Abstract
It is becoming increasingly evident that people with chronic, recurrent low back pain (LBP) exhibit changes in cerebrocortical activity that associate with altered postural coordination, suggesting a need for a better understanding of how the experience of LBP alters postural coordination and cerebrocortical activity. To characterize changes in postural coordination and pre-movement cerebrocortical activity related to the experience of acutely induced LBP, 14 healthy participants with no history of LBP performed sit-to-stand movements in 3 sequential conditions: (1) without experimentally induced LBP; NoPain1, (2) with movement-associated LBP induced by electrocutaneous stimulation; Pain, and (3) again without induced LBP; NoPain2. The Pain condition elicited altered muscle activation and redistributed forces under the seat and feet prior to movement, decreased peak vertical force exerted under the feet during weight transfer, longer movement times, as well as decreased and earlier peak hip extension. Stepwise regression models demonstrated that electroencephalographic amplitudes of contingent negative variation during the Pain condition significantly correlated with the participants’ change in sit-to-stand measures between the NoPain1 and Pain conditions, as well as with the subsequent difference in sit-to-stand measures between the NoPain1 and NoPain2 conditions. The results, therefore, identify the contingent negative variation as a correlate for the extent of an individual’s LBP-related movement modifications and to the subsequent change in movement patterns from before to after the experience of acutely induced LBP, thereby providing a direction for future studies aimed to understand the neural mechanisms underlying the development of altered movement patterns with LBP.
Keywords: contingent negative variation, low back pain, posture, sit-to-stand, electroencephalography
Introduction
Chronic, recurrent low back pain (LBP) represents a common and costly disorder (Andersson 1999; Katz 2006) for which a structurally based diagnosis is often unidentified (Hart et al. 1995; Hiebert et al. 2007a; Hiebert et al. 2007b; Rom and Markowitz 2007), thereby requiring a better understanding of the mechanisms that contribute to LBP. Altered movement patterns have been proposed as one possible mechanism for developing chronic, recurrent LBP by generating injurious tissue loads that perpetuate pain-inducing musculoskeletal pathology (Sahrmann 2002; Langevin and Sherman 2007; Hammill et al. 2008). In support of this model, people with LBP exhibit altered postural coordination during voluntary tasks (Gioftsos and Grieve 1996; Kuriyama and Ito 2005; Shum et al. 2005b; Shum et al. 2005a; Mok et al. 2007; MacDonald et al. 2009; Scholtes et al. 2009), and correcting their symptom-provoking movement patterns decreases the LBP (Harris-Hayes et al. 2005; Van Dillen et al. 2005; Van Dillen et al. 2009). In addition, changes in postural coordination persist when evaluating people with a history of recurrent LBP during a remission of pain (MacDonald et al. 2009), and these changes in postural coordination are concomitant with altered motor function within the cerebral cortex (Tsao et al. 2008; Jacobs et al. 2010). Therefore, the altered postural coordination associated with LBP represents a long-term modification in the central neural control of posture that may contribute to chronic, recurrent LBP symptoms.
Models of experimentally induced LBP have provided insight into the mechanisms by which an acute experience of LBP affects postural coordination (Moseley and Hodges 2005; Moseley and Hodges 2006), demonstrating that painful stimulation of the lumbar region elicits changes in postural coordination that are similar to those exhibited by people with a history of chronic, recurrent LBP (Hodges and Richardson 1999; Jacobs et al. 2009). In addition, the persistence of the postural modifications following the pain experience associates with higher pain-related anxiety (Moseley and Hodges 2006). The neurophysiologic mechanisms associated with acute pain-related modifications of postural coordination, however, remain unclear.
The electroencephalographic (EEG) potential of contingent negative variation (CNV) offers a potential measure for understanding the neural mechanisms that underlie pain-related changes in postural coordination. The CNV potential is a slow negative shift in the EEG signal that occurs prior to movements performed in response to an imperative stimulus preceded by a warning stimulus (Walter et al. 1964) and represents cerebrocortical activity related to the anticipation of the imperative stimulus, pre-movement sensory-motor status, and motor preparation for the response (Kok 1978; Haagh and Brunia 1985; van Boxtel and Brunia 1994; Pfeuty et al. 2008). The neural generators of the CNV include motor, cognitive and sensory components from the primary, premotor and supplementary motor cortex, as well as the prefrontal and parietal-temporal cortex (Lamarche et al. 1995; Hamano et al. 1997; Bares et al. 2007). The amplitude of the CNV is sensitive to changes in movement force, speed, and complexity as well as attention and experimentally induced pain (Low and McSherry 1968; Kok 1978; Grünewald et al. 1979; Cui et al. 2000; Stude et al. 2003; Babiloni et al. 2004; Babiloni et al. 2005). Thus, the CNV offers a potential measure for understanding changes in the neural control of posture associated with LBP.
This study investigated the changes in postural coordination and CNV potentials associated with experimentally induced LBP experienced during a sit-to-stand movement. The sit-to-stand movement was chosen due to its ecological relevance as a common activity of daily living, and because people with LBP exhibit altered coordination patterns, movement times, forces, and energy transfer when performing a sit-to-stand task (Coghlin and McFadyen 1994; Gioftsos and Grieve 1996; Simmonds et al. 1998; Shum et al. 2005a; Shum et al. 2007; Shum et al. 2009). We hypothesized that (A) experimentally induced LBP elicits changes in postural coordination of the sit-to-stand movement similar to those previously reported to associate with chronic LBP, and (B) cerebrocortical activity prior to painful movement, as measured by the CNV potential, associates with pain-related changes in postural coordination as well as with the extent of postural modification that remains after pain cessation.
Methods
Participants
Fourteen healthy participants with no self-reported history of LBP gave written informed consent in compliance with the Declaration of Helsinki to participate in the protocol, which was approved by the local Institutional Review Board. The participant sample included 8 females and 6 males with a mean (range) age of 28 (19-47) years, height of 165 (151-178) cm, and weight of 56 (43-75) kg. Based on an intake questionnaire, participants were included if they additionally reported no history of neurological, cardiovascular, respiratory, or psychiatric disorders, as well as no history or diagnosis of neuromuscular or joint disorders, dizziness, diabetes, systemic infection, alcoholism, tumor or suspected carcinoma, surgery in the previous three months, any back surgery, spinal fracture or dislocation, herniated disc, stenosis, kyphosis, or scoliosis.
Protocol
The task was to stand up from a seated position (illustrated in Fig. 1A) in response to an auditory movement cue that was preceded 2 seconds earlier by a warning cue (each presented at 2000 Hz for 100 ms and 35 dB above each participant’s hearing threshold). The participants initially sat on a force plate mounted on an adjustable frame with their feet placed on another force plate located on the floor. The seat height was adjusted to that of each participant’s lateral femoral epicondyle. The participants sat with the midpoint of their thigh (defined from the lateral femoral epicondyle to the greater trochanter) aligned to the front edge of the seat as well as with their trunk (defined from the greater trochanter to the acromium) oriented vertically and perpendicular to the seat. The feet were placed in parallel at a stance width of 10 cm between the medial aspects of the feet and were positioned in the anterior-posterior plane so as to create 10 degrees of ankle dorsiflexion. The participants also maintained their arms crossed in front of their torso. This initial seated position was confirmed prior to every trial.
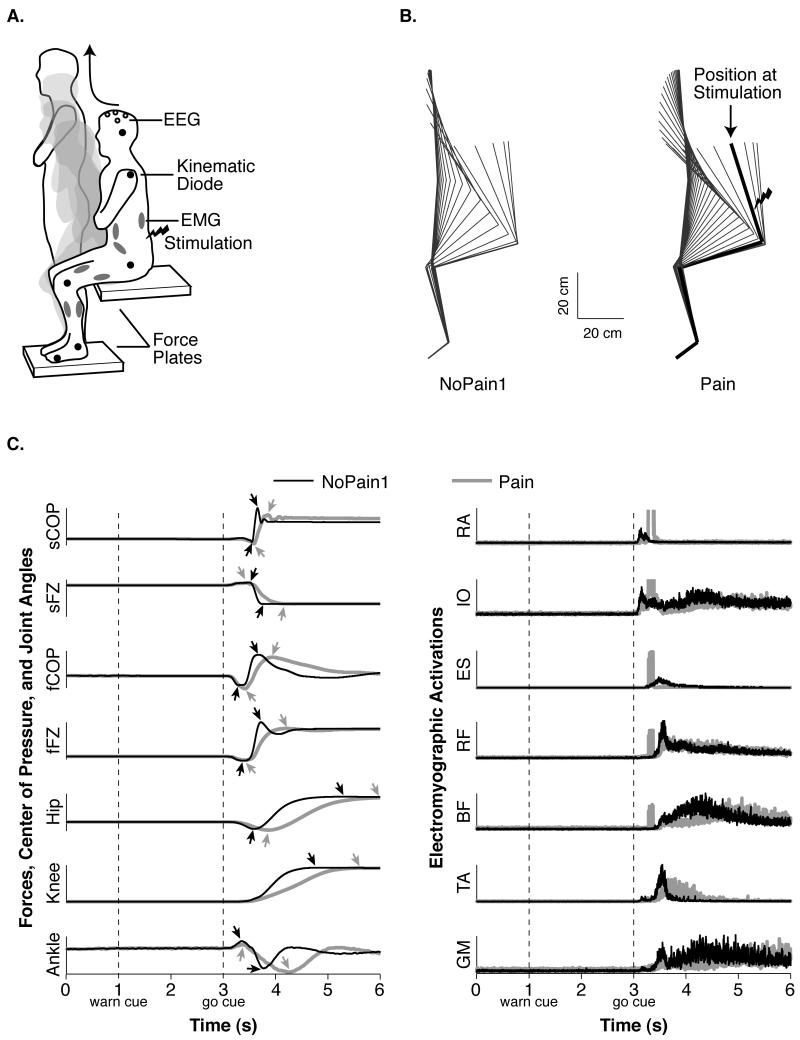
Fig. 1.
Illustration of the task and representative behavior. (A) The participants performed the sit-to-stand task amidst EEG, EMG, kinematic, and kinetic recordings. (B) Average sagittal kinematics of a representative participant from markers placed on the shoulder, hip, knee, ankle, and foot during the NoPain1 and Pain conditions. The lines span the total movement time and are separated by 100 ms (more lines denote a longer movement time). The thick line for the Pain condition’s example demonstrates this participant’s position when the painful stimulation occurred. (C) The graphs on the left illustrate average traces of a representative participant’s center of pressure under the seat (sCOP) and feet (fCOP), vertical forces under the seat (sFZ) and feet (fFZ), as well as joint angles of the hip, knee, and ankle during the NoPain1 (black traces) and Pain (gray traces) conditions. The vertical dotted lines denote the time of the warning and movement cues, and the arrowheads denote maxima and minima. The chart on the right illustrates representative EMG responses of the rectus abdominus (RA), internal oblique (IO), erector spinae (ES), rectus femoris (RF), biceps femoris (BF), tibialis anterior (TA), and gastrocnemius medialis (GM). The square-wave artifacts in the gray traces were generated by the electrocutaneous stimulations of the Pain condition.
Once positioned, the participants were instructed to pay attention to the auditory cues, remain still without blinking their eyes prior to the movement cue, stand up as soon as possible at a self-selected speed in response to the movement cue, and then to hold their standing position until asked to be reseated at the end of the trial. The participants performed 5 practice trials prior to recording to ensure they understood the task’s instructions. The participants then performed the task in 3 conditions presented in the following order: (1) without induced LBP – the NoPain1 condition, (2) with LBP induced by electrocutaneous stimulation – the Pain condition, and (3) again without induced LBP – the NoPain2 condition. The NoPain1 and Pain conditions permitted analysis of pain-related changes in sit-to-stand behavior and CNV amplitudes, and the NoPain2 condition permitted analysis of persistent or novel modifications in sit-to-stand behavior and CNV amplitudes after the experience of pain.
Prior to the Pain condition, we established the intensity of electrocutaneous stimulation for each participant. The stimulation consisted of a 60-Hz, 100-ms train of 1-ms pulses delivered bilaterally via surface electrodes that were placed over the posterior superior iliac spines. The participants were shown a number scale from 0-10, with 0 representing no pain, 3 representing mild pain, 5 representing moderate and uncomfortable pain, 7 representing awful pain, and 10 representing maximum and unbearable pain. Starting from low stimulus intensity, stimulations were then presented with incrementally increasing intensity until the participants rated the pain as a 5 on the scale. We asked the participants to confirm the intensity of the pain after every 5-10 trials during the Pain condition and, if necessary, the intensity was increased if the participant reported a value below a 5 in order to re-establish the target pain rating. Stimulations were presented 200 ms after the onset of each participant’s first muscle activation (rectus abdominus or tibialis anterior), which was determined from the average onset recorded during the NoPain1 condition. Although the electrocutaneous stimulations may not induce muscular pain as felt with chronic, recurrent LBP, this model provided precise temporal and spatial control in order to isolate the pain to the low back and associate the pain with the act of standing up. The painful stimulation could then be withdrawn in the same session to assess changes in postural coordination after the cessation of pain. In addition, electrocutaneous stimulations of the same characteristics were found to induce changes in postural coordination that are also evident in people with a natural history of chronic, recurrent LBP (Moseley and Hodges 2005; Moseley and Hodges 2006).
The participants performed the minimum number of trials necessary to generate 20 trials in each condition without ocular artifacts in the EEG signals or without anticipatory movement prior to the movement cue; up to 40 trials were performed in each condition to reach these criteria. The participants were aware that no painful stimulations would occur in the NoPain1 and NoPain2 conditions and that painful stimulations would occur with every trial in the Pain condition. The participants rested, at minimum, after every 10 trials and were instructed to request additional rest whenever needed. Following each condition, the participants rated the level of their anxiety for performing the task on a 0-10 scale, zero representing no anxiety and 10 representing maximum anxiety.
Data Collection and Processing
All data were recorded with 16-bit resolution and at 1000 Hz from 3 seconds before the movement cue to 4 seconds after the movement cue.
Forces and Kinematics
To record the ground reaction forces associated with the participants’ sit-to-stand movement, vertical forces and the anterior-posterior center of pressure (CoP) were generated from the force plates under the participants’ seat and feet (Fujiwara et al. 2003). In addition, the participants’ sagittal joint angles were quantified from the positions of infrared emitting diodes placed on the participants’ joints. Hip angle was defined from the segments adjoining the diodes placed on the lateral femoral epicondyle, greater trochanter, and acromium; knee angle was defined from diodes at the lateral maleolus, lateral femoral epicondyle, and greater trochanter; ankle angle was defined from diodes at the fifth metatarsal, lateral maleolus, and lateral femoral epicondyle. The signals were then low-pass filtered offline to 10 Hz. Onset of movement was determined as the moment the CoP of the seat’s force plate displaced greater than 3 standard deviations from the mean baseline of the 500-ms interval that immediately preceded the first warning cue. The end of the sit-to-stand movement was determined as the moment that the velocity of hip extension first reached zero following peak extension velocity. The time of onset was then subtracted from that of the end of the movement to calculate the sit-to-stand’s movement time. The peak and time-to-peak (as a percentage of movement time) displacements of the vertical forces, CoP, and joint angles additionally quantified the sit-to-stand movement. Initial forces, CoP positions, and joint angles were quantified from the 100-ms epochs that preceded the warning and movement cues to quantify pain-related changes in preparatory state.
Electromyography
Surface electromyography (EMG) was recorded from silver/silver-chloride electrodes placed in a bipolar montage over the right tibialis anterior, gastrocnemius medialis, rectus femoris, biceps femoris, internal oblique, rectus abdominus, and lumbar erector spinae. The EMG signals were amplified, band-pass filtered at 30-400 Hz (Drake and Callaghan 2006), and then rectified for analysis. The onset of EMG activation was determined as the moment the rectified EMG signal displaced greater than 3 standard deviations from baseline, which was defined from the 500-ms interval that immediately preceded the first warning cue. Amplitudes of initial EMG activity were determined from the integrated EMG evaluated over the 100-ms epochs that immediately preceded the warning and movement cues.
Electroencephalography
To record the CNV potential prior to movement, silver/silver-chloride EEG electrodes were affixed to the scalp at the Fz, Cz, C3, C4, and Pz locations defined by the 10/20 system of electrode placement (Jasper 1958) and were referred to linked electrodes affixed to the earlobes. In addition to the EEG electrodes, electrodes were affixed above and below the left orbit for electrooculographic (EOG) recordings in order to identify trials with ocular artifacts, remove them from analysis, and continue to collect trials until 20 artifact-free trials were achieved per condition; trials were considered to exhibit artifact if an EEG or EOG signal displaced greater than ± 100 μV prior to the movement cue. The EEG signals were band-pass filtered from 0.05-60 Hz, and the EOG signals were filtered from 0.05-30 Hz. Electrode impedance was kept below 5 kΩ for the EEG, EOG, and EMG electrodes. After collection, the 20 artifact-free trials of EEG signals were then low-pass filtered offline to 5 Hz in order to isolate the CNV signal of interest and then averaged to generate the mean CNV potential for each participant within each condition. The amplitude of this mean CNV potential was quantified as the average amplitude exhibited during the 100-ms epoch immediately preceding the movement cue.
Statistical Analysis
The initial forces, CoP positions, joint angles, and EMG activations were compared over the 100-ms epochs prior to the warning and movement cues as well as across the three sit-to-stand conditions by 2-factor repeated-measures ANOVA. Peak and time-to-peak force, CoP, and joint-angle displacements, as well as movement times, were compared across the three sit-to-stand conditions by 1-factor repeated-measures ANOVA. CNV amplitudes were compared across the five electrodes and the three sit-to-stand conditions by 2-factor repeated-measures ANOVA. A Greenhouse-Geisser correction was applied to the repeated-measures ANOVA statistics. To confirm the EMG patterns of the sit-to-stand movement (Goulart and Valls-Solé 1999) using the standardized positioning of this study, the EMG onset times of every muscle were compared by a one-way ANOVA followed by post-hoc paired comparisons to establish the activation pattern exhibited in the NoPain1 condition. The EMG onset times were not compared across the experimental conditions, however, due to artifacts generated by the electrocutaneous stimulations.
In addition to identifying consistent group differences among conditions in postural coordination and cerebrocortical activity, correlation analysis was expected to be instructive because multiple strategies are often employed to achieve the sit-to-stand movement (Coghlin and McFadyen 1994) and because changes in postural coordination associated with LBP can vary by group or individual; either increases or decreases in movement velocity and lumbar flexion are evident during a sit-to-stand task (Gioftsos and Grieve 1996; Shum et al. 2005a) and individuals with LBP can exhibit tendencies for increased lumbo-pelvic extension, flexion, or rotation across multiple movement tasks (Van Dillen et al. 2003). In addition, amplitudes of sensory-motor cortical function correlate with measures of postural coordination (Tsao et al. 2008; Jacobs et al. 2010), even when differences between participants with and without LBP are not consistent enough to elicit statistical significance (Jacobs et al. 2010). Thus, due to high inter-individual variability, it may be expected to see no significant differences in outcome measures across conditions for a group of participants, but to have pain-related modifications in the individual participants’ postural coordination correlate with their CNV amplitudes.
In order to determine whether the participants’ pain-related changes in postural coordination associate with their CNV amplitudes prior to LBP-inducing movements, stepwise linear regressions were employed to identify the subset of variables whose changes in the Pain and NoPain2 conditions from the NoPain1 condition independently associate with the participants’ CNV amplitudes. Four stepwise models were generated: one each to predict CNV amplitudes at the CZ and C4 electrodes from differences in outcome measures either between the Pain and NoPain1 condition or between the NoPain2 and NoPain1 condition. The variables tested in the model included peak and time-to-peak hip flexion and extension, knee flexion and extension, ankle plantar-flexion and dorsi-flexion, and vertical force under the feet, as well as movement time, anxiety scores, and initial EMG amplitudes and forces under the feet. The models testing CNV amplitudes at the CZ electrode represent planned analyses because this location elicits the maximum CNV amplitude for this task and overlies cortex that is somatotopically most relevant to the sit-to-stand task. The models on CNV amplitudes at the C4 electrode, however, were generated post hoc based on findings that the Pain condition elicited changes in the CNV potential at that electrode location.
Results
Initial Conditions Prior to the Sit-to-Stand Movement
The participants maintained similar initial joint angles and CoP positions prior to movement across the three conditions (main effect of condition: range of F = 0.50-2.07, P = 0.17-0.52; condition-by-epoch interaction: range of F = 0.01-2.14, P = 0.14-0.98), and only the knee angle demonstrated a significant shift in initial position between the warning and movement cues (main effect of epoch: F = 4.68, P = 0.05) (Table 1). The average change in knee angle from before the warning cue to before the movement cue, however, never exceeded 0.1 degrees for any participant.
Table 1. Mean (SD) Initial Position, Force and EMG Prior to the Warning and Movement Cues.
| Measure | 100-ms Epoch | Condition |
|||
|---|---|---|---|---|---|
| NoPain1 | Pain | NoPain2 | Significance | ||
| Hip Angle (deg) |
Before Warning | 106.6 (3.5) | 106.4 (3.5) | 106.5 (3.6) | NS |
| Before Movement | 106.6 (3.5) | 106.4 (3.5) | 106.5 (3.6) | ||
| Knee Angle (deg) |
Before Warning | 94.9 (4.1) | 95.1 (4.2) | 95.0 (4.3) | NS |
| Before Movement | 94.9 (4.1) | 95.1 (4.2) | 95.0 (4.3) | ||
| Ankle Angle (deg) |
Before Warning | 112.7 (4.7) | 112.4 (4.8) | 112.4 (5.0) | NS |
| Before Movement | 112.7 (4.8) | 112.4 (4.8) | 112.4 (5.0) | ||
| Anterior CoP Under Feet (cm) |
Before Warning | 10.6 (3.0) | 10.6 (3.0) | 10.7 (3.0) | NS |
| Before Movement | 10.6 (3.0) | 10.6 (3.0) | 10.7 (3.0) | ||
| Anterior CoP Under Seat (cm) |
Before Warning | 20.9 (1.9) | 20.7 (1.8) | 20.7 (1.7) | NS |
| Before Movement | 20.9 (2.0) | 20.7 (1.8) | 20.7 (1.7) | ||
| Feet Vertical Force (% wgt) |
Before Warning | 13.9 (1.6) | 14.3 (1.5) | 14.5 (1.7) | P < 0.000005 |
| Before Movement | 13.8 (1.6) | 14.2 (1.5) | 14.4 (1.7) | ||
| Seat Vertical Force (% wgt) |
Before Warning | 86.6 (2.8) | 86.0 (2.8) | 86.0 (3.1) | P < 0.05 |
| Before Movement | 86.7 (2.8) | 86.1 (2.8) | 86.0 (3.1) | ||
| Rectus Abdom. (uV*s) |
Before Warning | 97 (21) | 107 (17) | 94 (10) | NS |
| Before Movement | 97 (21) | 108 (15) | 97 (13) | ||
| Internal Oblique (uV*s) |
Before Warning | 269 (218) | 264 (186) | 243 (186) | NS |
| Before Movement | 275 (237) | 261 (180) | 246 (212) | ||
| Erector Spinae (uV*s) |
Before Warning | 623 (376) | 687 (429) | 771 (559) | NS |
| Before Movement | 622 (409) | 680 (458) | 731 (545) | ||
| Rectus Femoris (uV*s) |
Before Warning | 97 (28) | 118 (33) | 92 (20) | P < 0.0005 |
| Before Movement | 100 (38) | 122 (37) | 93 (21) | ||
| Biceps Femoris (uV*s) |
Before Warning | 83 (15) | 108 (27) | 85 (13) | P < 0.0005 |
| Before Movement | 83 (16) | 107 (28) | 84 (14) | ||
| Tibialis Anterior (uV*s) |
Before Warning | 231 (277) | 152 (115) | 107 (38) | NS |
| Before Movement | 248 (305) | 170 (162) | 119 (49) | ||
| Gastrocnemius Medialis (uV*s) |
Before Warning | 86 (13) | 107 (27) | 86 (15) | P < 0.005 |
| Before Movement | 88 (15) | 107 (29) | 88 (13) | ||
NS - not significant (P > 0.05); wgt – weight; abdom – abdominus
Although the initial joint and CoP positions were similar across the 3 conditions, the Pain condition elicited significant modifications in the distribution of vertical force under the feet and seat, as well as in the amplitudes of initial EMG activation (Table 1). Specifically, the participants significantly increased the initial vertical force under their feet and decreased the force under the seat during the Pain and NoPain2 conditions compared to during the NoPain1 condition (main effect of condition: F = 25.31, P < 0.000005 for under the feet; F = 6.38, P < 0.05 for under the seat). In addition, initial EMG amplitudes increased in the Pain condition compared to the other conditions at the rectus femoris (main effect of condition: F = 14.12, P < 0.0005), biceps femoris (main effect of condition: F = 22.62, P < 0.0005), and gastrocnemius medialis muscles (main effect of condition: F = 13.84, P < 0.005).
Sit-to-Stand Characteristics Before Experiencing Induced LBP: the NoPain1 Condition
To characterize the sit-to-stand behavior (Fig. 1), the participants began moving by flexing the hips and slightly redistributing their weight from the feet to the seat. The participants subsequently loaded their weight under the feet, and extended the hips and knees until they achieved a standing position. An ankle dorsiflexion and then plantarflexion coincided with the hip and knee extensions. Some small and slow adjustments in hip-joint angle occurred after reaching a zero velocity of hip extension at the end of the movement. Thus, maximum hip extension was not always evident within the defined movement time.
Significant differences in EMG onset times (F = 23.46, P < 0.000001) were evident among the recorded muscles in the NoPain1 condition. The tibialis anterior, rectus abdominus, and internal oblique first activated with statistically similar onset times (mean onset ± the standard deviation = 187 ± 71, 195 ± 91, and 238 ± 144 ms after the movement cue, respectively). The onset of the tibialis anterior, however, reflects an initially small increase in activation amplitude that was later proceeded by a much larger burst of activity. The rectus femoris and gastrocnemius medialis subsequently activated (mean onset ± the standard deviation = 298 ± 104 and 384 ± 172 ms, respectively), followed by the erector spinae (mean onset ± the standard deviation = 483 ± 79 ms) and, lastly, the biceps femoris (mean onset ± the standard deviation = 555 ± 81 ms).
Differences Among The Sit-To-Stand Conditions
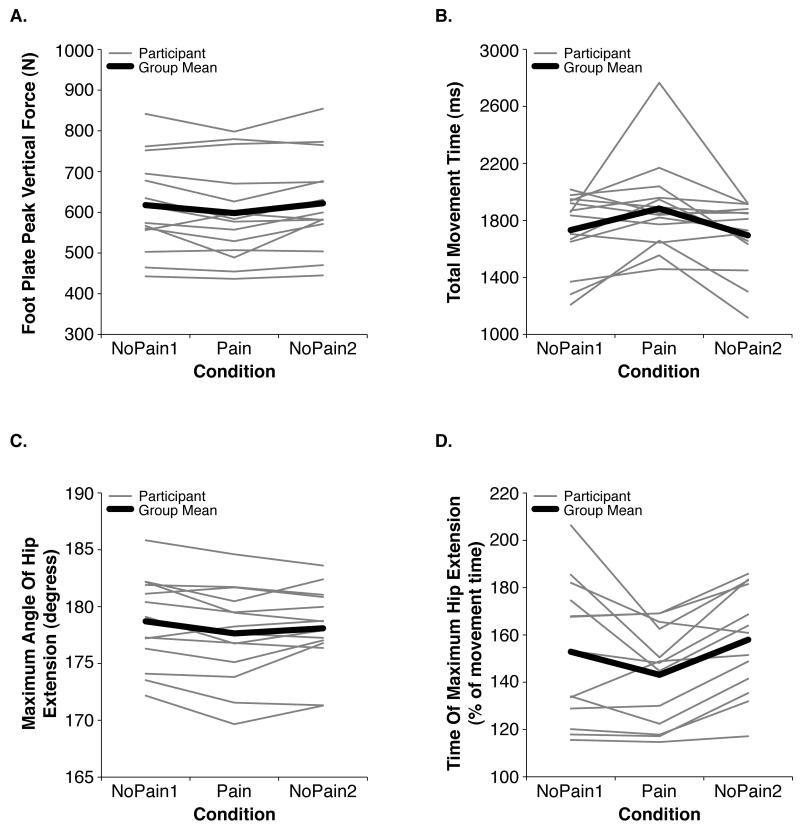
The Pain condition associated with significantly decreased maximum vertical force under the feet (F = 5.87, P < 0.05) (Fig. 2A), significantly increased movement times (F = 5.26, P < 0.05) (Fig. 2B), and significantly decreased peak hip extension (F = 4.43, P < 0.05) that occurred at an earlier time relative to the total movement time (F = 5.24, P < 0.05) (Fig. 2C and D). The participants all reported no anxiety (rating = 0) after performing the NoPain1 and NoPain2 conditions, and reported a median rating of 3 (range = 0-5) out of 10 after the Pain condition (F = 53.61, P < 0.000001).
Fig. 2.
Mean differences among conditions in the (A) maximum vertical force under the feet, (B) movement time, (C) maximum angle of hip extension, and (D) the time of maximum hip extension as a percentage of the total movement time. The thin gray lines represent average values for each participant, and the thick black line represents the group mean. All of the illustrated variables exhibited significant (P < 0.05) changes in the Pain condition relative to one or both of the NoPain conditions. Notice the variability in the participants’ absolute values and in the extent of the condition-related changes in behavior.
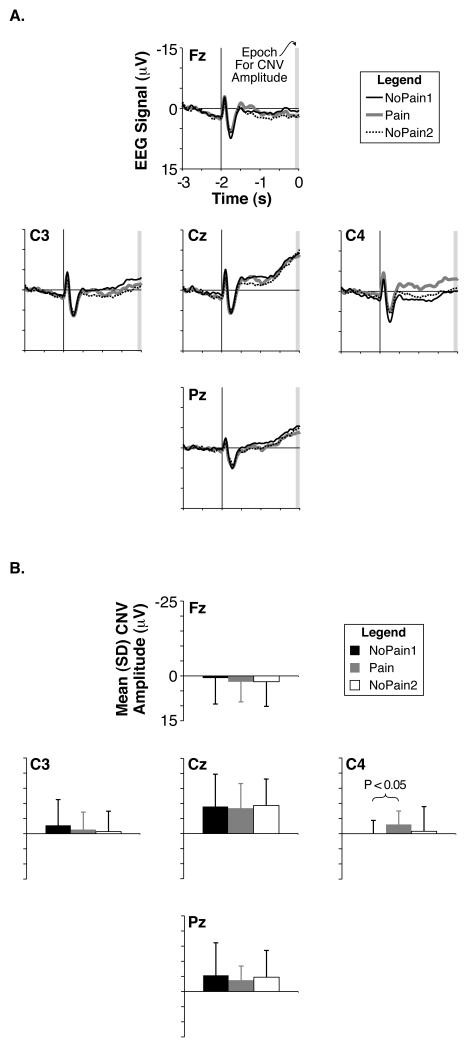
The participants’ CNV amplitudes were not statistically different among the conditions (main effect of condition: F = 0.05, P = 0.92; condition-by-electrode interaction: F = 0.64, P = 0.61) and were largest at the Cz electrode (main effect of electrode: F = 13.15, P < 0.000005) (Fig. 3). Although not significant in the ANOVA statistic comparing all electrodes and conditions, increased average CNV amplitudes were visually evident at the C4 electrode in the Pain condition (Fig. 3), and a post-hoc comparison by a paired t-test demonstrated significantly increased CNV amplitudes in the Pain Condition compared to the NoPain1 condition at the C4 location (T = 2.32; P < 0.05).
Fig. 3.
Grand mean CNV potentials. (A) Grand average EEG waveforms at frontal, central, and parietal midline electrodes (Fz, Cz, and Pz, respectively) as well as left-hemisphere and right-hemisphere dorsolateral central electrodes (C3 and C4, respectively) during the NoPain1 (thin black traces), Pain (thick gray traces), and NoPain2 (dotted black traces) conditions. The time scale is relative to the movement cue, with the warning cue illustrated at −2 s by a vertical line. The gray rectangle denotes the final 100-ms epoch prior to the movement cue used to calculate the average CNV amplitude. (B) Group mean CNV amplitudes calculated from the mean EEG amplitude of the final 100-ms epoch preceding the movement cue for each electrode and condition. Color and name schemes are the same as in (A) except the NoPain2 condition is represented by white bars.
Associations Among CNV Amplitudes And Sit-To-Stand Characteristics
When examining changes in sit-to-stand behavior between the NoPain1 and Pain conditions by stepwise regression, the participants’ maximum vertical force exerted under the feet provided the only significant independent predictor of CNV amplitudes at the Cz electrode, whereas the participants’ initial activation of the erector spinae muscle, movement time, peak hip flexion, relative time of peak ankle dorsiflexion, and maximum vertical force exerted under the feet all provided significant independent predictors of CNV amplitudes at the C4 electrode (Table 2). For the regression model on CNV amplitudes at the C4 electrode, single-variable Pearson’s correlations identified maximum vertical force under the feet as the only variable of the 5 significant independent predictors of the regression model listed above to be significantly correlated with CNV amplitudes (Table 3). Thus, the other four variables provided significant independent contributions to predicting CNV amplitudes only with adjustment for the other variables in the multiple-variable model.
Table 2. Regression Models Associating Sit-to-Stand Modifications With CNV Amplitudes.
| Condition Difference |
Electrode | Sit-to-Stand Modification |
Coefficient of Determination |
Change in F Statistic |
Significance of F Change |
|---|---|---|---|---|---|
| NoPain1 Minus Pain |
Cz | ||||
| Maximum Vertical Force Under Feet |
0.30 | 5.08 | P = 0.044 | ||
| C4 | |||||
| Maximum Vertical Force Under Feet |
0.49 | 11.50 | P = 0.005 | ||
| Initial Erector Spinae Activity |
0.72 | 8.85 | P = 0.013 | ||
| Time of Peak Ankle Flexion |
0.85 | 8.36 | P = 0.016 | ||
| Movement Time |
0.91 | 6.15 | P = 0.035 | ||
| Peak Hip Flexion |
0.95 | 5.43 | P = 0.048 | ||
| NoPain1 Minus NoPain2 |
Cz | ||||
| Time of Peak Hip Extension |
0.48 | 10.97 | P = 0.006 | ||
| C4 | |||||
| Peak Ankle Flexion |
0.52 | 12.81 | P = 0.004 |
Table 3. Correlation Matrix of Significant Independent Predictors of CNV Amplitudes at C4.
| CNV Amplitude at C4 |
Max Vertical Force Under Feet |
Initial Erector Spinae Activity |
Time of Peak Ankle Flexion |
Movement Time |
|
|---|---|---|---|---|---|
| Max Vertical | R = 0.70 | ||||
| Force Under Feet | P = 0.005 | ||||
| Initial Erector | R = −0.38 | R = 0.14 | |||
| Spinae Activity | P = 0.18 | P = 0.65 | |||
| Time of Peak | R = 0.42 | R = −0.05 | R = −0.24 | ||
| