma cells, we previously demonstrated that adherens junctions (AJs) are impaired in schwannoma cells because of a ubiquitous, upregulated Rac activity. However, the mechanism by which loss of contact inhibition leads to proliferation remains obscure in merlin-deficient tumors. In this study, we show that proliferative Wnt/β-catenin signaling is elevated as active β-catenin (dephosphorylated at serine 37 and threoine 41) localizes to the nucleus and the Wnt targets genes c-myc and cyclin D1 are upregulated in confluent human schwannoma cells. We demonstrate that Rac effector p21-activated kinase 2 (PAK2) is essential for the activation of Wnt/β-catenin signaling because depletion of PAK2 suppressed active β-catenin, c-myc, and cyclin D1. Most importantly, the link between the loss of the AJ complex and the increased proliferation in human schwannoma cells is connected by Src and platelet-derived growth factor receptor-induced tyrosine 654 phosphorylation on β-catenin and associated with degradation of N-cadherin. We also demonstrate that active merlin maintains β-catenin and N-cadherin complex at the plasma membrane through direct regulation. Finally, we demonstrate that phosphorylation of tyrosine 654 is critical for the increased proliferation in human schwannoma cells because overexpression of a Y654F mutant β-catenin reduces hyperproliferation of schwannoma cells. We suggest a model that these pathways are coordinated and relevant for proliferation in merlin-deficient tumors.
Introduction
Neurofibromatosis type 2 (NF2) is an inherited predisposition cancer syndrome characterized by the development of multiple benign tumors in the nervous system including schwannomas, meningiomas, and ependymomas. NF2 affects 1 in 30,000 people and leads to significant medical problems, namely, substantial morbidity and reduced life span. The tumor suppressor protein merlin, which belongs to the ezrinradixin-moesin (ERM) family, is the protein product of the NF2 gene. Loss of merlin also causes sporadic tumors, namely, all schwannomas, a proportion of ependymomas, and most meningiomas [1]. Like other ERM proteins, merlin can forma C-terminal tail-FERM (Band 4.1, ezrin, radixin, and moesin) interaction and switch between open and closed conformation through changing phosphorylation status on serine 518. Merlin localizes to the cortical cytoskeleton [2] and the nucleus [3]. At the plasma membrane, merlin exerts inhibitory effects by regulating growth factor receptors, integrins, and downstream signaling pathways, for example, Rac/PAK/JNK, integrins/Src/FAK, PI3K/Akt, mTOR, and Raf/MEK/ERK pathways [4–10]. In the nucleus, merlin suppresses tumorigenesis by binding to the E3 ubiquitin ligase CRL4DCAF1, inhibiting its activity [3].
Loss of contact inhibition is a hallmark of merlin-deficient tumors. The potential mechanism for this has been investigated by analyzing the relationship between merlin and Rac/PAK, CD44, Paxillin, epidermal growth factor receptor, and other growth factor receptors [5,8,11,12]. Although a colocalization and a physical interaction between merlin and the core members of the adherens junction (AJ) complex, namely, cadherins and catenins, had been observed [13], it is still unclear how merlin directly inhibits proliferation by regulating cadherins and catenins.
The Wnt signaling transduction pathway plays an important role in tumorigenesis [14]. The stability of β-catenin, the core player of the canonical Wnt pathway, is strictly regulated in the cell cytoplasm. Tyrosine phosphorylation, which was detected on β-catenin in schwannoma cells [15], is the first step of β-catenin translocation from the AJs to the cytoplasm [16]. In the absence of Wnt signaling, a deconstruction complex comprising axin, GSK-3, and APC is formed, leading to the proteolytic degradation of β-catenin. Nevertheless, while Wnt is present, serine 37 and threoine 41 on cytoplasmic β-catenin are dephosphorylated and β-catenin is shuttled into the nucleus [17]. Nuclear translocation of β-catenin is dependent on the phosphorylation of Ser191 and Ser605 mediated by the active Rac1/JNK2 pathway; mutations of these residues significantly affect β-catenin nuclear accumulation and Wnt signaling [18]. In the nucleus, β-catenin will then interact with the TCF/LEF family of transcription factors and activate Wnt transcription target genes, for example, cyclin D1 and c-myc [14].
In a recent study, Wnt/β-catenin signaling, which was measured by the expressions of Wnt target genes and TCF activity, was found to be significantly increased in NF2 knockout mouse embryonic fibroblast in confluent cell cultures, which was reduced by knockdown or inhibition of Rac1 [19]. The increase in Wnt signaling had also been correlated with merlin deficiency in human malignant gliomas [20]. In human primary schwannoma cells, increased activity of Rac1/PAK/JNK [7,15,21] and colocalization of phosphorylated tyrosine (antibody) and β-catenin [15] prompted us to examine the fate and roles of β-catenin and related growth factor signaling in schwannoma cells. More importantly, the mechanism by which merlin loss leads to increased Wnt/β-catenin in NF2 was investigated. We show that increased nuclear β-catenin signaling is initiated at the AJs and is regulated by tyrosine kinase receptors PDGFR/Src and the Rac1/PAK/JNK pathway in human schwannoma cells.
Materials and Methods
Cell Culture
Human schwannoma cells were kindly provided by NF2 patients after informed consent. Diagnosis of NF2 was based on clinical criteria defined by the National Institutes of Health consensus conference on neurofibromatosis. Normal human Schwann cells from healthy nerve donors were used in this research. Isolation and culturing were carried out as previously described [22]. Briefly, cells were cultured in proliferation medium and grown on poly-l-lysine/laminin-coated six-well plates (Greiner, Bio One, Stonehouse, United Kingdom) or eight-well permanox chamber slides (Lab-Tek, Nunc, Wiesbaden, Germany). HEK293T cells were cultured in standard conditions. All experiments were performed on starved confluent cells unless otherwise stated, and all experiments were repeated with cells from at least three different patients.
Expression Vectors and Transfection
We used expression vectors pcDNA3.2/V5 (Invitrogen, Carlsbad, CA) encoding merlin I mutants S518D and S518A (a gift from David H Gutmann, Washington University, St. Louis). β-Catenin wild-type (WT) full-length complementary DNA was purchased from Open Biosystems Abgene (Surrey, United Kingdom) and then cloned into pENTR11 vector (Invitrogen). β-Catenin mutants Y654F was generated by the GeneTailor site-directed mutagenesis kit (Invitrogen) according to the manufacturer's instructions. The primers were designed as previously described [23]. The WT and mutation were then subcloned into a GFP-tagged gateway cloning vector pDEST53 (Invitrogen). All transfections were carried out with Lipofectamine 2000 (Invitrogen) or Fugene 6 (Roche, Applied Sciences, Indianapolis, IN).
Coimmunoprecipitation and Immunoblot Analysis
Immunoprecipitation and immunoblot analysis were performed using standard protocols with NP40 lysis buffer (20 mM Tris, 137 mM NaCl, 10% glycerol, and 1% NP40). Proteins were detected using ECL Plus Western Blotting Detection System (Amersham Biosciences, Buckinghamshire, United Kingdom). RhoGDI, which has been shown not to be regulated in our system [24], was used as loading control. Densities of bands were quantified using FluorS-Multi-Imager and Quantity One software (BioRad Laboratories, Hercules, CA). The following antibodies were used: β-catenin 1:1000, N-cadherin mouse 1:500 (BD Biosciences, Palo Alto, CA); active β-catenin (ABC) 1:250 (Millipore, Billerica, MA); β-catenin Y654 1:500 (Abcam, Cambridge, MA); anti-cyclin D1 1:2000, p21-activated kinase 2 (PAK2) 1:1000 (Cell Signaling Technology, Danvers, MA); and c-myc 1:250, merlin (C-18) 1:500, RhoGDI 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA).
Immunofluorescence
In brief, cells were fixed with 4% paraformaldehyde, permeablized with 0.2% Triton X-100, and blocked with 10% normal goat serum. Cells were incubated overnight with primary antibodies: β-catenin 1:500, N-cadherin mouse 1:500, ABC 1:250, N-cadherin rabbit 1:500 (Santa Cruz Biotechnology), PAK2 1:250, and anti-GFP 1:500 (Invitrogen). Appropriate Alexa Fluor 488- or Alexa Fluor 594-(Invitrogen) labeled secondary antibodies were used. Alexa Fluor 488-labeled phalloidin (1:100; Invitrogen) was used to visualize filamentous actin. 4,6-Diamidino-2-phenylindole (DAPI; Sigma-Aldrich) was used for nuclear staining. Cells were mounted with VectaShield (Vector Laboratories, Burlingame, CA). As a control, the primary antibody was omitted once in every experiment. Cells were examined with a Zeiss Meta510 confocal microscope using a 403 or 633 oil objectives and the Zeiss LSM software package (Zeiss, Jena, Germany).
Proliferation Assay
Schwannoma cells were seeded into Lab-Tek at 80% to 90% confluency, then transfected with empty vector pDEST53-GFP and β-catenin-Y654F, respectively. Cells were then starved for 24 hours and stimulated with GFM for another 24 hours before immunostaining as described previously. The DAPI- and Ki67- (Dako UK Ltd., Ely, United Kingdom) stained cells were counted manually. The percentage of Ki67-positive cells was calculated. Experiments were repeated three times and with different tumors.
Pervanadate and Inhibitors Treatment
To detect specific tyrosine phosphorylation signal of β-catenin, starved cells were treated with freshly prepared pervanadate (40 mM sodium orthovandate, 0.3% [wt/wt] H2O2, and 200 µg/ml catalase) for 30 minutes. Small molecule inhibitors imatinib (10 µM; Novartis, Surrey, United Kingdom) and SU6656 (4 µM, a gift from Jyrki Kukkonen, University of Helsinki) were added into the growth medium for 30 minutes of treatment at the same time as pervanadate. For immunostaining, cells were treated with imatinib (10 µM) and SU6656 (4 µM) for 24 hours before fixation. The proteasome inhibitor MG132 (1 µM; Sigma-Aldrich) was added into growth medium for 24 hours of treatment.
Lentiviral Short Hairpin RNA Knockdown
A set of pLKO.1-shRNA plasmid encoding a short hairpin RNA (shRNA) with scrambled sequence or sequences targeting human PAK2 (NM_002577), was purchased from Open Biosystems. After first-round selection in 293T cells, shRNA clones sh-PAK2a TRCN0000002118 (5′-TGGGAATGGAAGGATCTGTTA-3′) and sh-PAK2b TRCN0000002115 (5′-CTCTAGGAACCAAAGTGATTT-3′) were chosen for lentivirus production based on knockdown efficiency following the protocol described in the RNAi Consortium Protocols Section II (The RNA: Consortium). To infect schwannoma cells, on day 1, the appropriate amount of cells was seeded in a six-well plate or Lab-Tek to reach 70%confluency; on day 2, cells were infected with 50% virus (vol/vol) in the full growth medium containing 8 µg/ml protamine sulfate; and on day 3, growth media were replaced, 4 µg/ml puromycin was added into medium for selection for 48 hours. The knockdown efficiency of each individual clone against the scramble control was verified by Western blot analysis.
