Abstract
Consumption of spicy foods containing capsaicin, the major pungent principle in hot peppers, reportedly promotes negative energy balance. However, many individuals abstain from spicy foods due to the sensory burn and pain elicited by the capsaicin molecule. A potential alternative for nonusers of spicy foods who wish to exploit this energy balance property is consumption of nonpungent peppers rich in capsiate, a recently identified nonpungent capsaicin analog contained in CH-19 Sweet peppers. Capsiate activates transient receptor potential vanilloid subtype 1 (TRPV1) receptors in the gut but not in the oral cavity. This paper critically evaluates current knowledge on the thermogenic and appetitive effects of capsaicin and capsiate from foods and in supplemental form. Meta-analyses were performed on thermogenic outcomes, with a systematic review conducted for both thermogenic and appetitive outcomes. Evidence indicates that capsaicin and capsiate both augment energy expenditure and enhance fat oxidation, especially at high doses. Furthermore, the balance of the literature suggests that capsaicin and capsiate suppress orexigenic sensations. The magnitude of these effects is small. Purposeful inclusion of these compounds in the diet may aid weight management, albeit modestly.
Keywords: appetite, burn, oral irritation, palatability, pungency, thermogenesis
Introduction
The prevailing view is that obesity results from a small sustained positive energy balance (Hill et al. 2009). It has been proposed that modest diet and/or physical activity adjustments equivalent to 10 (Veerman et al. 2007) to 50 kcal/day or less (Hill et al. 2003) are sufficient to reverse the epidemic and that these minor changes will be more simply implemented and sustained in the long-term than radical dietary or behavioral modifications (Mattes 2008). Consequently, growing attention is being placed on the manipulation of foods and/or weight loss products with noncaloric bioactive ingredients that may influence energy balance modestly by altering energy expenditure (EE), substrate oxidation, and/or appetitive sensations (Kovacs and Mela 2006; Westerterp-Plantenga et al. 2006).
When individual food components are demonstrated to promote negative energy balance, palatability is a key consideration for encouraging their long-term inclusion in the diet. If followed, energy-restricted diets of varied nutrient compositions are capable of producing weight loss (Shai et al. 2008; Sacks et al. 2009). However, individuals with the resources to choose from an array of foods generally select the ones they consider most palatable (Glanz et al. 1998). Recommendations to increase consumption of bioactive food ingredients may be made, but if they are not implemented, there will be no impact on health (Westerterp-Plantenga et al. 2006).
There is a widespread desire among the public, healthcare providers, and third-party payers for managing overweight and obesity with dietary modifications (Pavlovich et al. 2004). Adoption of a healthful diet presents a highly cost-effective strategy for weight loss, with the potential for fewer negative side effects than lifetime pharmacological therapies or invasive surgical interventions (Dalziel and Segal 2007). Thus, if bioactive food ingredients influence energy balance and can be tolerated without significant side effects or the development of resistance to thermogenic and/or appetitive outcomes with long-term use, they offer great potential as dietary approaches to weight management.
The health effects of capsaicin-containing foods have been heralded in traditional medicine for centuries (Cichewicz and Thorpe 1996; Yamamoto and Nawata 2009), but their pungency, noted as a sensory burn, and propensity for eliciting gastrointestinal side effects (Lejeune et al. 2003; Belza and Jessen 2005) limit consumption in many individuals. Levels of intake are widely varied both within and between cultures. For example, in Mexico where chili peppers are a hallmark of the cuisine, a study classified high capsaicin consumers as those eating the equivalent of 9–25 jalapeno peppers per day (90–250 mg capsaicin) and low consumers as those who eat less than 3 jalapeno peppers per day (<30 mg capsaicin) (Lopez-Carrillo et al. 2003). In contrast, only 10.5% of individuals in the United States consume peppers of any kind on a daily basis (Smiciklas-Wright et al. 2002) and a study found that regular spicy food users, who consumed spicy foods 3 times a week or more, preferred a mean concentration of 3.6 ± standard error (SE) 0.6 mg capsaicin at spicy food–containing meals. Nonusers, who consumed spicy foods less than once a month, preferred a mean concentration of 0.6 ± SE 0.2 mg capsaicin at spicy food–containing meals (Ludy and Mattes 2011b) (Table 1).
Table 1.
Studies on the effect of capsaicin on thermogenesis and appetite
| Study | Participants | Experimental design/intervention | Thermogenesis | Appetite |
| Ludy and Mattes (2011b) | 14 ♂ and 11 ♀ in the United States (IN) Age 23.0 ± 0.5 years BMI 22.6 ± 0.3 kg/m213 regular spicy food users and 12 nonusers (mean ± SE) |
Randomized crossover Lunch containing: 1) 1 g RP (following high-FAT diet) 2) 1 g RP (following high-CHO diet) 3) 0 (control, following high-FAT diet) 4) 0 (control, following high-CHO diet) 5) Preferred dose RP orally (1.8 ± 0.3 g in users; 0.3 ± 0.1 g in nonusers) 6) Preferred dose RP in capsule form (1.8 ± 0.3 g in users; 0.3 ± 0.1 g in nonusers) RP: 1995 μg/g capsaicin, 247 μg/g nordihydrocapsaicin, and 1350 μg/g dihydrocapsaicin; 53 800 SHU(mean ± SE) |
↑ EE, core body temperature, and FAT oxidation (when consumed orally compared with in capsule form) ↓ Skin temperature |
↓ Preoccupation with food and desire to eat fatty/salty/sweet foods in nonusers but not users ↓ EI in nonusers, but not users No effect on desire to eat in general, fullness, prospective food intake, thirst, or hunger |
| Reinbach et al. (2010) | 17 ♂ and 23 ♀ in Denmark Age 24.6 ± 2.5 years BMI 22.5 ± 7 kg/m2 Likers of hot spices (mean ± SD) |
Randomized crossover (10 visits) “Starter meal”: 1) With or without chili peppers (0.3 g RP; 0.375 mg capsaicin) 2) With or without ginger 3) With or without mustard 4) With or without horseradish 5) With or without wasabi Ad libitum dinner |
n/a | ↑ Desire to eat sweet foods ↓ Desire to eat hot foodsNo effect on EI, food intake (g), hunger, satiety, or desire to eat bitter/fatty/salty/sour(results for RP only) |
| Smeets and Westerterp-Plantenga (2009) | 11 ♂ and 19 ♀ in the Netherlands Age 31 ± 14 years BMI 23.8 ± 2.8 kg/m2 (mean ± SD) |
Randomized crossover Lunch containing: 1) Capsaicin (1.03 g RP; 80 000 SHU) 2) No capsaicin |
No effect on EE or RQ | ↑ GLP-1 Tended to ↓ ghrelin No effect on self-reported satiety or PYY |
| Shin and Moritani (2007) | 10 sedentary ♂ in Japan Age 24.4 ± 1.9 years BMI 20.2 ± 1.7 kg/m2 (mean ± SE) |
Randomized crossover Supplement 1 h before exercise containing: 1) Capsaicin (150 mg) (capsules) 2) Placebo 30 min on cycle |
↑ FAT oxidation No effect on ANS activity |
n/a |
| Ahuja et al. (2007) | 14 ♂ and 22 ♀ in Australia Age 46 ± 12 years BMI 26.3 ± 4.6 kg/m2 <daily chili (∼ 90% naïve/infrequent consumers) (mean ± SD) |
Randomized crossover 4-week dietary periods containing: 1) Chili (30 g/day chili blend, 55% cayenne RP; 33 mg/day capsaicin) 2) Bland spice free |
No effect on RMR, RQ, FAT oxidation, BMI, FAT mass, or lean mass | No effect on total energy, PRO, FAT, or CHO intake |
| Ahuja et al.(2006) | 14 ♂ and 22 ♀ in Australia Age 46 ± 12 years BMI 26.3 ± 4.6 kg/m2 <daily chili (∼ 90% naïve/infrequent consumers) (mean ± SD) |
Randomized crossover 4-week dietary periods containing: 1) Chili (30 g/day chili blend, 55% cayenne RP; 33 mg/day capsaicin) 2) Bland spice free |
↓ EE BMI ≥ 26.3 kg/m2 No effect on EE using all BMIs or BMI < 26.3 kg/m2 |
n/a |
| Westerterp-Plantenga et al. (2005) | 12 Caucasian ♂ and 12 Caucasian ♀ in the Netherlands Age 25–45 years BMI 25 ± 2.4 kg/m2 Used to eating spicy foods ≥ 1×/week (mean ± SD) |
Randomized crossover 2-day treatments 30 min before every meal containing: 1) 0.9 g RP in tomato juice 2) 0.9 g RP in 2 capsules 3) Placebo in tomato juice 4) Placebo in 2 capsules RP (0.9 g): 2.25 mg capsaicin; 80 000 SHU |
n/a | ↓ EI (juice > capsules) ↑ Satiety and CHO intake ↓ Hunger, energy density, and FAT intake |
| Yoshioka et al. (2004) | 16 ♂ in Japan Age 22.4 ± 3.2 years Wt 79.4 ± 19.4 kg Ht 176.1 ± 6.7 cm (mean ± SD) |
Randomized crossover Soup (prelunch) containing: 1) Self-perceived moderate RP soup (0.064 ± 0.046 g; 0.192 ± 0.138 mg capsaicin; 55 000 SHU) and capsule (placebo) 2) Self-perceived strong RP soup (0.923 ± 1.377 g; 2.769 ± 4.131 mg capsaicin; 55 000 SHU) and capsule (placebo) 3) Soup (placebo) and self-perceived strong RP capsules (0.923 ± 1.377 g; 2.769 ± 4.131 mg capsaicin; 55 000 SHU) 4) Soup (placebo) and capsule (placebo)Ad libitum lunch until satiated(mean ± SD) |
Biphasic effect on SNS:PSNS (moderate = maximum tolerable > strongest/excessive) | Dose-dependent ↓ EI (soup = capsules) Dose-dependent ↓ FAT intake (soup = capsules) Positive correlation between EI and FAT intake Negative correlation between change in EI and RP ingested |
| Chaiyata et al. (2003) | 12 ♀ in Thailand Consumers of <10 g/day chili peppers |
Glucose drink containing: 5 g fresh chili pepper (Capsicum frutescens): 3.5 mg capsaicin |
↑ EE above RMR immediately after ingestion | n/a |
| Lejeune et al. (2003) | 91 subjects in the Netherlands Capsaicin: 12 ♂ and 30 ♀Placebo: 11 ♂ and 38 ♀ Age 18–60 years BMI 25–35 kg/m2 Not habitual capsaicin users |
Randomized double blind 4-week very low–energy diet intervention (mean ↓ 6.6 ± 2 kg or 7.8 ± 1.8% body wt) 3-month wt-maintenance period with supplements containing: 1) 135 mg/day capsaicin (capsules with 3 meals) 2) 0 (placebo) (mean ± SD) |
↑ REE during wt maintenance ↑ FAT oxidation No effect on percent or rate of regain |
No effect on hunger or satiety |
| Matsumoto et al. (2000) | 8 lean ♀ in Japan Age 19.6 ± 0.26 years BMI 21.0 ± 0.57 kg/m2 8 overweight/obese ♀ in Japan Age 20.1 ± 0.40 years BMI 28.8 ± 1.01 kg/m2 No long-term history of eating spices (mean ± SE) |
Randomized crossover Breakfast containing: 1) 3 mg capsaicin 2) 0 (control) |
↑ EE in lean but not overwt/obese ↑ Total, very low, low, and very low:total frequency heart waves (SNS activity) in lean but not overwt/obese Trend toward ↑ CHO oxidation in lean but no effect in entire group No effect on high-frequency heart waves (PSNS activity) in either group |
n/a |
| Yoshioka et al. (1999) (Study 1) | 13 Japanese ♀ Age 25.8 ± 2.8 years Wt 54.2 ± 6.4 kg Ht 157 ± 4 cm Accustomed to eating spicy foods (mean ± SD) |
Randomized crossover Breakfast containing: 1) 10 g RP (high FAT, 30 mg capsaicin) 2) 10 g RP (high CHO, 30 mg capsaicin) 3) 0 (high FAT, control) 4) 0 (high CHO, control) Ad libitum lunch buffet until satiated |
n/a | ↓ Lunch intake 1) PRO (HF > HC) 2) FAT (HF > HC) Self-reported appetitive sensations 1) ↓ Prospective food consumption before lunch, but ↑ prospective food consumption after lunch 2) ↓ Desire to eat and hunger immediately after breakfast and before lunch |
| Yoshioka et al. (1999) (Study 2) | 10 Caucasian ♂ Age 32.9 ± 7.8 years Wt 72.5 ± 10.1 kg Ht 175 ± 6 cm (mean ± SD) |
Randomized crossover Lunch appetizers containing: 1) 6 g RP (18 mg capsaicin) 2) 0 (control) Ad libitum lunch and snack buffet until satiated |
↑ SNS:PSNS Trend toward a negative correlation between SNS:PSNS and EI |
↓ Total energy and CHO intakes during lunch and snack |
| Yoshioka et al. (1998) | 13 Japanese ♀ Age 25.8 ± 2.8 years Wt 54.2 ± 6.4 kg Ht 157.3 ± 4.5 cm Hotness of habitual meals between RP and control meals (mean ± SD) |
Randomized crossover Breakfast containing: 1) 10 g RP (high FAT) 2) 10 g RP (high CHO) 3) 0 (control, high FAT) 4) 0 (control, high CHO) |
↑ EE (VO2) and FAT oxidation | ↓ Appearance, taste, and smell ↑ Hot sensation |
| Lim et al. (1997) | 8 ♂ long distance runners in Korea Age 20.8 ± 0.5 years Wt 58.5 ± 2.1 kg Ht 169.5 ± 1.8 cm (mean ± SE) |
Randomized crossover Breakfast containing: 1) 10 g RP (30 mg capsaicin) 2) 0 (control) Followed by 2.5 h rest, then 1 h cycling |
↑ CHO oxidation both at rest and during exercise ↑ Epinephrine and norepinephrine 30 min after meal No effect on VO2 |
n/a |
| Yoshioka et al. (1995) (Study 1) | 8 ♂ long distance runners Age 20.5 ± 1 years Wt 58.5 ± 5.6 kg Ht 169.5 ± 4.7 cm (mean ± SD) |
Randomized crossover Breakfast containing: 1) 10 g RP (30 mg capsaicin) 2) 0 (control) |
↑ CHO oxidationTended to ↑ EE (VO2) | n/a |
| Yoshioka et al. (1995) (Study 2) | 7 ♂ long distance runners Age 21.6 ± 0.7 years Wt 61.3 ± 11.6 kg Ht 170.9 ± 6.3 cm (mean ± SD) |
Randomized crossover Breakfast containing 10 g RP (30 mg capsaicin) followed by: 1) β-adrenergic blocker (propranolol) 2) Placebo |
Propranolol inhibited the initial ↑ in EE caused by RP | n/a |
Abbreviations: autonomic nervous system (ANS), carbohydrate (CHO), energy intake (EI), glucagon-like peptide 1 (GLP-1), not applicable (n/a), oxygen consumption (VO2), parasympathetic nervous system (PSNS), peptide YY (PYY), protein (PRO), red pepper (RP), resting energy expenditure (REE), resting metabolic rate (RMR), and Scoville Heat Unit (SHU).
The emergence of capsiate, a recently identified nonpungent capsaicin analog, presents a promising alternative for those who abstain from capsaicin-containing foods due to pungency. Currently, capsiate consumption among the general population is limited to dietary supplements. In the United States, capsiate is available as “CH-19 Sweet Extract” with recommendations to consume three 1 mg capsules once daily by mouth to “naturally increase basal metabolic rate” (Pellicore 2007). This paper critically reviews knowledge of the thermogenic and appetitive effects of capsaicin and capsiate administered to humans in both oral and encapsulated forms.
Overview of capsaicin and capsiate
Source
Red peppers are members of the Capsicum genus (Iwai et al. 2003) that contains over 200 pepper varieties with differing degrees of pungency (0–13 000 mg/kg capsaicin) (Kozukue et al. 2005). Hot red peppers contain pungent compounds called capsaicinoids. These include capsaicin, dihydrocapsaicin, nordihydrocapsaicin, and trace amounts of other compounds. Capsaicin is the major pungent principle, responsible for about 70% of the burn, of hot red peppers. The newly bred nonpungent red pepper variety, CH-19 Sweet (Capsicum annuum L.), contains capsinoids. These are nonpungent capsaicin analogs and include capsiate, dihydrocapsiate, and nordihydrocapsiate. Capsiate is the primary capsinoid present in CH-19 Sweet peppers (Iwai et al. 2003).
Physical and chemical properties
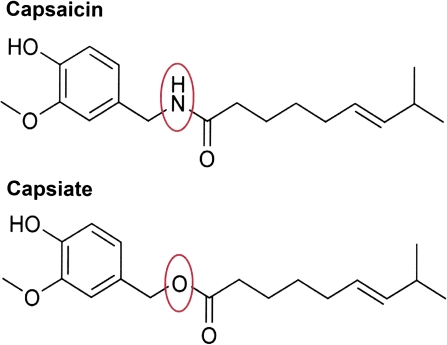
The chemical structures of capsaicin, (E)-N-(4-hydroxy-3-methoxybenzyl)-8-methylnon-6-enamide, and capsiate, 4-hydroxy-3-methoxybenzyl (E)-8-methyl-6-nonenoate, are similar, varying only at the center linkage (Figure 1). Capsaicin contains an amide bond, whereas capsiate contains an ester bond (Iwai et al. 2003).
Figure 1.
Chemical structures of capsaicin and capsiate vary only at the center linkage. Capsaicin (top) contains an amide bond between the vanillyl group and the fatty acid chain, whereas capsiate (bottom) contains an ester bond. This figure appears in color in the online version of Chemical Senses.
Pungency
When placed in the oral cavity, capsaicin diffuses across the lingual epithelium and selectively binds to transient receptor potential vanilloid subtype 1 (TRPV1) receptors on heat and pain sensitive sensory neurons. This receptor is a nonspecific calcium channel and when capsaicin binds to it, the channel opens (Caterina and Julius 2001). The initial influx of calcium leads to neurotransmitter release and sensations of warmth with low concentrations of capsaicin and burning pain with higher concentrations. Prolonged activation results in depletion of the presynaptic neurotransmitter, substance P, and desensitization causes reduced responsiveness to capsaicin (Iwai et al. 2003). Although the precise mechanisms are unknown, somatosensory experiences following ingestion of capsaicin vary between individuals (Astrup et al. 2010). One mechanism entails exposure frequency as capsaicin's rated irritancy is lower in regular spicy food users (Lawless et al. 1985; Cowart 1987; Stevenson and Yeomans 1993; Stevenson and Prescott 1994).
Absorption, distribution, and systemic activity
Both capsaicin and capsiate bind with high affinity to the TRPV1 receptor and are passively absorbed, with greater than 80% efficiency, in the stomach and upper portion of the small intestine (Kawada et al. 1984; Iwai et al. 2003). Limited pharmacological data suggest that capsaicin is initially more stable than capsiate and is metabolized by various cytochrome P450 enzymes in the liver. Capsiate is cleaved earlier and is nondetectable in the portal circulation (Takanohashi et al. 2010). Derivatives of capsaicin and capsiate, including vanillylamine, vanillin, vanillyl alcohol, and vanillic acid, are generated in both free and glucuronidated forms (Iwai et al. 2003; Takanohashi et al. 2010). However, the activity of these derivatives, and whether they are the bioactive forms or inactive metabolites, is currently unknown.
Once released in the circulation, albumin transports capsaicin to the adrenal gland where it stimulates the release of catecholamines (Kawada, Watanabe, et al. 1986; Kawada et al. 1988; Reinbach 2008). The assessment of catecholamine concentration in humans has been limited due to the challenge of the crude, indirect measurements provided by pharmacologic blockade/stimulation, and the requirement for radioactive tracer infusion to assess steady-state turnover (Ravussin and Tataranni 1996). The sole study exploring catecholamine secretion following capsaicin ingestion in humans reported that the increase in EE following a 30 mg capsaicin breakfast was abolished by concurrent administration of the β-adrenergic blocker propranolol in young male long distance runners. This implies that capsaicin's effects are dependent on β-adrenergic stimulation (Yoshioka et al. 1995) (Table 1). These findings have been supported by animal studies indicating that capsaicin-stimulated catecholamine release enhances lipolysis in adipocytes, glycogenolysis in the liver, and substrate oxidation in muscle (Kawada et al. 1984; Watanabe et al. 1994; Reinbach 2008).
Differences in the perceived pungency of capsaicin and capsiate are related to the site of TRPV1 activation. Capsaicin activates TRPV1 receptors on neurons located in the tongue, whereas capsiate is hydrolyzed as it crosses the oral mucosa, rendering it an ineffective sensory stimulus. Both capsaicin and capsiate have equal potency for activating TRPV1 receptors in the gut, leading to similar increases in sympathetic nervous system (SNS) activation (Snitker et al. 2009).
Most, but not all (Shin and Moritani 2007), studies involving capsaicin (Lim et al. 1997; Yoshioka et al. 1999, 2004; Matsumoto et al. 2000; Hachiya et al. 2007) and capsiate (Hachiya et al. 2007; Josse et al. 2010) (Tables 1–3) report enhanced SNS activation. Weight management effects are purportedly derived from the increase in SNS activity, as generally it stimulates thermogenesis and fat oxidation while decreasing energy intake in both humans and rodents (Bray 2000).
Table 2.
Studies on the effect of capsiate on thermogenesis and appetite
| Study | Participants | Experimental design/intervention | Thermogenesis | Appetite |
| Galgani and Ravussin (2010) | 78 ♂ in the United States (LA) Young adults Overweight |
Parallel-arm double blind Randomized to 1 of 3 supplements for 4 weeks: 1) 3 mg/day dihydrocapsiate (capsules) 2) 9 mg/day dihydrocapsiate (capsules) 3) 0 (placebo) |
↑ RMR 54 kcal/day (3 and 9 mg groups combined) No effect on FAT oxidation, body wt, fat mass, or fat-free mass |
n/a |
| Galgani et al. (2010) | 13 ♂ in the United States (LA) Age 28.4 ± 1.4 years BMI 27.1 ± 1.0 kg/m2 (mean ± SE) |
Randomized crossover double blind 1) 1 mg capsinoids (capsules) 2) 3 mg capsinoids (capsules) 3) 6 mg capsinoids (capsules) 4) 12 mg capsinoids (capsules) 5) 0 (placebo) Capsinoids (capsiate, dihydrocapsiate, nordihydrocapsiate in a 70:23:7 ratio) |
No effect on RMR, FAT oxidation, or axillary temperature | n/a |
| Josse et al. (2010) | 12 ♂ in Canada (Ontario) Age 24.3 ± 3 years BMI 25.5 ± 1.7 kg/m2 (mean ± SD) |
Randomized crossover double blind Ingested capsules 30 min before 90 min cycling and 30 min recovery 1) 10 mg capsinoids (capsules) 2) 0 (placebo) Capsinoids (capsiate, dihydrocapsiate, nordihydrocapsiate in a 70:23:7 ratio) |
↑ SNS activation, EE (VO2), and FAT oxidation | n/a |
| Lee et al. (2010) | 26 ♂ and 20 postmenopausal ♀ in the United States (CA) BMI 26.9–38 kg/m2 |
Parallel-arm double blind Randomized to 1 of 3 supplements for 4 weeks (while following an 800 kcal/day, 120 g/day PRO diet): 1) 3 mg/day dihydrocapsiate (capsules) 2) 9 mg/day dihydrocapsiate (capsules) 3) 0 (placebo) |
↑ PPEE (9 mg > 3 mg > placebo) ↑ FAT oxidation (3 and 9 mg > placebo) No effect on body wt, fat mass, or BMR |
n/a |
| Snitker et al. (2009) | 40 ♂ and 40 ♀ in the United States (NJ) Age 42.6 ± 8 y BMI 25–35 kg/m2 (mean ± SD) |
Parallel-arm double blind Randomized to 1 of 2 supplements for 12 weeks: 1) 6 mg/day capsinoids (capsules) 2) 0 (placebo) Capsinoids (capsiate, dihydrocapsiate, nordihydrocapsiate in a 70:23:7 ratio) |
↓ Abdominal adiposity (correlated with change in body wt) Tended to ↑ FAT oxidation No effect on RMR |
n/a |
| Inoue et al. (2007) | 44 ♂ and postmenopausal ♀ in Japan Age 30–65 years BMI ≥ 23 kg/m2 (BMI ≥ 25, n = 28) |
Parallel-arm double blind Randomized to 1 of 3 supplements for 4 weeks: 1) 3 mg/day capsinoids (capsules) 2) 10 mg/day capsinoids (capsules) 3) 0 (placebo)Capsinoids (capsiate, dihydrocapsiate, nordihydrocapsiate) |
↑ FAT oxidation positively correlated with BMI ↑ VO2 (10 mg, BMI ≥ 25 kg/m2 only) Tended to ↑ EE and FAT oxidation (3 and 10 mg, BMI ≥ 25 kg/m2 only) Tended to ↓ body wt and BMI (3 and 10 mg) |
Tended to ↑ PRO intake (10 mg only) No effect on energy, FAT, CHO, cholesterol, or fiber |
| Kawabata et al. (2006) | 7 ♂ (capsiate group) and 5 ♂ (control) in Japan Young adults BMI 17–30 kg/m2 |
Parallel arm 1) Capsinoids (0.4 g/kg/day for 2 weeks (divided between 3 meals, ingested uncooked, and frozen before meals) 2) Control (received no supplemental food) |
↓ Body wt, BMI, fat mass, fat-free mass, total fat area, and RQ Tended to ↑ FAT oxidation and SNS activity Tended to ↓ visceral FAT area No effect on subcutaneous fat area, resting VO2, CHO oxidation, RMR, or PSNS activity |
n/a |
| Ohnuki et al. (2001) | 7 ♂ and 4 ♀ in JapanAge 21–32 years | Crossover 1) CH-19 Sweet: 0.1 g/kg (containing 0.3–1 mg/g capsiate ingested orally) 2) California-Wandar: 0.1 g/kg (containing neither capsiate or capsaicin ingested orally) |
↑ Core body (tympanic membrane of ear) and body surface (forehead, wrist, and neck) temperature ↑ VO2 No effect on substrate oxidation |
n/a |
Abbreviations: basal metabolic rate (BMR), carbohydrate (CHO), not applicable (n/a), oxygen consumption (VO2), parasympathetic nervous system (PSNS), protein (PRO), red pepper (RP), and resting metabolic rate (RMR).
Table 3.
Studies on the effect of both capsaicin and capsiate on thermogenesis and appetite
| Study | Participants | Experimental design/intervention | Thermogenesis | Appetite |
| Reinbach et al. (2009) | 10 ♂ and 17 ♀ in the Netherlands Age 26.9 ± 6.3 years BMI 22.2 ± 2.7 kg/m2 Likers of hot spices (mean ± SD) |
Randomized crossover (3 weeks positive and 3 weeks negative EB) 5 test days in which the following treatments were consumed in capsule form with 3 meals (standardized breakfast, standardized lunch, and ad libitum dinner): 1) Capsaicin (0.510 g RP; 40 000 SHU) 2) Green tea and capsaicin (0.510 g RP; 40 000 SHU) 3) Green tea (19.2 g) 4) CH-19 Sweet (2.3 mg capsiate) 5) Placebo |
n/a | CH-19 Sweet ↓ EI (positive EB) No effect on EI (negative EB); desire to eat, fullness, hunger, and satiety (positive or negative EB) Capsaicin ↑ Fullness and satiety (negative EB) ↓ Hunger (positive EB) No effect on hunger (negative EB); fullness or satiety (positive EB); EI and desire to eat (positive or negative EB) (results for CH-19 Sweet and capsaicin only) |
| Hachiya et al. (2007) | 5 ♂ and 7 ♀ in Japan Age 22–25 years |
Crossover 0.1 g/kg body wt (0.1 mg/kg dry weight capsiate and capsaicin) (ingested at 37 °C with water, in 2 min, after 30 chews): 1) CH-19 Sweet (capsinoids) 2) Cayenne Long Slim (capsaicinoids) 3) California Wonder (control, containing neither capsinoids or capsaicinoids) |
↑ SNS activity ↑ Forehead and neck temperature (capsaicinoids > capsinoids > control) ↑ Tympanic (core) temperature (capsaicinoids = capsinoids > control) No effect on PSNS activity |
n/a |
Abbreviations: energy balance (EB), parasympathetic nervous system (PSNS), red pepper (RP), and Scoville Heat Unit (SHU).
Despite reports that capsaicin-containing foods promote negative energy balance (Yoshioka et al. 1995, 1998, 1999, 2004; Matsumoto et al. 2000; Chaiyata et al. 2003; Lejeune et al. 2003; Westerterp-Plantenga et al. 2005; Ahuja et al. 2006; Hachiya et al. 2007; Shin and Moritani 2007; Reinbach et al. 2009; Smeets and Westerterp-Plantenga 2009; Ludy and Mattes 2011b) (Tables 1 and 3), many individuals abstain from their consumption due to the sensory burn they impart. Even among individuals accustomed to ingesting capsaicin-containing foods, self-perceived high doses of capsaicin affect sensory evaluations. For example, when the taste, smell, and appearance of a capsaicin-rich stir fry (10 g red pepper) was evaluated by Japanese women customarily consuming spicy foods, hedonic ratings were decreased by 19, 11, and 23 units, respectively, on 100 unit visual analog scales, whereas hotness was increased by 74 units (Yoshioka et al. 1998) (Table 1). The sensation of gastric distress elicited when capsaicin is provided in capsule form presents additional obstacles to consumption (Lejeune et al. 2003; Belza and Jessen 2005). Thus, the nonpungent capsaicin analog, capsiate, presents an alternative weight management option for nonusers or those with limited tolerance of capsaicin-containing foods (Ohnuki et al. 2001; Kawabata et al. 2006; Hachiya et al. 2007; Inoue et al. 2007; Reinbach et al. 2009; Snitker et al. 2009; Galgani and Ravussin 2010; Josse et al. 2010; Lee et al. 2010) (Tables 2 and 3).
Reported effects of capsaicin and capsiate on energy balance
Thermogenesis and substrate oxidation
Capsaicin and capsiate stimulate thermogenesis by increasing EE (Yoshioka et al. 1995, 1998; Matsumoto et al. 2000; Chaiyata et al. 2003; Lejeune et al. 2003; Inoue et al. 2007; Galgani and Ravussin 2010; Josse et al. 2010; Lee et al. 2010; Ludy and Mattes 2011b) as well as enhancing core body and skin temperature (Ohnuki et al. 2001; Hachiya et al. 2007; Ludy and Mattes 2011b). Furthermore, both capsaicin and capsiate are reported to influence substrate oxidation (Yoshioka et al. 1995; 1998; Lim et al. 1997; Matsumoto et al. 2000; Lejeune et al. 2003; Kawabata et al. 2006; Inoue et al. 2007; Shin and Moritani 2007; Josse et al. 2010; Lee et al. 2010; Ludy and Mattes 2011b). However, there have been conflicting reports on these outcomes, including studies that note no effect on substrate oxidation (Ohnuki et al. 2001; Ahuja et al. 2007; Smeets and Westerterp-Plantenga 2009; Galgani and Ravussin 2010; Galgani et al. 2010) or EE (Ahuja et al. 2006; Snitker et al. 2009; Galgani et al. 2010), increased EE (Ahuja et al. 2006), and decreased skin temperature (Ludy and Mattes 2011b) (Tables 1–3).
One potential explanation for inconsistent findings is that the thermogenic effects may vary with body composition, as has been demonstrated with other bioactive food components (Acheson et al. 1980; Bracco et al. 1995). The majority of studies reporting that capsaicin increases EE have been conducted in lean individuals (Yoshioka et al. 1995, 1998; Matsumoto et al. 2000; Ludy and Mattes 2011b) or did not report body composition (Chaiyata et al. 2003). Only one study reported no effect on EE in lean individuals (Ahuja et al. 2006). Among overweight and obese individuals, capsaicin's effects on EE have been inconsistent. During the 13 weeks following a 4-week weight loss intervention in overweight and obese individuals (body mass index [BMI] 25–35 kg/m2), a study in the Netherlands found that when 135 mg/day capsaicin was consumed in capsules with meals, resting EE was increased by 119 kcal/day compared with placebo (Lejeune et al. 2003). An Australian study reported that EE was suppressed following consumption of a 33 mg/day capsaicin spice blend for 4 weeks in overweight and obese men and women (BMI ≥ 26.3 ± standard deviation [SD] 4.6 kg/m2) but did not vary in lean men and women (BMI < 26.3 ± SD 4.6 kg/m2) (Ahuja et al. 2006). A Japanese study reported that EE was augmented in lean women (BMI 21.0 ± SE 0.57 kg/m2) following a 3 mg capsaicin breakfast but did not vary in overweight and obese women (BMI 28.8 ± SE 1.01 kg/m2) matched for age and height (Matsumoto et al. 2000). Therefore, the effects of capsaicin may be blunted in individuals at high weights.
All studies evaluating the effects of capsiate on EE have included overweight and/or obese individuals (Inoue et al. 2007; Snitker et al. 2009; Galgani and Ravussin 2010; Galgani et al. 2010; Josse et al. 2010; Lee et al. 2010), with 4 reporting increases (Inoue et al. 2007; Galgani and Ravussin 2010; Josse et al. 2010; Lee et al. 2010) and 2 reporting no effect (Snitker et al. 2009; Galgani et al. 2010) (Table 2). Only one study (Inoue et al. 2007) stratified thermogenic effects by body composition. In contrast to reports of increased EE among lean individuals supplemented with capsaicin (Yoshioka et al. 1995, 1998; Matsumoto et al. 2000; Ludy and Mattes 2011b), 3 and 10 mg/day doses of encapsulated capsinoids tended to increase resting EE in overweight middle-aged Japanese individuals (BMI ≥ 25 kg/m2) by 0.8 and 0.9 kcal/kg/day, respectively, over 2 weeks and 0.4 and 0.6 kcal/kg/day, respectively, over 4 weeks compared with baseline. The declining effect over time may reflect the development of tolerance to its action. However, no effect was observed in analyses with the range of body compositions studied (BMI ≥ 23 kg/m2) (Inoue et al. 2007). Among other studies reporting increases in EE, one entailed a single exposure to encapsulated capsinoids (Josse et al. 2010) and 2 administered encapsulated dihydrocapsiate supplements for 4 weeks (Galgani and Ravussin 2010; Lee et al. 2010). The single exposure study (Josse et al. 2010), conducted in young men (BMI 25.5 ± SD 1.7 kg/m2) at rest and during exercise, reported that 10 mg capsinoids ingested 30 min before cycling increased metabolic rate 0.24 kcal/min more than placebo. Both 4-week studies (Galgani and Ravussin 2010; Lee et al. 2010) involved supplementation with 3 and 9 mg/day encapsulated dihydrocapsiate. Resting metabolic rate was increased by 54 kcal/day in young, overweight men after a 4-week ad libitum diet when the 3 and 9 mg/day groups were combined and compared with placebo (Galgani and Ravussin 2010). Additionally, postprandial EE (normalized for fat-free mass) was increased in a dose-dependent manner among men and postmenopausal women (BMI 26.9–38 kg/m2) following a 4-week very low–calorie diet by ∼1 and ∼2 kcal/kg/day in the 3 and 9 mg/day groups, respectively, compared with placebo (Lee et al. 2010). The 2 studies reported no effect on EE (Snitker et al. 2009; Galgani et al. 2010). One study in young men (BMI 27.1 ± SE 1.0 kg/m2) involved single exposures to 1, 3, 6, and 12 mg encapsulated capsinoids compared with placebo (Galgani et al. 2010). The other study was conducted in middle-aged men and women (BMI 25–35 kg/m2) and compared 12-week supplementation with 6 mg/day of capsinoids to placebo (Snitker et al. 2009). Given that all the studies assessing capsiate's thermogenic effects included overweight and/or obese individuals (Inoue et al. 2007; Snitker et al. 2009; Galgani and Ravussin 2010; Galgani et al. 2010; Josse et al. 2010; Lee et al. 2010), and results were stratified by weight in only one study, the issue of body composition-specific effects cannot be adequately addressed. Thus, further investigation in both lean and overweight/obese individuals will be required to determine whether capsaicin and capsiate are more appropriate agents for prevention or treatment of overweight and obesity.
Another possibility for mixed results is that characteristics of the subject population, such as customary exposure to capsaicin-containing foods, affect thermogenic outcomes. Although a previous study in the United States demonstrated weaker or nonsignificant effects on energy balance in regular users compared with nonusers (Ludy and Mattes 2011b), the definitions of “users” were not consistent between studies. For instance, a Thai study excluded those consuming ≥10 g/day chili peppers (Chaiyata et al. 2003). However, the study in the United States indicated that the mean preference at spicy food–containing meals was ∼2 mg capsaicin and classified regular users as consuming spicy foods 3 times per week or more (Ludy and Mattes 2011b). Among other studies, “naïve or infrequent consumers” ingested chili less than daily (Ahuja et al. 2006, 2007), were classified as “not used to capsaicin in their habitual diet” (Lejeune et al. 2003), or “did not have a long-term dietary history of eating spices” (Matsumoto et al. 2000). Several other studies did not classify individuals by history of capsaicin use (Yoshioka et al. 1995, 1999, 2004; Lim et al. 1997; Shin and Moritani 2007; Smeets and Westerterp-Plantenga 2009). Given that differential outcomes have been noted among users and nonusers of capsaicin (Ludy and Mattes 2011b) and that differences in metabolism and clearance have been noted among users and nonusers of other bioactive food components (Hursel et al. 2009), it is vital that future studies characterize customary capsaicin use and maintain consistent definitions.
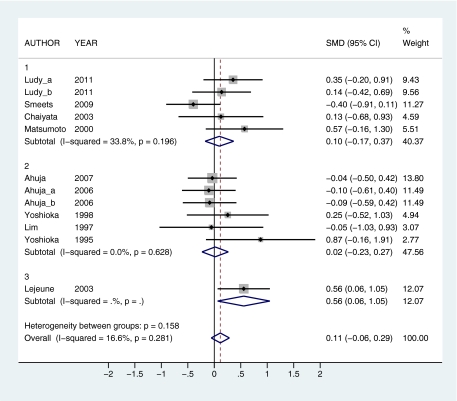
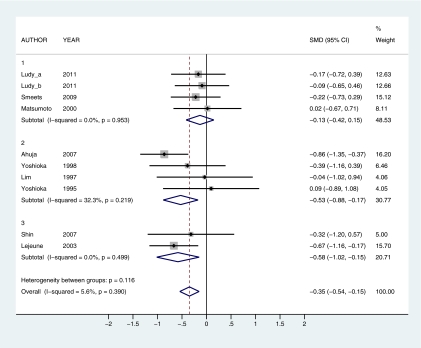
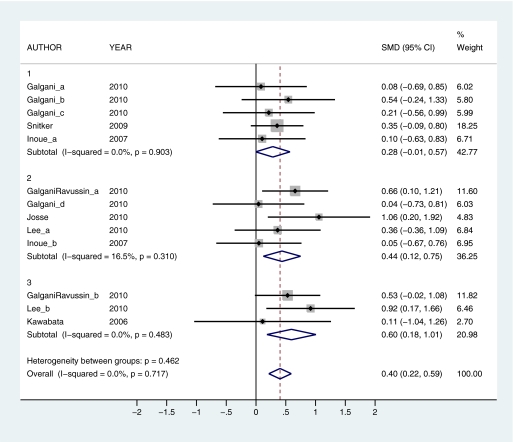
To provide a quantitative assessment of the effects of capsaicin and capsiate intake on thermogenic outcomes, a series of meta-analyses were conducted. The effects of capsaicin and capsiate on EE and substrate oxidation (respiratory quotient [RQ]) were compared in 4 overall analyses: 1) capsaicin EE (12 observations; Figure 2), 2) capsaicin RQ (10 observations; Figure 3), 3) capsiate EE (13 observations; Figure 4), and 4) capsiate RQ (9 observations; Figure 5). To explore dose–response effects, studies were subdivided into self-selected low (≤7 mg capsaicin or ≤1.5 mg dihydrocapsiate), intermediate (20–35 mg capsaicin or 2–3 mg dihydrocapsiate), and high (135–150 mg capsaicin or 6–9 mg dihydrocapsiate) doses. Subanalyses were also calculated at each dose level: 1) low capsaicin EE (5 observations), 2) intermediate EE (6 observations), 3) high capsaicin EE (1 observation; Figure 2), 4) low capsaicin RQ (4 observations), 5) intermediate capsaicin RQ (4 observations), 6) high capsaicin RQ (2 observations; Figure 3); 7) low capsiate EE (5 observations), 8) intermediate capsiate EE (5 observations), 9) high capsiate EE (3 observations; Figure 4), 10) low capsiate RQ (1 observation), 11) intermediate capsiate RQ (5 observations), and 12) high capsiate RQ (3 observations; Figure 5). Corresponding authors were contacted to obtain raw data. This was provided by 4 authors (Yoshioka et al. 1998; Matsumoto et al. 2000; Josse et al. 2010; Ludy and Mattes 2011b). In cases where the raw data were not available, results were extracted from published papers (Yoshioka et al. 1995; Lim et al. 1997; Chaiyata et al. 2003; Lejeune et al. 2003; Ahuja et al. 2006, 2007; Kawabata et al. 2006; Inoue et al. 2007; Shin and Moritani 2007; Smeets and Westerterp-Plantenga 2009; Snitker et al. 2009; Galgani and Ravussin 2010; Galgani et al. 2010; Lee et al. 2010). In 5 instances, summary findings were reported but could not be included due to lack of data (i.e., capsaicin had no significant effect on EE (Ahuja et al. 2006) and capsiate had no significant effect on substrate oxidation (Ohnuki et al. 2001)) or inability to convert data to standardized units (i.e., capsaicin had no significant effect on substrate oxidation (Shin and Moritani 2007), capsiate increased EE (Ohnuki et al. 2001), and capsiate enhanced fat oxidation (Snitker et al. 2009)). Thus, in these 5 reports, findings were mixed on thermogenic outcomes. Standardized mean difference (SMD) with 95% confidence interval (CI) was used as an effect size indicator. Significant differences were indicated when the 95% CI did not include 0. I-squared, a measure of heterogeneity, was used to assess the proportion of inconsistency in individual studies that could not be explained by chance. I-squared can potentially range between 0% and 100% with lower values representing less heterogeneity. Nonsignificant I-squared P values (i.e., P > 0.05) indicate that heterogeneity was likely to occur by chance alone, suggesting pooled data would not likely lead to bias. Forest plots were generated with STATA SE version 11.1 (StataCorp, College Station, TX).
Figure 2.
Forest plots comparing studies on the effects of capsaicin on EE by low (1), intermediate (2), and high (3) dose. The weight that the study received in the overall association is indicated by the size of the gray squares. SMD was used as an effect size indicator. The 95% CIs are represented by error bars. The pooled estimates for each analysis are represented by diamonds. SMD ± 95% CIs show that capsaicin increases EE (indicated by SMD > 0) at high doses. Capsaicin had no significant effect on EE overall or at low or intermediate doses. I-squared was used to assess heterogeneity. All I-squared values (subtotal and overall) were not statistically significant when subdivided by dose. This indicates that SMD with 95% CI can be interpreted separately by dose or when all 3 dose levels are combined into a single overall estimate. Multiple subscripts following author name indicate the use of more than one pepper dose within the same study. This figure appears in color in the online version of Chemical Senses.
Figure 3.
Forest plots comparing studies on the effects of capsaicin on substrate oxidation (RQ) by low (1), intermediate (2), and high (3) dose. The weight that the study received in the overall association is indicated by the size of the gray squares. SMD was used as an effect size indicator. The 95% CIs are represented by error bars. The pooled estimates for each analysis are represented by diamonds. SMD ± 95% CIs show that capsaicin enhances fat oxidation (indicated by SMD < 0) overall as well as at intermediate and high doses. Capsaicin had no significant effect on substrate oxidation at low doses. I-squared was used to assess heterogeneity. All I-squared values (subtotal and overall) were not statistically significant when subdivided by dose. This indicates that SMD with 95% CI can be interpreted separately by dose or when all 3 dose levels are combined into a single overall estimate. Multiple subscripts following author name indicate the use of more than one pepper dose within the same study. This figure appears in color in the online version of Chemical Senses.
Figure 4.
Forest plots comparing studies on the effects of capsiate on energy expenditure (EE) by low (1), intermediate (2), and high (3) dose. The weight that the study received in the overall association is indicated by the size of the gray squares. SMD was used as an effect size indicator. The 95% CIs are represented by error bars. The pooled estimates for each analysis are represented by diamonds. SMD ± 95% CIs show that capsiate increases EE (indicated by SMD > 0) overall as well as at intermediate and high doses. Capsiate had no significant effect on EE at low doses. I-squared was used to assess heterogeneity. All I-squared values (subtotal and overall) were not statistically significant when subdivided by dose. This indicates that SMD with 95% CI can be interpreted separately by dose or when all 3 dose levels are combined into a single overall estimate. Multiple subscripts following author name indicate the use of more than one pepper dose within the same study. This figure appears in color in the online version of Chemical Senses.
Figure 5.
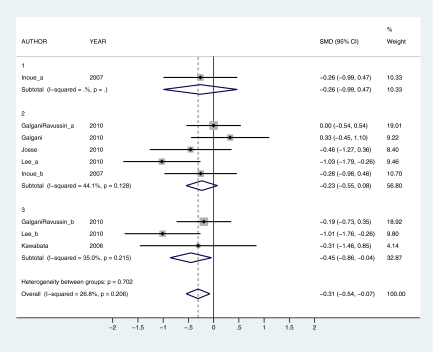
Forest plots comparing studies on the effects of capsiate on substrate oxidation (RQ) by low (1), intermediate (2), and high (3) dose. The weight that the study received in the overall association is indicated by the size of the gray squares. SMD was used as an effect size indicator. The 95% CIs are represented by error bars. The pooled estimates for each analysis are represented by diamonds. SMD ± 95% CIs show that capsiate enhances fat oxidation (indicated by SMD < 0) overall as well as at high doses. Capsiate had no significant effect on substrate oxidation at low or intermediate doses. I-squared was used to assess heterogeneity. All I-squared values (subtotal and overall) were not statistically significant when subdivided by dose. This indicates that SMD with 95% CI can be interpreted separately by dose or when all 3 dose levels are combined into a single overall estimate. Multiple subscripts following author name indicates the use of more than one pepper dose within the same study. This figure appears in color in the online version of Chemical Senses.
Forest plots for capsaicin and capsiate's overall effects on EE and RQ are shown in Figures 2–5. The meta-analyses revealed that capsiate increased EE (SMD = 0.40, 95% CI = 0.22–0.59; Figure 4), whereas both capsaicin and capsiate enhanced fat oxidation (SMD = −0.35, 95% CI = −0.54 to −0.15 and SMD = −0.31, 95% CI = −0.54 to −0.07; Figures 3 and 5, respectively). Capsaicin had no overall effect on EE (SMD = 0.11, 95% CI = −0.06 to 0.29; Figure 2). Neither the overall nor the subtotal I-squared P values for any attribute (capsaicin EE, capsaicin RQ, capsiate EE, or capsiate RQ) were statistically significant. This indicates that it is permissible to combine all dose levels for each attribute into the same analysis (e.g., low-, intermediate-, and high-dose capsaicin's effect on EE into a pooled capsaicin EE group) as well as interpret each dose level for that attribute separately (e.g., low dose capsaicin's effect on EE alone). There were 4 pooled analyses (capsaicin EE, Figure 2; capsaicin RQ, Figure 3; capsiate EE, Figure 4; capsiate RQ, Figure 5) and 12 subtotal analyses by dose (i.e., 3 dose levels for each of 4 attributes).
When subdivided by dose, capsaicin increased EE at high doses (SMD = 0.56, 95% CI = 0.06–1.05) but had no effect at low or intermediate doses (SMD = 0.10, 95% CI = −0.17 to 0.37 and SMD = 0.02, 95% CI = −0.23 to 0.27, respectively) (Figure 2). Capsaicin enhanced fat oxidation at intermediate and high doses (SMD = −0.53, 95% CI = −0.88 to −0.17 and SMD = −0.58, 95% CI = −1.02 to −0.15, respectively) but had no effect at low doses (SMD = −0.13, 95% CI = −0.42 to 0.15) (Figure 3). Capsiate increased EE at intermediate and high doses (SMD = 0.44, 95% CI = 0.12–0.75 and SMD = 0.60, 95% CI = 0.18–1.01, respectively) but had no effect at low doses (SMD = 0.28, 95% CI = −0.01 to 0.57) (Figure 4). Capsiate enhanced fat oxidation at high doses (SMD = −0.45, 95% CI = −0.86 to −0.04) but had no effect at low or intermediate doses (SMD = −0.26, 95% CI = −0.99 to 0.47 and SMD = −0.23, 95% CI = −0.55 to 0.08, respectively) (Figure 5).
Although the meta-analyses suggest the presence of a dose-dependent effect (i.e., that higher doses are more effective for thermogenic outcomes), these results should be interpreted with caution. The data in Figures 2–5 represent average effects over all study lengths and environments, subject populations, forms, and compositions. The potential problems associated with these issues are discussed throughout this manuscript. Furthermore, these findings may be affected by publication bias in which selective reporting of positive results may lead to an overestimation of energy balance effects (Easterbrook et al. 1991).
Appetite regulation
Research concerning the role of pepper consumption on appetite regulation has centered on capsaicin (Yoshioka et al. 1998, 1999, 2004; Lejeune et al. 2003; Westerterp-Plantenga et al. 2005; Ahuja et al. 2007; Reinbach et al. 2009; Smeets and Westerterp-Plantenga 2009; Reinbach et al. 2010; Ludy and Mattes 2011b) (Tables 1 and 3), with only 2 studies evaluating the appetitive effects of capsiate (Inoue et al. 2007; Reinbach et al. 2009) (Tables 2 and 3). Capsaicin suppresses self-reported orexigenic sensations, including the desire to eat fatty foods (Ludy and Mattes 2011b), hot foods (Reinbach et al. 2010), sweet foods (Ludy and Mattes 2011b), salty foods (Ludy and Mattes 2011b), and desire to eat generally (Yoshioka et al. 1999); preoccupation with food (Yoshioka et al. 1999; Ludy and Mattes 2011b); prospective food consumption (Yoshioka et al. 1999); and hunger during positive energy balance (Reinbach et al. 2009) and hunger generally (Yoshioka et al. 1999; Westerterp-Plantenga et al. 2005) (Tables 1 and 3). Supporting this, concentrations of the orexigenic hormone ghrelin are suppressed with capsaicin ingestion (Smeets and Westerterp-Plantenga 2009) (Table 1). Additionally, capsaicin is reported to enhance anorexigenic sensations, including satiety during negative energy balance (Reinbach et al. 2009), satiety generally (Westerterp-Plantenga et al. 2005), and fullness during negative energy balance (Reinbach et al. 2009) while increasing concentrations of the anorexigenic hormone glucagon-like peptide 1 (Smeets and Westerterp-Plantenga 2009) (Tables 1 and 3). At subsequent eating occasions, capsaicin decreases total energy (Yoshioka et al. 1999, 2004; Westerterp-Plantenga et al. 2005; Ludy and Mattes 2011b), fat (Yoshioka et al. 1999, 2004; Westerterp-Plantenga et al. 2005), carbohydrate (Yoshioka et al. 1999), and protein intake (Yoshioka et al. 1999) as well as energy density by decreasing fat intake (Westerterp-Plantenga et al. 2005) (Table 1).
Consistent with the capsaicin findings, capsiate decreases energy intake during positive energy balance (Reinbach et al. 2009) (Table 3). However, not all previous studies are in agreement. Some report that capsaicin has no effect on desire to eat salty, bitter, or sour foods (Reinbach et al. 2010); desire to eat generally (Reinbach et al. 2010; Ludy and Mattes 2011b); prospective food consumption (Ludy and Mattes 2011b); satiety (Lejeune et al. 2003; Reinbach et al. 2010); fullness during positive energy balance (Reinbach et al. 2009); fullness generally (Ludy and Mattes 2011b); thirst (Ludy and Mattes 2011b); peptide YY (Smeets and Westerterp-Plantenga 2009); total energy (Ahuja et al. 2007; Reinbach et al. 2010), fat (Ahuja et al. 2007), carbohydrate (Ahuja et al. 2007), or protein intake (Ahuja et al. 2007); and/or energy density (Reinbach et al. 2010) (Table 1 and 3). Furthermore, 2 studies report that capsaicin may promote a positive energy balance by increasing the desire to eat sweet foods (Reinbach et al. 2010) or intake of foods rich in carbohydrate (Westerterp-Plantenga et al. 2005) (Table 1). In contrast, capsiate exerts no effect on the desire to eat, fullness, hunger, or satiety during positive or negative energy balance (Reinbach et al. 2009); total energy intake during negative energy balance (Reinbach et al. 2009); and total energy, fat, carbohydrate, cholesterol, or fiber intake in energy balance (Inoue et al. 2007) (Tables 2 and 3).
A potential explanation for discrepant findings on appetitive sensation is that they may reflect culturally based sex differences. Of the 10 published articles (Yoshioka et al. 1998, 1999, 2004; Lejeune et al. 2003; Westerterp-Plantenga et al. 2005; Ahuja et al. 2006; Reinbach et al. 2009, 2010; Smeets and Westerterp-Plantenga 2009; Ludy and Mattes 2011b) (Tables 1 and 3) evaluating capsaicin's appetitive effects, 2 (Yoshioka et al. 1998, 1999) focused on women and 2 (Yoshioka et al. 1999, 2004) focused on men, whereas the remainder (Lejeune et al. 2003; Westerterp-Plantenga et al. 2005; Ahuja et al. 2007; Reinbach et al. 2009, 2010; Smeets and Westerterp-Plantenga 2009; Ludy and Mattes 2011b) were mixed and not powered to stratify results by sex. Both studies in women (Yoshioka et al. 1998, 1999) used the same data set. As described in the above pungency section, the ingestion of greater-than-usual doses of capsaicin led the Japanese women participants accustomed to consuming capsaicin to provide reduced hedonic ratings compared with a bland breakfast (Yoshioka et al. 1998). Furthermore, although ad libitum lunch protein (20% and 6% following high-fat and high-carbohydrate capsaicin-containing breakfasts, respectively) and fat (17% and 11% following high-fat and high-carbohydrate capsaicin-containing breakfasts, respectively) intakes were suppressed, total energy intake was not affected (Yoshioka et al. 1999) (Table 1). In contrast, when Caucasian men ingested a capsaicin-containing prelunch appetizer, total energy and carbohydrate intakes were reduced by 11% and 18%, respectively, when an ad libitum lunch and snack were served (Yoshioka et al. 1999) (Table 1). Similarly, a study in Japanese men demonstrated that self-perceived “strong” capsaicin doses ingested as an appetizer reduced total energy and fat intake (8% and 14%, respectively) at an ad libitum lunch (Yoshioka et al. 2004) (Table 1). These findings are consistent with culturally based gender biases in which the consumption of capsaicin-containing foods is believed to confer ideals of strength and manliness (Rozin and Schiller 1980).
Based on a review of the studies listed in Tables 1–3, meta-analyses were not deemed appropriate to address the effects of capsaicin and capsiate intake on appetitive outcomes. There were only 2 investigations concerning capsiate (Inoue et al. 2007; Reinbach et al. 2009), and the effects of red pepper intake on appetite regulation were so divergent that no common outcome measure could be identified across studies.
Body composition
Reported effects of capsaicin and capsiate on body composition are limited, and those that exist are of short duration. Most studies on capsaicin have been short term (≤1 day) (Yoshioka et al. 1995, 1998, 1999, 2004; Lim et al. 1997; Matsumoto et al. 2000; Chaiyata et al. 2003; Hachiya et al. 2007; Shin and Moritani 2007; Smeets and Westerterp-Plantenga 2009; Reinbach et al. 2010; Ludy and Mattes 2011b), with 2 others lasting only 2 (Westerterp-Plantenga et al. 2005) and 5 days (Reinbach et al. 2009) so cannot provide insights on changes of body composition. Four longer term studies (3 weeks to 3 months) have been reported (Lejeune et al. 2003; Ahuja et al. 2006; 2007; Reinbach et al. 2009) (Tables 1 and 3). Although appearing efficacious for promoting negative energy balance in short-term highly controlled environments (Yoshioka et al. 1995, 1998, 1999, 2004; Lim et al. 1997; Matsumoto et al. 2000; Chaiyata et al. 2003; Hachiya et al. 2007; Shin and Moritani 2007; Smeets and Westerterp-Plantenga 2009; Ludy and Mattes 2011b), capsaicin's long-term effectiveness for modifying body composition under free-living conditions is uncertain. Of the longer term studies, only 2 evaluated body composition and neither led to changes compared with control treatments (i.e., placebo capsules (Lejeune et al. 2003) or a bland spice-free diet (Ahuja et al. 2007)). A 3-month Dutch study exploring the effects of 135 mg/day encapsulated capsaicin in overweight and obese (BMI 25–35 kg/m2), nonhabitual capsaicin consumers demonstrated no effect on weight regain following a 4-week very low–energy diet intervention. However, perceived pungency interfered with the ability to achieve full compliance. All individuals in the test group reported that stomach burning accompanied capsule consumption, which lead 24% of individuals to decrease their daily capsaicin dose by half (Lejeune et al. 2003) (Table 1). Similarly, a 4-week ad libitum diet with 33 mg/day capsaicin as part of an orally incorporated chili blend in Australians who were primarily naïve/infrequent capsaicin consumers (BMI 26.3 ± SD 4.6 kg/m2) reported no effects on BMI, fat mass, or lean mass (Ahuja et al. 2007) (Table 1). Whether this lack of effect on body composition is due to 1) poor compliance with recommendations to ingest an unacceptably high capsaicin dose; 2) individuals becoming desensitized to capsaicin's effects with longer term intake (Ludy and Mattes 2011b); 3) the need for oral sensory stimulation (Westerterp-Plantenga et al. 2005; Ludy and Mattes 2011b); 4) differential effects due to body composition (Matsumoto et al. 2000; Ahuja et al. 2006); or 5) limited study duration requires clarification. These issues will be important considerations in future investigations.
The effects of capsiate on body composition have been explored more thoroughly. Whereas 4 studies were short term (Ohnuki et al. 2001; Hachiya et al. 2007; Galgani et al. 2010; Josse et al. 2010) and 1 study was 5 days (Reinbach et al. 2009), several have been longer term (2–12 weeks) (Kawabata et al. 2006; Inoue et al. 2007; Snitker et al. 2009; Galgani and Ravussin 2010; Lee et al. 2010) (Tables 2 and 3). Effects have been conflicting. Capsiate has been reported to decrease body weight (Kawabata et al. 2006; Inoue et al. 2007), BMI (Kawabata et al. 2006; Inoue et al. 2007), fat-free mass (Kawabata et al. 2006), fat mass (Kawabata et al. 2006), total fat (Kawabata et al. 2006), and visceral fat (Kawabata et al. 2006; Snitker et al. 2009). However, other reports have demonstrated that capsiate has no effect on body weight (Galgani and Ravussin 2010; Lee et al. 2010), fat mass (Galgani and Ravussin 2010; Lee et al. 2010), fat-free mass (Galgani and Ravussin 2010), or subcutaneous fat (Kawabata et al. 2006). As is the case with capsaicin, a major limitation of studies exploring body composition effects of capsiate is that the maximum study duration was 12 weeks. Exposure duration is a relevant concern, given that modest, long-term dietary adjustments are reported to be the most effective for weight management (Mattes 2008), and it is unknown whether long-term use of capsiate will result in tolerance and loss of effect at acceptable dietary levels.
The strongest, albeit still of very limited duration, study exploring body composition effects was conducted in normal to overweight Japanese young adults who were provided a laboratory-controlled diet that promoted energy balance during the week prior to initiating capsinoid supplementation (Kawabata et al. 2006). Over a 2-week period, the addition of 0.4 g/kg/day capsinoids ingested uncooked and frozen before 3 meals per day decreased body weight by 1.77 kg, BMI by 0.6 kg/m2, fat-free mass by 1.15 kg, and total fat area of the umbilicus by 15.77 cm2. Furthermore, total fat mass was decreased 1.38 kg more, and visceral fat tended to be decreased by 13.98 cm2 more, among individuals in the capsinoid group compared with those in the control group (Table 2). Similar, but weaker, body composition effects were noted in a study of free-living middle-aged Japanese individuals (BMI ≥ 23 kg/m2). This report noted that supplementation with 10 mg/kg/day encapsulated capsinoids nonsignificantly decreased weight by 0.21 kg and BMI by 0.08 kg/m2 over 2 weeks and tended to decrease weight by 0.46 kg and BMI by 0.17 kg/m2 over 4 weeks. The same study found that supplementation with 3 mg/kg encapsulated capsinoids tended to decrease weight by 0.34 kg/day and BMI by 0.12 kg/m2 over 2 weeks and nonsignificantly decreased weight by 0.29 kg and BMI by 0.10 kg/m2 over 4 weeks (Inoue et al. 2007) (Table 2). An additional study conducted in free-living middle-aged overweight and obese adults in the United States reported that supplementation with 6 mg/day capsinoids for 12 weeks decreased visceral fat 0.93% more than placebo, which was correlated with the change in body weight (Snitker et al. 2009) (Table 2). In contrast, two 4-week studies conducted in overweight and obese individuals in the United States reported no body composition effects when 3 or 9 mg/day dihydrocapsiate were consumed as part of an 800 kcal/day very low–energy diet (Lee et al. 2010) or self-selected free-living diet (Galgani and Ravussin 2010) compared with placebo (Table 2).
In addition to length of study issues, other potential explanations for these discrepant results may involve the form of capsinoid and level of dietary control. Whereas the 3 studies (Kawabata et al. 2006; Inoue et al. 2007; Snitker et al. 2009) reporting body composition effects involved supplementation with total capsinoids, of which capsiate is predominant, the 2 studies (Galgani and Ravussin 2010; Lee et al. 2010) demonstrating no effect included only dihydrocapsiate. Additionally, the strong body composition effects produced in highly controlled environments (Kawabata et al. 2006) may not be easily replicable under free-living conditions. To provide results that can be translated into public health recommendations for weight management, it is imperative that long-term studies (ideally ≥1 year) are undertaken to reflect the small weight gain (∼0.2 to 0.8 kg/person/year) deemed responsible for the obesity epidemic (Williamson 1993; Jeffrey and French 1997; Hill et al. 2003) and the popular recommendation for minor changes to reverse the epidemic (Mattes 2008). Furthermore, a comparison of various capsinoid forms, in combination with other strategies to achieve negative energy balance, should be explored to promote maximal long-term acceptability and compliance.
Potential concerns regarding the long-term sustainability of capsaicin and capsiate's effects on energy balance
Dose, form, and composition
The dose, form, and composition of capsaicin (Tables 1 and 3) and capsiate (Tables 2 and 3) provided in previous studies were widely divergent. With the exception of one study where capsaicin was provided in whole pepper form with water (Hachiya et al. 2007), all other studies evaluating the effects of orally consumed capsaicin incorporated it into meals as ground red pepper (Yoshioka et al. 1995, 1998, 1999; Lim et al. 1997; Matsumoto et al. 2000; Chaiyata et al. 2003; Westerterp-Plantenga et al. 2005; Ahuja et al. 2006; 2007; Reinbach et al. 2009, 2010; Smeets and Westerterp-Plantenga 2009; Ludy and Mattes 2011b). Capsaicin doses in these studies have ranged from ∼0.2 mg at a single meal (Yoshioka et al. 2004) to 33 mg/day for 4 weeks (Ahuja et al. 2007). When administered in capsule form, capsaicin has been included in modest doses as ground red pepper containing capsaicin (0.2–7 mg [Westerterp-Plantenga et al. 2005; Reinbach et al. 2009; Ludy and Mattes 2011b]) and markedly higher doses as pure capsaicin (135–150 mg [Lejeune et al. 2003; Shin and Moritani 2007]). In one study, 150 mg was ingested before a single bout of exercise (Shin and Moritani 2007) and in another, treatment entailed consuming 135 mg/day for 3 months (Lejeune et al. 2003). Although these broad dosage variances mirror cross-cultural trends in capsaicin consumption (e.g., 1.5 mg/day in Europe vs. 25–200 mg/day in India [Astrup et al. 2010]), they also generate concern about the long-term sustainability of recommendations to increase intake, especially in nonusers of spicy foods. Furthermore, despite being the food industry standard for quantifying the pungency of hot peppers (Betts 1999), Scoville Heat Unit (SHU) values are reported for only 5 (Yoshioka et al. 2004; Westerterp-Plantenga et al. 2005; Reinbach et al. 2009; Smeets and Westerterp-Plantenga 2009; Ludy and Mattes 2011b) of the 19 studies evaluating capsaicin's thermogenic and appetitive effects (Tables 1 and 3). Nonpungent peppers, containing capsiate, may lead to increased compliance and long-term acceptability. However, capsiate doses used in studies are not easily determined. They are inconsistently reported as capsiate, dihydrocapsiate, or total capsinoids (which includes variable percentages of capsiate and dihydrocapsiate as well as nordihydrocapsiate). Concentrations of these compounds in chili peppers are affected by factors such as genetics, weather, growing conditions, and age (Reinbach 2008), so it is not possible to convert to capsiate and dihydrocapsiate concentrations without study-specific ratios. For capsiate, the only reported oral test doses were consumed in the form of whole peppers in quantities of ∼2 to 7 (Ohnuki et al. 2001) and ∼7 mg (Hachiya et al. 2007) at single test loads. In capsule form, single capsiate doses ranged from 0.7 (Galgani et al. 2010) to 7 mg (Josse et al. 2010), with longer term doses of 2.3 mg/day for 5 days (Reinbach et al. 2009) and 4.2 mg/day for 12 weeks (Snitker et al. 2009). For dihydrocapsiate, only data on capsule consumption is available, with single test loads ranging from 0.2 to 2.8 mg (Galgani et al. 2010) and longer term doses of 1.4 mg/day for 12 weeks (Snitker et al. 2009) and 9 mg/day for 4 weeks (Galgani and Ravussin 2010; Lee et al. 2010). For total capsinoids, the only reported oral test dose was ∼28 g/day of whole peppers for 2 weeks (Kawabata et al. 2006). In capsule form, single test doses ranged from 1 (Galgani et al. 2010) to 10 mg (Inoue et al. 2007; Josse et al. 2010), with longer term doses of 3 (Inoue et al. 2007; Lee et al. 2010) to 10 mg/day (Inoue et al. 2007) for 4 weeks. Given that other components of red pepper's food matrix (e.g., polyphenols) may influence thermogenic and/or appetitive outcomes, the impacts of dose, form (oral vs. capsule), and composition (red pepper vs. pure capsaicin) will be crucial areas of future investigation.
Palatability and consumer acceptability
The ability of the population at large to tolerate varying doses of capsaicin and capsiate presents a key concern for generating sustainable recommendations regarding their consumption. It is well established that palatability impacts food choice (Glanz et al. 1998) and is directly related to intake (Sorensen et al. 2003). Furthermore, consumers rate palatability as the most important factor in food selection (Glanz et al. 1998). Additionally, cephalic phase thermogenic responses are generally increased with palatable foods (LeBlanc and Cabanac 1989). Full compliance with recommendations to consume capsaicin may be problematic, especially among individuals who abstain from its intake due to pungency. Although acceptability of less palatable foods is generally enhanced with repeated exposure and can match hedonic ratings for initially preferred foods (Zandstra et al. 2000), high capsaicin doses are associated with intolerable gastric distress and anal burning when voiding, even with repeated exposure and when provided in capsule form (Lejeune et al. 2003; Belza and Jessen 2005) (Table 1). Moreover, as described in the pungency and appetite regulation sections, habitual spicy food consumers report that the palatability of foods is decreased with higher-than-normal capsaicin levels (Yoshioka et al. 1998) (Table 1). Due to its lack of pungency, capsiate presents a possible alternative.
In a 3-month double-blind weight maintenance study following a very low–energy diet intervention (Lejeune et al. 2003), the administration of 135 mg/day capsaicin in capsule form was compared with placebo. Researchers noted that “all participants who appeared to have consumed the capsaicin capsules had mentioned a somewhat burning feeling in their stomach.” Despite a more sustained fat oxidation, no significant differences in weight regain were noted between the capsaicin and the placebo groups. Since 24% of participants consumed only half the prescribed dose, a possible explanation is that the gastric distress associated with high-dose supplements reduces compliance, subsequently diminishing capsaicin's effectiveness as an agent for weight management (Table 1). In a 12-week double-blind weight loss study (Snitker et al. 2009), the daily intake of 6 mg of capsinoids in capsule form was compared with placebo. In contrast to the side effects experienced by all participants in the study with capsaicin (Lejeune et al. 2003), side effects in the study with capsinoids (Snitker et al. 2009) were limited to dyspepsia in 2/41 participants, bowel irregularities in 1/41 participants, and diarrhea in 1/41 participants. Furthermore, as detailed in the body composition section, abdominal adiposity decreased more and fat oxidation tended to be higher in the capsinoid group compared with the placebo group (Table 2). Decreased side effects, and thus improved compliance, likely contributed to the effectiveness of capsiate compared with capsaicin.
Whether the effects of capsaicin and capsiate on energy balance occur in a dose-dependent manner is unclear. Although high capsaicin doses have been associated with gastric discomfort, their effectiveness compared with lower doses is not known. A study in young healthy Japanese males demonstrated a dose-dependent relationship between amount of capsaicin consumed and subsequent energy intake, with the maximum tolerable dose suppressing fat intake (Yoshioka et al. 2004) (Table 1). This is supported by a rat study showing a dose-dependent decrease in fat mass when capsaicin was added to a high-fat diet (Kawada, Hagihara, et al. 1986). Regarding capsiate, a study in overweight and obese men and women following a 4-week very low–energy diet in the United States reported that a dihydrocapsiate intake increased postprandial EE in a dose-dependent manner (Lee et al. 2010) (Table 2). Similarly, another study found that oxygen consumption was increased in overweight Japanese women consuming 10 mg/day capsinoids for 4 weeks but not among women consuming 3 mg or placebo (Inoue et al. 2007) (Table 2). However, a study in overweight young men in the United States demonstrated that supplementation with 3 and 9 mg/day dihydrocapsiate for 4 weeks increased resting metabolic rate but failed to demonstrate a dose-dependent relationship (Galgani and Ravussin 2010) (Table 2). Of the 2 studies where capsaicin showed no effects on energy balance, one was low dose (0.4 mg) (Reinbach et al. 2010) and the other was high dose (33 mg) (Ahuja et al. 2007) (Table 1). Similarly, with capsiate the only study showing no effects on energy balance involved a range of doses (1, 3, 6, and 12 mg dihydrocapsiate) (Galgani et al. 2010) (Table 2). A potential explanation underlying these discrepant findings is that habitual consumption of ingredients, such as capsaicin and capsiate that influence TRPV1 activity may modify thermogenic and appetitive effects (Ludy and Mattes 2011a).
Sensory stimulation
Whereas capsaicin evokes oral sensory burning, capsiate does not. Reports concerning the importance of oral sensory stimulation in inducing thermogenic and appetitive effects are conflicting (Yoshioka et al. 2004; Westerterp-Plantenga et al. 2005; Ludy and Mattes 2011b) (Table 1). A short-term study in healthy lean individuals reported that when preferred capsaicin concentrations (2.1 ± SE 0.4 mg) were consumed with oral stimulation, compared with in capsule form, fat oxidation was enhanced, skin temperature was greater, and postprandial EE tended to be higher (Ludy and Mattes 2011b). Similarly, another short-term study in normal and overweight spicy food consumers (≥1 time per week) in the Netherlands demonstrated that decreases in energy intake were greater with oral, compared with gastrointestinal, exposure to capsaicin (2.3 mg) (Westerterp-Plantenga et al. 2005). In contrast, a short-term study among healthy lean Japanese men reported that ingestion of a self-rated “maximum tolerable” dose of capsaicin (2.8 ± SD 4.1 mg) in 150 mL consommé decreased fat intake to a similar extent when delivered both orally and gastrointestinally. The same study noted a biphasic response in SNS activity. Whereas SNS activity was greatest with intermediate, that is, “moderate” (0.04 ± SD 0.03 mg capsaicin/30 mL consommé) to “strong” (0.6 ± SD 0.8 mg) doses, it was suppressed to baseline levels with doses deemed “too spicy” (1.3 ± SD 0.9 mg) (Table 1) (Yoshioka et al. 2004). A possible explanation for these contradictory results is that palatable exposures are required for maximum effects on energy balance. Due to its lack of sensory burn, capsiate presents a potential means to achieve energy balance effects for those who abstain from spicy foods as a result of their pungency. However, despite capsiate being documented to promote negative energy balance when provided alone (Ohnuki et al. 2001; Hachiya et al. 2007), uncooked, frozen (Kawabata et al. 2006), and in capsule form (Inoue et al. 2007; Reinbach et al. 2009; Snitker et al. 2009; Galgani and Ravussin 2010; Josse et al. 2010; Lee et al. 2010) (Tables 2 and 3), its consumption as part of a traditional meal has not been investigated.
Conclusion
Collectively, the studies reviewed provide supportive evidence for roles of capsaicin and capsiate in weight management. However, the magnitude of these thermogenic and appetitive effects is small and their long-term sustainability is uncertain. A 10 kcal negative energy balance (predicted for hedonically acceptable capsaicin doses [Ludy and Mattes 2011b]) in an average weight, middle-aged man would produce an ultimate weight loss of 0.5 kg over 6.5 years, whereas a 50 kcal negative energy balance (predicted for encapsulated dihydrocapsiate [Galgani and Ravussin 2010]) would yield a total weight reduction of 2.6 kg over 8.5 years (Hall et al. 2009; Hall 2010). Although seemingly inconsequential capsaicin and capsiate are food ingredients and cannot be expected to exert pharmacologic effects. On a population scale, modest sustained weight loss can be predicted to generate substantial health and economic benefits by increasing disease-free years, reducing disease incidence, increasing life expectancy, and decreasing medical costs (Oster et al. 1999). Nevertheless, the long-term acceptability and effectiveness of capsaicin and capsiate are unclear. Published studies occurred under controlled conditions and may not apply to environments with free choice of foods and dietary supplements. Furthermore, regular users may become desensitized with long-term intake (Ludy and Mattes 2011b), either by a reduction in peripheral sensitivity to pungent stimuli or by adaptive changes in absorption and/or metabolism of active compounds. To aid weight management, additional investigation is warranted to explore: 1) the sustainability of energy balance effects; 2) the need to alter dose, form, and/or composition to improve long-term efficacy; 3) the combination of capsaicin and capsiate with other weight management strategies; and 4) the mechanisms by which capsaicin and capsiate can be most successfully incorporated into the diet.
Funding
This work was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award [5T32DK076540] and the McCormick Science Institute.
Acknowledgments
The authors thank Mario Ferruzzi for comments on the manuscript.
References
- Acheson KJ, Zahorska-Markiewicz B, Pittet PH, Anantharaman K, Jéquier E. Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal and obese individuals. Am J Clin Nutr. 1980;33:989–997. doi: 10.1093/ajcn/33.5.989. [DOI] [PubMed] [Google Scholar]
- Ahuja KDK, Robertson IK, Geraghty DP, Ball MJ. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am J Clin Nutr. 2006;84:63–69. doi: 10.1093/ajcn/84.1.63. [DOI] [PubMed] [Google Scholar]
- Ahuja KDK, Robertson IK, Geraghty DP, Ball MJ. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur J Clin Nutr. 2007;61:326–333. doi: 10.1038/sj.ejcn.1602517. [DOI] [PubMed] [Google Scholar]
- Astrup A, Kristensen M, Gregersen N, Belza A, Lorenzen J, Due A, Larsen T. Can bioactive foods affect obesity? Ann N Y Acad Sci. 2010;1190:25–41. doi: 10.1111/j.1749-6632.2009.05272.x. [DOI] [PubMed] [Google Scholar]
- Belza A, Jessen AB. Bioactive food stimulants of sympathetic activity: effect on 24-h energy expenditure and fat oxidation. Eur J Clin Nutr. 2005;59:733–741. doi: 10.1038/sj.ejcn.1602121. [DOI] [PubMed] [Google Scholar]
- Betts TA. Pungency quantitation of hot pepper sauces using HPLC. J Chem Educ. 1999;76:240–244. [Google Scholar]
- Bracco D, Ferrarra J-M, Arnaud MJ, Jéquier E, Schutz Y. Effects of caffeine on energy metabolism, heart rate and methylxanthine metabolism in lean and obese women. Am J Physiol. 1995;269:E671–E678. doi: 10.1152/ajpendo.1995.269.4.E671. [DOI] [PubMed] [Google Scholar]
- Bray GA. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes. 2000;24:S8–S17. doi: 10.1038/sj.ijo.0801269. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Chaiyata P, Puttadechakum S, Komindr S. Effect of chili pepper (Capsicum frutescens) ingestion on plasma glucose response and metabolic rate in Thai women. J Med Assoc Thai. 2003;86:854–860. [PubMed] [Google Scholar]
- Cichewicz RH, Thorpe PA. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J Ethnopharmacol. 1996;52:61–70. doi: 10.1016/0378-8741(96)01384-0. [DOI] [PubMed] [Google Scholar]
- Cowart B. Oral chemical irritation: does it reduce perceived taste intensity? Chem Senses. 1987;12:467–479. [Google Scholar]
- Dalziel K, Segal L. Time to give nutrition interventions a higher profile: cost-effectiveness of 10 nutrition interventions. Health Promot Int. 2007;22:271–283. doi: 10.1093/heapro/dam027. [DOI] [PubMed] [Google Scholar]
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- Galgani JE, Ravussin E. Effect of dihydrocapsiate on resting metabolic rate in humans. Am J Clin Nutr. 2010;92:1089–1093. doi: 10.3945/ajcn.2010.30036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgani JE, Ryan DH, Ravussin E. Effect of capsinoids on energy metabolism in human subjects. Br J Nutr. 2010;103:38–42. doi: 10.1017/S0007114509991358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc. 1998;98:1118–1126. doi: 10.1016/S0002-8223(98)00260-0. [DOI] [PubMed] [Google Scholar]
- Hachiya S, Kawabata F, Ohnuki K, Inoue N, Yoneda H, Yazawa S, Fushiki T. Effects of CH-19 Sweet, a non-pungent cultivar of red pepper, on sympathetic nervous activity, body temperature, heart rate, and blood pressure in humans. Biosci Biotechnol Biochem. 2007;71:671–676. doi: 10.1271/bbb.60359. [DOI] [PubMed] [Google Scholar]
- Hall KD. Mechanisms of metabolic fuel selection: modeling human metabolism and body weight change. IEEE Eng Med Biol Mag. 2010;29:36–41. doi: 10.1109/MEMB.2009.935465. [DOI] [PubMed] [Google Scholar]
- Hall KD, Guo M, Dore M, Chow CC. The progressive increase of food waste in America and its environmental impact. PLoS One. 2009;4:e7940. doi: 10.1371/journal.pone.0007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Peters JC, Wyatt HR. Using the energy gap to address obesity: a commentary. J Am Diet Assoc. 2009;109:1848–1853. doi: 10.1016/j.jada.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Hursel R, Viechtbauer W, Westerterp-Plantega MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes. 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- Inoue N, Matsunaga Y, Satoh H, Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids) Biosci Biotechnol Biochem. 2007;71:380–389. doi: 10.1271/bbb.60341. [DOI] [PubMed] [Google Scholar]
- Iwai K, Yazawa A, Watanabe T. Roles as metabolic regulators of the non-nutrients, capsaicin and capsiate, supplemented to diets. Proc Jpn Acad. 2003;79:207–212. [Google Scholar]
- Jeffrey RW, French SA. Preventing weight gain in adults: design, methods and one year results from the Pound of Prevention study. Int J Obes Relat Metab Disord. 1997;21:457–464. doi: 10.1038/sj.ijo.0800431. [DOI] [PubMed] [Google Scholar]
- Josse AR, Sherriffs SS, Holwerda AM, Andrews R, Staples AW, Phillips SM. Effects of capsinoid ingestion on energy expenditure and lipid oxidation at rest and during exercise. Nutr Metab. 2010;7:65. doi: 10.1186/1743-7075-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata F, Inoue N, Yazawa S, Kawada T, Inoue K, Fushiki T. Effects of CH-19 sweet, a non-pungent cultivar of red pepper, in decreasing the body weight and suppressing body fat accumulation by sympathetic nerve activation in humans. Biosci Biotechnol Biochem. 2006;70:2824–2835. doi: 10.1271/bbb.60206. [DOI] [PubMed] [Google Scholar]
- Kawada T, Hagihara K-I, Iwai K. Effects of capsaicin on lipid metabolism in rats fed high fat diet. J Nutr. 1986;116:1272–1278. doi: 10.1093/jn/116.7.1272. [DOI] [PubMed] [Google Scholar]
- Kawada T, Sakabe S, Watanabe T, Yamamoto M, Iwai K. Some pungent principles of spices cause the adrenal medulla to secrete catecholamine in anesthetized rats. Proc Soc Exp Biol Med. 1988;188:229–233. doi: 10.3181/00379727-188-2-rc1. [DOI] [PubMed] [Google Scholar]
- Kawada T, Suzuki T, Takahashia M, Iwai K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol Appl Pharmacol. 1984;72:449–456. doi: 10.1016/0041-008x(84)90121-2. [DOI] [PubMed] [Google Scholar]
- Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med. 1986;183:250–256. doi: 10.3181/00379727-183-42414. [DOI] [PubMed] [Google Scholar]
- Kovacs EMR, Mela DJ. Metabolically active functional food ingredients for weight control. Obes Rev. 2006;7:59–78. doi: 10.1111/j.1467-789X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Kozukue N, Han JS, Kozukue E, Lee SJ, Kim JA, Lee KR, Levin CE, Friedman M. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography-mass spectrometry. J Agric Food Chem. 2005;53:9172–9181. doi: 10.1021/jf050469j. [DOI] [PubMed] [Google Scholar]
- Lawless H, Rozin P, Shenker J. Effects of oral capsaicin on gustatory, olfactory, and irritant sensations and flavor identification in humans who regularly or rarely consume chili pepper. Chem Senses. 1985;10:579–589. [Google Scholar]
- LeBlanc J, Cabanac M. Cephalic postprandial thermogenesis in human subjects. Physiol Behav. 1989;46:479–482. doi: 10.1016/0031-9384(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Lee AL, Li Z, Zerlin A, Heber D. Effects of dihydrocapsiate on adaptive and diet-induced thermogenesis with a high protein very low calorie diet: a randomized control trial. Nutr Metab. 2010;7:78. doi: 10.1186/1743-7075-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune MPGM, Kovacs EMR, Westerterp-Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr. 2003;90:651–659. doi: 10.1079/bjn2003938. [DOI] [PubMed] [Google Scholar]
- Lim K, Yoshioka M, Kikuzato S, Kiyonaga A, Tanaka H, Shindo M, Suzuki M. Dietary red pepper ingestion increases carbohydrate oxidation at rest and during exercise in runners. Med Sci Sport Exerc. 1997;29:355–361. doi: 10.1097/00005768-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Lopez-Cervantes M, Robles-Diaz G, Ramirez-Espitia A, Mohar-Betancourt A, Meneses-Garcia A, Lopez-Vidal Y, Blair A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer. 2003;106:277–282. doi: 10.1002/ijc.11195. [DOI] [PubMed] [Google Scholar]
- Ludy MJ, Mattes RD. Comparison of sensory, physiological, personality, and cultural attributes in regular spicy food users and non-users. 2011a doi: 10.1016/j.appet.2011.09.018. Appetite. doi:10.1016/j.appet.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Ludy MJ, Mattes RD. The effects of hedonically acceptable red pepper doses on thermogenesis and appetite. Physiol Behav. 2011b;102:251–258. doi: 10.1016/j.physbeh.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD. Dietary approaches to exploit energy balance utilities for body weight control. In: Coulston A, Boushey C, editors. Nutrition in the prevention and treatment of disease. Burlington, MA: Elsevier Academic Press; 2008. pp. 457–468. [Google Scholar]
- Matsumoto T, Miyawaki C, Ue H, Yuasa T, Miyatsuji A, Moritani T. Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. J Nutr Sci Vitaminol. 2000;46:309–315. doi: 10.3177/jnsv.46.309. [DOI] [PubMed] [Google Scholar]
- Ohnuki K, Niwa S, Maeda S, Inoue N, Yazawa S, Fushiki T. CH-19 sweet, a non-pungent cultivar of red pepper, increased body temperature and oxygen consumption in humans. Biosci Biotechnol Biochem. 2001;65:2033–2036. doi: 10.1271/bbb.65.2033. [DOI] [PubMed] [Google Scholar]
- Oster G, Thompson D, Edelsberg J, Bird AP, Colditz G. Lifetime health and economic benefits of weight loss among obese persons. Am J Public Health. 1999;89:1536–1542. doi: 10.2105/ajph.89.10.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich WD, Waters H, Weller H, Weller W, Bass EB. Systematic review of literature on the cost-effectiveness of nutrition services. J Am Diet Assoc. 2004;104:226–232. doi: 10.1016/j.jada.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Pellicore LS. Food and drug administration notification for CH-19 sweet extract. College Park (MD): 2007. Food and Drug Administration. Available from: http://www.capsiatenatura.com/fda_letter.aspx. Last accessed: 14 October 2011. [Google Scholar]
- Ravussin E, Tataranni PA. The role of altered sympathetic nervous system activity in the pathogenesis of obesity. Proc Nutr Soc. 1996;55:793–802. doi: 10.1079/pns19960079. [DOI] [PubMed] [Google Scholar]
- Reinbach HC. Hot spices: effects on appetite, energy intake and sensory properties of a meal. Copenhagen (Denmark): Department of Food Science, Faculty of Life Sciences, University of Copenhagen; 2008. p. 140. [Google Scholar]
- Reinbach HC, Martinussen T, Møller P. Effects of hot spices on energy intake, appetite, and sensory specific desires in humans. Food Qual Prefer. 2010;21:655–661. [Google Scholar]
- Reinbach HC, Smeets A, Martinussen T, Møller P, Westerterp-Plantenga MS. Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance. Clin Nutr. 2009;28:260–265. doi: 10.1016/j.clnu.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Rozin P, Schiller D. The nature and acquisition of a preference for chili pepper by humans. Motiv Emot. 1980;4:77–101. [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, et al. Weight loss with a low-carbohydrate, Mediterranean, or low fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- Shin KO, Moritani T. Alterations of autonomic nervous activity and energy metabolism by capsaicin ingestion during aerobic exercise in healthy men. J Nutr Sci Vitaminol. 2007;53:124–132. doi: 10.3177/jnsv.53.124. [DOI] [PubMed] [Google Scholar]
- Smeets A, Westerterp-Plantenga M. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur J Clin Nutr. 2009;48:229–234. doi: 10.1007/s00394-009-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiciklas-Wright H, Mitchell DC, Mickle SJ, Cook AJ, Goldman JD. Foods commonly eaten in the United States: quantities consumed per eating occasion and in a day, 1994–1996. Beltsville, MD: US Department of Agriculture; 2002. NFS Report No 96-5. [Google Scholar]
- Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr. 2009;89:45–50. doi: 10.3945/ajcn.2008.26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen LB, Møller P, Flint A, Martens M, Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes. 2003;27:1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Prescott J. The effects of prior experience with capsaicin on ratings of its burn. Chem Senses. 1994;19:651–656. doi: 10.1093/chemse/19.6.651. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Yeomans MR. Differences in ratings of intensity and pleasantness for the capsaicin burn between chili likers and non-likers: implications for liking development. Chem Senses. 1993;18:471–482. [Google Scholar]
- Takanohashi T, Isaka M, Ubukata K, Mihara R, Bernard BK. Studies of the toxicological potential of capsinoids XIII: inhibitory effects of capsaicin and capsinoids on cytochrome P450 3A4 in human liver microsomes. Int J Toxicol. 2010;29:22S–26S. doi: 10.1177/1091581809360282. [DOI] [PubMed] [Google Scholar]
- Veerman JL, Barendregt JJ, Van Beeck F, Seidell JC, Mackenbach JP. Stemming the obesity epidemic: a tantalizing prospect. Obesity. 2007;15:2365–2370. doi: 10.1038/oby.2007.280. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kawada T, Kato T, Harada T, Iwai K. Effects of capsaicin analogs on adrenal catecholamine secretion in rats. Life Sci. 1994;54:369–374. doi: 10.1016/0024-3205(94)00793-4. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Diepvens K, Joosen AMCP, Bérubé-Parent S, Tremblay A. Metabolic effects of spices, teas, and caffeine. Physiol Behav. 2006;89:85–91. doi: 10.1016/j.physbeh.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Smeets A, Lejeune MPG. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int J Obes. 2005;29:682–688. doi: 10.1038/sj.ijo.0802862. [DOI] [PubMed] [Google Scholar]
- Williamson DF. Descriptive epidemiology of body weight and weight change in U.S. adults. Ann Intern Med. 1993;119:646–649. doi: 10.7326/0003-4819-119-7_part_2-199310011-00004. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Nawata E. Use of Capsicum frutescens L. by the indigenous peoples of Taiwan and the Batanes Islands. Econ Bot. 2009;63:43–59. [Google Scholar]
- Yoshioka M, Imanaga M, Ueyama H, Yamane M, Kubo Y, Boivin A, St-Amand J, Tanaka H, Kiyonaga A. Maximum tolerable dose of red pepper decreases fat intake independently of spicy sensation in the mouth. Br J Nutr. 2004;91:991–995. doi: 10.1079/BJN20041148. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Lim K, Kikuzato S, Kiyonaga A, Tanaka H, Shindo M, Suzuki M. Effects of red-pepper diet on the energy metabolism in men. J Nutr Sci Vitaminol. 1995;41:647–656. doi: 10.3177/jnsv.41.647. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, St-Pierre S, Drapeau V, Dionne I, Doucet E, Suzuki M, Tremblay A. Effects of red pepper on appetite and energy intake. Br J Nutr. 1999;82:115–123. [PubMed] [Google Scholar]
- Yoshioka M, St-Pierre S, Suzuki M, Tremblay A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br J Nutr. 1998;80:503–510. doi: 10.1017/s0007114598001597. [DOI] [PubMed] [Google Scholar]
- Zandstra EH, De Graaf C, Mela DJ, Van Staveren WA. Short- and long-term effects of changes in pleasantness on food intake. Appetite. 2000;34:253–260. doi: 10.1006/appe.1999.0304. [DOI] [PubMed] [Google Scholar]