Results
Wnt Signaling Is Increased in Primary Human Schwannoma Cells in a PAK2-Dependent Manner
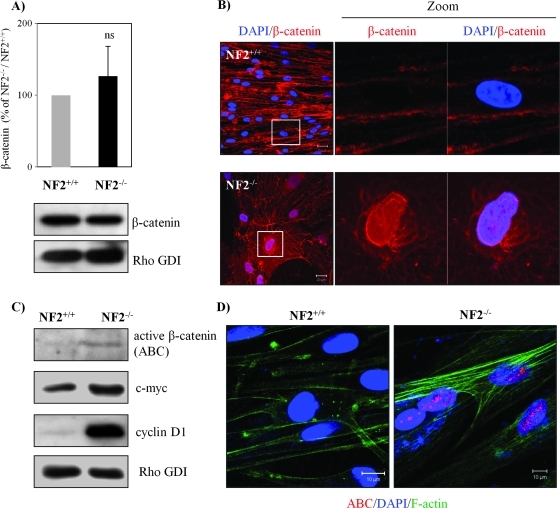
To confirm whether Wnt signaling is involved in merlin-deficient tumors and to analyze primary human tumor cells, we used normal Schwann cells (NF2+/+) and schwannoma cells (NF2-/-). Merlin accumulates under serum-deprived or -confluent conditions in normal Schwann cells [25], and therefore, to address how the loss of merlin leads to loss of contact inhibition of proliferation, all our experiments were performed with serum starved and confluent cells. Our investigation initiated from comparing the expression of β-catenin between Schwann and schwannoma cells; however, no significant difference was observed (Figure 1A), which supports previous findings [13,15,19]. We next investigated the localization of β-catenin by immunostaining. As expected, the localization of β-catenin at AJ was disrupted. β-Catenin was, instead, ubiquitously distributed in the cytoplasm and in the nucleus in schwannoma cells but not in Schwann cells (Figure 1B). To confirm the increase of Wnt signaling in schwannoma cells, we compared the expression levels of the components of the Wnt signaling pathway including ABC (dephosphorylated on Ser37 and Thr41), c-myc, and cyclin D1. In comparison to Schwann cells, a strong elevation of all Wnt signaling markers was detected in schwannoma cells (Figure 1C) that agrees with previous findings in NF2 knockout mouse embryonic fibroblast. In addition, the fact that ABC is shuttled into the nucleus was proven by immunostaining using anti-ABC antibodies detecting nuclear localization of ABC in schwannoma cells (Figure 1D). Taken together, our data show that canonical Wnt signaling is indeed elevated in primary human schwannoma cells and not in normal human Schwann cells.
Figure 1.
Wnt signaling is elevated in human schwannoma cells. (A) Western blot analyses were carried out to compare the levels of β-catenin between Schwann and schwannoma cells. RhoGDI was used as a loading control between Schwann and schwannoma cells. No significant difference (ns) was observed after quantification and statistical analysis (n = 5). Error bars represent the mean ± SEM. (B) β-Catenin localizes in the nucleus of schwannoma but not Schwann cells. Confluent and 24-hour starved human Schwann (NF2+/+) and schwannoma cells (NF2-/-) were immunostained with DAPI (nuclear marker) and β-catenin. Scale bar, 20 µm. (C) ABC and Wnt target genes are elevated in schwannoma. Western blot analyses were carried out to compare the level of ABC, c-myc, and cyclin D1. (D) The nuclear portion of β-catenin in schwannoma is ABC. Immunostaining was carried out for ABC, DAPI, and cytoskeleton marker F-actin in Schwann and schwannoma cells. Scale bar, 10 µm.
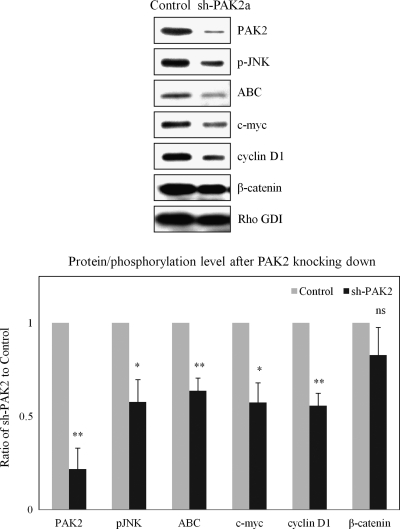
A recent study confirmed that Wnt signaling promotes the loss of contact inhibition of proliferation in a Rac-dependent manner [19]. In primary human endothelial cells, a loop connecting merlin, Rac, and PAK has been proposed to be responsible to the loss of contact inhibition [8]. Therefore, we suppressed Rac/PAK with a shRNA construct to determine whether the Wnt pathway is dependent on the Rac/PAK pathway in schwannoma cells. Our data suggested that PAK2 is dominant in human schwannoma cells (Figure W1A) and PAK2 is activated as phosphorylation at Ser192/197, which could be detected in schwannoma cells, because of a negative feedback loop with merlin [26]. Thus, PAK2 was selected as a target of Rac/PAK/JNK pathway here. The knockdown of PAK2 was confirmed by Western blot (Figure 2, top panel). As expected, the pJNK was decreased after knocking down PAK2. More importantly, significant reductions were observed in Wnt signaling markers (ABC, c-myc, and cyclin D1) in sh-PAK2a cells compared with the scrambled control. These results were further supported by the second shRNA construct in both Western blot (Figure W1B, upper panel) and immunostaining (Figure W1B, lower panel) because ABC in the nucleus was largely reduced after PAK2 knock down. These data suggest that PAK2, as a part of the Rac/JNK pathway, indeed play a critical role in the enhanced Wnt/β-catenin signaling in human schwannoma cells.
Figure 2.
Knockdown PAK2 suppresses Wnt/β-catenin signaling. Schwannoma cells were infected with shRNA encoding a sequence targeting human PAK2 (sh-PAK2a) or a matched scramble sequence (Control). Western blot analyses were carried out to compare the levels of pJNK, ABC, c-myc, cyclin D1, and β-catenin between sh-PAK2a and control. Densities of bands were quantified and compared. Error bars represent the mean ± SEM. n = 3. *P < .05. **P < .01. ns indicates no significant difference. pJNK, ABC, c-myc, and cyclin D1 were significantly reduced after knocking down PAK2.
N-cadherin Is Degraded in Schwannoma Cells
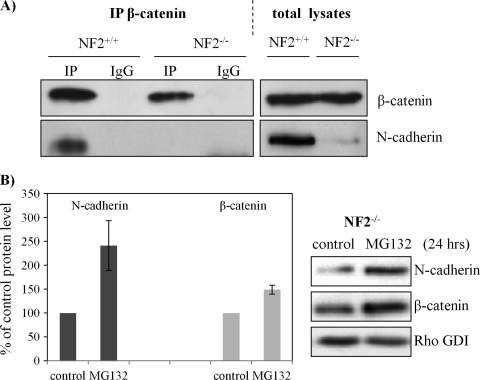
To further understand how Wnt/β-catenin is regulated by merlin in confluent cells, we investigated the AJ complex including β-catenin and N-cadherin in schwannoma cells by immunoprecipitation (E-cadherin not being expressed in our human schwannoma model [15]). The AJ complex was precipitated using an anti-β-catenin antibody (Figure 3A). We observed that N-cadherin is dramatically reduced in the β-catenin complex of schwannoma cells. Interestingly, we observed N-cadherin to be decreased in total lysates of schwannoma cells compared with Schwann cells.
Because N-cadherin is decreased in human schwannoma cells, we asked whether down-regulation of N-cadherin is a consequence of posttranslational modification. We tested whether N-cadherin is degraded through a proteasome-dependent pathway. The protein level of N-cadherin increased significantly after treatment with the proteasome inhibitor MG132 for 24 hours (Figure 3B). This observation supports previous studies showing that the ubiquitin proteasome system is required for degradation of transmembrane receptors, for example, cadherin proteins [27,28]. As expected, a small portion of cytoplasmic β-catenin was also degraded through a proteasome-dependent proteolysis (Figure 3B), which suggests that the maintenance of β-catenin stability is an ongoing process.
Figure 3.
N-cadherin reduced in schwannoma cells and its degradation. (A) Schwann (NF2+/+) and schwannoma cells (NF2-/-) were lysed in NP40 buffer and immunoprecipitated with an antibody against β-catenin. IgG mouse without anti-β-catenin antibody served as control. Western blot analysis was carried out to detect the levels of β-catenin and N-cadherin in both co-IP complex and total lysates. (B) N-cadherin is accumulated after MG132 inhibition. Schwannoma cells were treated with MG132 (1 µM) for 24 hours, Western blots were then carried out for N-cadherin and β-catenin. RhoGDI served as loading control. Error bars represent the mean ± SEM.
β-Catenin Y654 Phosphorylation Is Enhanced in Schwannoma Cells and Mediated by Src and PDGFR at the Plasma Membrane
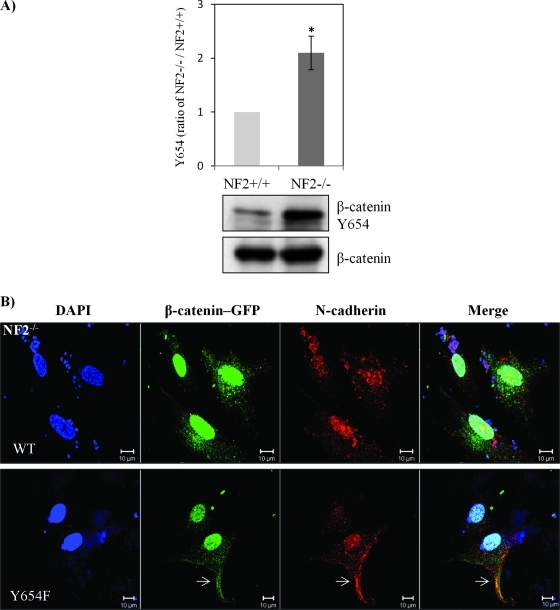
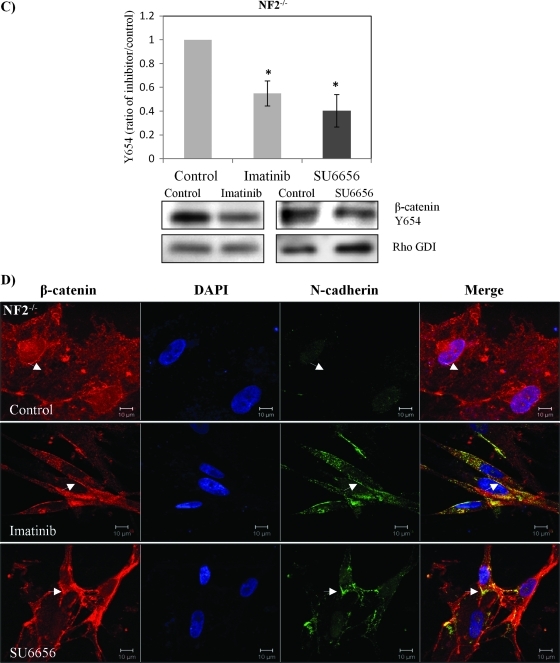
Despite β-catenin being turned over to some extent, we found evidence of increased nuclear localization of β-catenin in human schwannoma cells (Figure 1B). Phosphorylation of tyrosine 654 on β-catenin by the epidermal growth factor receptor or Src decreases its affinity to E-cadherin, therefore weakening the stability of AJs [29]. Because growth factor receptors (PDGFR and the EGF family) are activated in schwannoma [4] and activation of β-catenin by Rac/PAK requires phosphorylation at Y654, we wondered if β-catenin is phosphorylated at Y654 and if activation of growth factor receptors is linked to the tyrosine phosphorylation of β-catenin in schwannoma cells. As assessed by immunoblot analysis, Tyr654 phosphorylation was indeed elevated in human schwannoma compared with Schwann cells (Figure 4A). To confirm that Tyr654 phosphorylation is linked to N-cadherin localization, we constructed GFP-β-catenin chimeras containing either WT or a point mutation Y654F, which cannot be phosphorylated. To prove the specificity of the antibody used in immunostaining, we first transfected GFP-β-catenin-WT and Y654F constructs into HEK293T cells. As expected, β-catenin colocalized with N-cadherin at the AJ in those cells (Figure W2). Moreover, we transfected confluent human primary schwannoma cells with GFP-β-catenin-WT and GFP-β-catenin-Y654F (Figure 4B). After selection with antibiotic G418, cell's confluency was reduced, but the subcellular localization of proteins at the nucleus, and the plasma membrane, in cells having cell-cell contact were investigated. The staining shows that the WT and mutant forms of β-catenin localized to the nucleus in schwannoma cells, thus confirming the elevated Wnt signaling observed in Figure 1B. In addition, only overexpressed Y654F, but not WT, relocalizes endogenous N-cadherin back to the plasma membrane at least to some extent (Figure 4B). More than 80% of cells showed similar localization. Furthermore, using small molecule inhibitors, we tested whether Src and PDGFR, which are both overexpressed and activated in our primary human schwannoma cells [4], are upstream kinases of β-catenin. The Src inhibitor SU6656 reduced the Tyr654 phosphorylation by 40%, whereas the PDGFR inhibitor imatinib reduced Tyr654 signals by 55% (Figure 4C). Importantly, both imatinib and SU6626 restored the colocalization of β-catenin and N-cadherin back to the plasma membrane, especially at AJ sites (Figure 4D). Taken together, these data suggest that Src/PDGFR-mediated tyrosine phosphorylation of β-catenin at Y654 is involved in the disruption of AJs complex in human primary schwannoma cells.
Figure 4.
β-Catenin Y654 phosphorylation is enhanced in schwannoma cells and is mediated by Src and PDGFR at the plasma membrane. (A) β-Catenin Y654 phosphorylation is elevated in schwannomas. Confluent Schwann cells (NF2+/+) and schwannoma cells (NF2-/-) was starved for 24 hours before being treated with pervanadate for 30 minutes to reserve the phosphorylation signals. Western blot analysis was carried out to compare the level of phosphorylation of β-catenin at tyrosine 654. Total β-catenin served as control. Densities of bands were quantified and compared. (B) β-Catenin Y654F relocates N-cadherin to the membrane in schwannoma cells (NF2-/-). Cells were transfected with GFP-β-catenin-WT and GFP-β-catenin-Y654F, respectively. Immunostaining was carried out with DAPI, anti-GFP, and anti-N-cadherin. Scale bar, 10 µm. The sites at the plasma membrane are indicated by arrows. (C) PDGFR and Src inhibitors (imatinib and SU6656) reduced β-catenin Y654 phosphorylation. Schwannoma cells were starved for 24 hours and then treated with DMSO or different inhibitors as well as pervanadate for 30 minutes. Western blot analyses were carried out for β-catenin Y654. RhoGDI was used as a loading control. Densities of bands were quantified and compared. (D) PDGFR and Src inhibitors bring the colocalization of N-cadherin and β-catenin back to the plasma membrane. Cells were treated with control (DMSO), imatinib, and SU6656 for 24 hours. Immunostaining was then carried out with DAPI, anti-N-cadherin, and anti-β-catenin. The potential AJ sites are indicated by arrows. Scale bar, 10 µm.
Active Merlin Maintains Y654 Dephosphorylated β-Catenin and N-cadherin Complex at the Cell Surface to Inhibit Proliferation
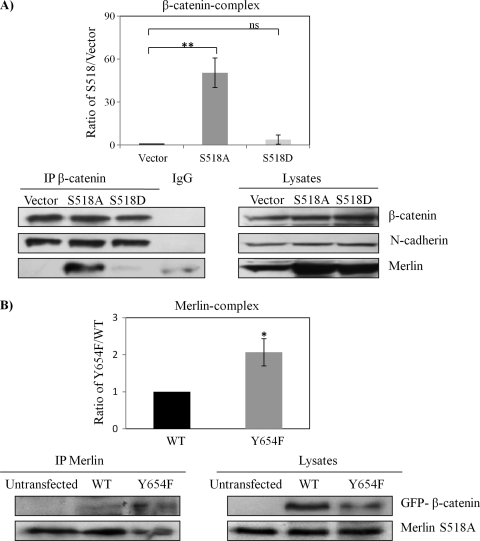
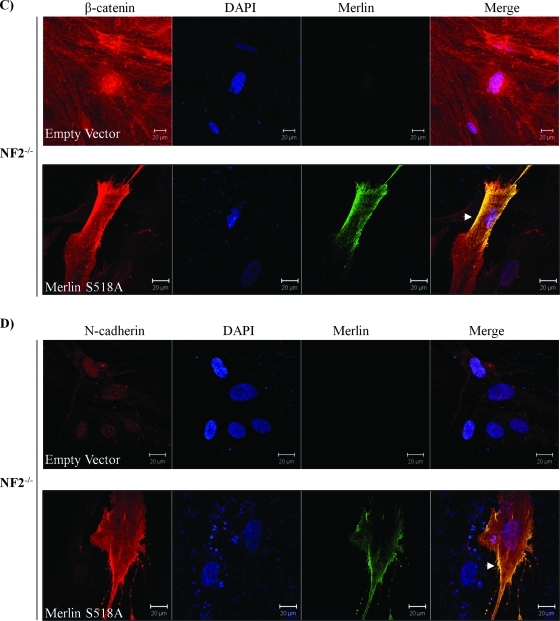
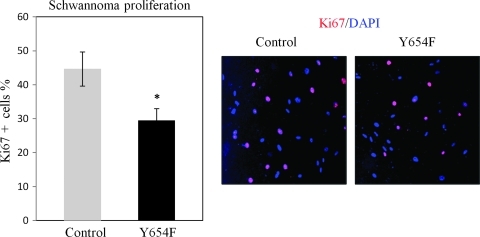
It is known that only Ser518 hypophosphorylated (active) merlin functions as tumor suppressor. If nuclear β-catenin is a main stimulator of transcriptional activity and proliferation in merlin-deficient tumors, one could hypothesize that Ser518 hypophosphorylated merlin would inhibit Wnt/β-catenin signaling through physical interaction and stabilization of β-catenin/N-cadherin complex at the AJs. We have therefore examined this hypothesis using traditional merlin mutants S518D (phosphomimic) and S518A (dephosphomimic). In coimmunoprecipitation (co-IP) experiments using HEK293T cells, we found that only merlin mutant S518A forms a complex with endogenous β-catenin, which constitutively binds to N-cadherin (Figure 5A). This interaction between S518A and β-catenin was confirmed by a co-IP with an antimerlin antibody in a cell line stably expressing Merlin-S518A stable line (Figure 5B). In addition, we observed that the S518A mutant of merlin preferentially binds to β-catenin-Y654F as shown in the immunoprecipitation assay (Figure 5B). In addition, the S518A mutant of merlin relocates β-catenin and N-cadherin back to the plasma membrane in human schwannoma cells (Figure 5, C and D). Finally and importantly, we tested whether Y654 β-catenin is important for the hyperproliferation of schwannoma cells. Because the PDGFR and Src are also upstream of other important mitogenic pathways, for example, the MEK/ERK pathway [4], their inhibitors are not suitable to address this question. Thus, we decided to overexpress β-catenin-Y654F in human primary schwannoma cells. Overexpression of β-catenin-Y654F significantly reduced proliferation, in contrast to the control overexpression of empty vector pDEST53-GFP (Figure 6) in schwannoma cells.
Figure 5.
Only Merlin S518A forms a complex with β-catenin and relocates AJs complex to the plasma membrane. (A) β-Catenin forms a complex with Merlin-S518A not Merlin S518D. HEK293T cells are transfected with empty vector, Merlin-S518A, and Merlin-S518D. Endogenous β-catenin complex was immunoprecipitated (IP) after treatment with pervanadate to reserve the phosphorylation status of β-catenin. IgG mouse without anti-β-catenin antibody served as a control. Immunoprecipitates were blotted for β-catenin. Merlin, N-cadherin, and RhoGDI served as controls. Densities of bands were quantified and compared. The protein level of merlin in the β-catenin complex was compared between Merlin S518 mutants and empty vector after being corrected with their count parts in total lysates. Error bars represent the mean ± SEM. n = 3. **P < .01. ns indicates no significant difference. (B) Merlin-S518A preferentially interacts with β-catenin-Y654F. Merlin-S518A stable line (HEK293T cells) was transfected with GFP-β-catenin WT and GFP-β-catenin-Y654F (Y654F), and untransfected cells. Merlin complex was immunoprecipitated and blotted for β-catenin and merlin. Densities of bands were quantified and compared. The protein level of β-catenin in the merlin complex was compared between WT and Y654F after being corrected with their count parts in total lysates. Error bars represent the mean ± SEM. n = 3. *P < .05. (C, D) Merlin-S518A relocates β-catenin and N-cadherin to the plasma membrane in schwannoma cells. Cells were transfected with either empty vector or Merlin-S518A. Immunostaining was carried out for merlin and β-catenin (C) or merlin and N-cadherin (D). DAPI was used as nuclear maker. The potential AJ sites are indicated by arrows. Scale bar, 20 µm.
Figure 6.
Overexpression of β-catenin Y654F inhibits the hyperproliferation of human schwannoma cells. Cells were transfected with GFP-β-catenin-Y654F (Y654F) and empty vector (control), starved for 24 hours, and stimulated with GFM for another 24 hours and then stained with DAPI and Ki67. The stained cells were counted manually; the Ki67-positive (ratio of Ki67 to DAPI) cells were calculated. Error bars represent the mean ± SEM. n = 3. *P < .05, t test.
Discussion
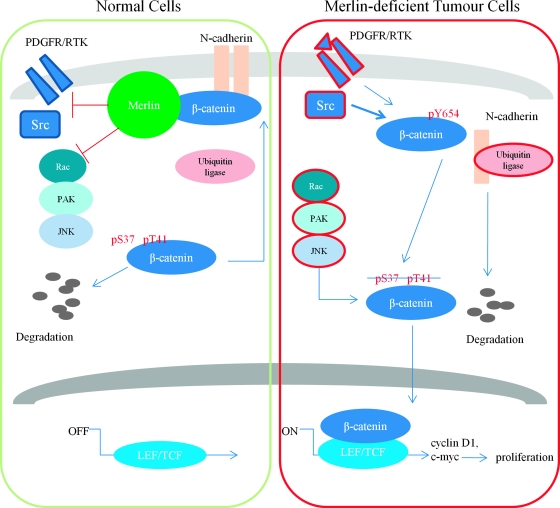
In this contribution, we show that, on confluency, canonical Wnt/β-catenin signaling is activated in primary human schwannoma cells, ABC localizes in the nucleus and Wnt target genes c-myc and cyclin D1 are overexpressed as a consequence of enhanced transcriptional activities (Figure 1). We also demonstrate that the elevated Wnt signaling is dependent on the Rac/PAK/JNK pathway (Figure 2). These observations agree well with previously published data in different cell types [19,20,30]. We then showed that hyperactivated Wnt signaling is initiated from tyrosine phosphorylation of β-catenin at the AJ sites (Figure 4, A and B) and associated with the degradation of N-cadherin (Figure 3). Furthermore, by using PDGFR inhibitor imatinib and Src inhibitor SU6656, we demonstrate the importance of Src/PDGFR kinases in activation of Wnt signaling in merlin-deficient schwannoma (Figure 4, C and D). Importantly, overexpression of β-catenin that cannot be phosphorylated at tyrosine 654 reduces proliferation of primary human schwannoma cells (Figure 6). Finally, we demonstrate that only merlin cannot be phosphorylated regulates AJ complex by maintaining the non-tyrosine phosphorylated β-catenin together with N-cadherin in the site of AJs (Figure 5). Taken together, our data suggest that Ser518 hypophosphorylated merlin inhibits Wnt/β-catenin signaling through regulation of Rac/PAK/JNK pathway and additionally Src/PDGFR kinases. Therefore, merlin loss leads to a coordinated activation of these pathways (Figure 7).
Figure 7.
Hypothetical model of merlin's role on inhibition of Wnt/β-catenin signaling. In normal cells, merlin inhibits Src/PDGFR, Rac/PAK/JNK, and maybe N-cadherin ubiquitination as well to keep Y654 unphosphorylated β-catenin staying at AJ together with N-cadherin. In addition, excess cytoplasmic β-catenin can be regulated by proteasome degradation. In merlin-deficient tumor cells, loss of merlin leads to activation of PDGFR and Src (red). β-Catenin is then phosphorylated by Src/PDGFR at Y654 and disassociated with N-cadherin. In further regulation by activated Rac/PAK/JNK (red), β-catenin shuttles into the nucleus to activate transcriptional factors LEF/TCF and then drive the expression of downstream targets, namely, cyclin D1, c-myc, and eventually, proliferation is increased in tumor cells.
Up-regulation/activation of β-catenin and down-regulation of E/N-cadherin have been reported in many tumors including meningiomas [31,32]. However, in a study of osteoblast and bone formation, it has been found that N-cadherin negatively regulates proliferation by decreasing Wnt/β-catenin signaling [33]. In mouse embryonic fibroblasts, Nf2 inactivation did not alter the expression of β-catenin and N-cadherin [13]. However, an increase of N-cadherin expression at the mRNA level and protein level was observed in primary Schwann cells derived from the Nf2flox/flox mouse model and human schwannoma tissue [34]. In our human primary schwannoma model, no change in N-cadherin expression was observed at the protein level in subconfluent schwannoma cells [15]. However, in accordance with data in different tumors including meningioma, a decrease in N-cadherin combined with strongly increased phosphorylation of β-catenin at Y654 was detected in confluent schwannoma cells, compared with Schwann cells (Figures 3A and 4A). It has been shown that after β-catenin phosphorylation at Y654, E-cadherin uncouples from β-catenin at the AJ and becomes a subject for endocytosis and E3 ligase-mediated ubiquitination and proteolysis [27]. In neurons, overexpression of the inactive β-catenin mutant Y654F prevents N-cadherin internalization, resulting in stabilization of surface N-cadherin molecules [35]. Therefore, we hypothesized that the down-regulation of N-cadherin in our schwannoma cells occurs by posttranslational degradation on confluency. This hypothesis was confirmed by the increased levels of N-cadherin on treatment with proteasome inhibitor MG132 (Figure 3B). In accordance with a previous study in human epidermoid carcinoma cells [36], our data suggest that β-catenin might dissociate from N-cadherin at the plasma membrane during N-cadherin internalization (Figures 3A and 4B, upper panel). Indeed, tyrosine phosphorylation of β-catenin at Y654, which is an Src phosphorylation site [37], plays a important role in AJ disassembly as overexpression of a mutant form of β-catenin (Y654F) restored the colocalization of N-cadherin and β-catenin (Figure 4B, lower panel). Importantly, overexpression of the mutant form of β-catenin reduced the hyperproliferation in human primary schwannoma cells (Figure 6). The importance of β-catenin Y654 phosphorylation in enhancing Wnt signaling supports previous in vivo findings in intestinal tumorigenesis, where overexpression of a phospho-mimicking β-catenin Y654E in a conditional knock-in mouse strongly potentiated Wnt signaling-dependent intestinal tumor initiation [38]. In addition, overexpression of the merlin mutant S518A in schwannoma cells also restored the localization of β-catenin and N-cadherin to the plasma membrane (Figure 5, C and D).
We have previously demonstrated that the Rac/PAK/JNK and Src/PDGFR pathways are activated [4,7] and linked Rac activity to the immature AJs in human schwannoma cells [15]. Our present data suggest that PAK2 is indeed a part of Rac/JNK signaling for the regulation of Wnt/β-catenin signaling (Figures 2 and W1B). This result supports previously report that elevation of Wnt/β-catenin signaling in NF2-/- mouse fibroblast is Rac dependent [19]. Importantly, for the first time, we demonstrate a link between Src/PDGFR and the loss of contact inhibition in human schwannoma cells (Figure 4, C and D). However, it is still unclear exactly how merlin inhibits phosphorylation of β-catenin through upstream kinases, for example, PDGFR and Src. One possible explanation could be the competition between merlin and Src for ErbB2 binding [39]. Immunoprecipitation experiments using traditional merlin mutants (S518A/D) suggest that only merlin mutant that cannot be phosphorylated forms a complex with β-catenin (Figure 5A). Merlin could compete with Src for binding to β-catenin as well as ErbB2. However, currently available data support a second possibility that merlin inhibits growth factor receptors or other RTK signaling pathways such as PDGFR by regulating their abundance on the cell surface [2]. Merlin forms a complex with AJ components through its binding partner, the Na+-H+ exchanger regulatory factor 1/ERM binding protein of 50 kDa (NHERF1/EBP50) rather than through direct interaction in Schwann cells [40]. It has been shown that NHERF1 connects components of the AJ complex to PDGFR to modulate the actin cytoskeleton [41]. More importantly, depletion of merlin causes a retarded degradation of PDGFR in schwannoma cells [4,42] and NHERF is thought to be involved in the regulation of PDGFR internalization in NF2-/- cells [42]. Therefore, it is likely that merlin inhibits the PDGFR abundance and disassembly of AJ through NHERF.
In summary, in primary human tumor cells from NF2 patients, merlin loss seems to lead to a coordinated increase of Rac/PAK/JNK and PDGFR/Src signaling, loss of contact inhibition including β-catenin Y654 phosphorylation, and N-cadherin degradation, resulting in increased Wnt/β-catenin signaling as shown in our model (Figure 7). This coordinated activation is relevant for proliferation of NF2 tumor cells.
Supplementary Material
Acknowledgments
The authors thank Professor David H. Gutmann for the constructs of merlin mutations and Professor Jyrki Kukkonen for SU6656 and Novartis for imatinib.
Abbreviations
- ABC
active β-catenin, dephosphorylated on Ser37 and Thr41
- AJ
adherens junction
- ERM
ezrin-radixin-moesin
- NF2
neurofibromatosis type 2
- NHERF1/EBP50
Na+-H+ exchanger regulatory factor 1/ERM binding protein
- PAK
p21-activated kinase
- PDGFR
platelet-derived growth factor receptor
Footnotes
The authors thank the Dr. Hadwen Trust for the funding support and the Dr. Hadwen Trust for Humane Research, the UK's leading medical research charity funding exclusively nonanimal research techniques to replace animal experiments. There are no conflicts of interest by any of the coauthors.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131:606–615. doi: 10.1093/brain/awm249. [DOI] [PubMed] [Google Scholar]
- 2.McClatchey AI, Fehon RG. Merlin and the ERM proteins—regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 5.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, Gusella JF, Ramesh V. NF2/Merlin is a novel negative regulator of mTOR complex 1 and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–1221. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 8.Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 10.Rong R, Tang X, Gutmann DH, Ye K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci USA. 2004;101:18200–18205. doi: 10.1073/pnas.0405971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Valle C, Tang Y, Ricard J, Rodenas-Ruano A, Taylor A, Hackler E, Biggerstaff J, Iacovelli J. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet. 2002;31:354–362. doi: 10.1038/ng930. [DOI] [PubMed] [Google Scholar]
- 12.Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlich P. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiyama T, Wnt/β-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–282. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 15.Flaiz C, Utermark T, Parkinson DB, Poetsch A, Hanemann CO. Impaired intercellular adhesion and immature adherens junctions in merlin-deficient human primary schwannoma cells. GLIA. 2008;56:506–515. doi: 10.1002/glia.20629. [DOI] [PubMed] [Google Scholar]
- 16.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 17.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosco EE, Nakai Y, Hennigan RF, Ratner N, Zheng Y. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene. 2010;29:2540–2549. doi: 10.1038/onc.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–5742. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68:7932–7937. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Mueller HW, Hanemann CO. Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis. 1998;5:55–64. doi: 10.1006/nbdi.1998.0179. [DOI] [PubMed] [Google Scholar]
- 23.Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in β-catenin are crucial in regulation of Met-β-catenin interactions. Exp Cell Res. 2006;312:3620–3630. doi: 10.1016/j.yexcr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanemann CO, Bartelt-Kirbach B, Diebold R, Kampchen K, Langmesser S, Utermark T. Differential gene expression between human schwannoma and control Schwann cells. Neuropathol Appl Neurobiol. 2006;32:605–614. doi: 10.1111/j.1365-2990.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 25.Shaw RJ, McClatchey AI, Jacks T, Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J Biol Chem. 1998;273:7757–7764. doi: 10.1074/jbc.273.13.7757. [DOI] [PubMed] [Google Scholar]
- 26.Flaiz C, Kaempchen K, Matthies C, Hanemann CO. Actin-rich protrusions and nonlocalized GTPase activation in merlin-deficient schwannomas. J Neuropathol Exp Neurol. 2007;66:608–616. doi: 10.1097/nen.0b013e318093e555. [DOI] [PubMed] [Google Scholar]
- 27.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 28.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem. 2003;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- 29.Huber AH, Weis WI. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 30.Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, Jhanwar S, Testa JR. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunner EC, Romeike BF, Jung M, Comtesse N, Meese E. Altered expression of β-catenin/E-cadherin in meningiomas. Histopathology. 2006;49:178–187. doi: 10.1111/j.1365-2559.2006.02440.x. [DOI] [PubMed] [Google Scholar]
- 32.Saydam O, Shen Y, Wurdinger T, Senol O, Boke E, James MF, Tannous BA, Stemmer-Rachamimov AO, Yi M, Stephens RM, et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathway. Mol Cell Biol. 2009;29:5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay E, Nouraud A, Marie PJ. N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling. PLoS One. 2009;4:e8284. doi: 10.1371/journal.pone.0008284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, Lampin A, Niwa-Kawakita M, Kalamarides M, Giovannini M. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–865. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 35.Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Lu Z, Ghosh S, Wang Z, Hunter T, Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 37.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 38.van Veelen W, Le NH, Helvensteijn W, Blonden L, Theeuwes M, Bakker ER, Franken PF, van Gurp L, Meijlink F, van der Valk MA, et al. β-Catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut. 2011;60:1204–1212. doi: 10.1136/gut.2010.233460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houshmandi SS, Emnett RJ, Giovannini M, Gutmann DH. The neurofibromatosis-2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol Cell Biol. 2008;29:1472–1486. doi: 10.1128/MCB.01392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangwala R, Banine F, Borg JP, Sherman LS. Erbin regulates mitogen-activated protein (MAP) kinase activation and MAP kinase-dependent interactions between Merlin and adherens junction protein complexes in Schwann cells. J Biol Chem. 2005;280:11790–11797. doi: 10.1074/jbc.M414154200. [DOI] [PubMed] [Google Scholar]
- 41.Theisen CS, Wahl JK, III, Johnson KR, Wheelock MJ. NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell. 2007;18:1220–1232. doi: 10.1091/mbc.E06-10-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraenzer JT, Pan H, Minimo L, Jr, Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.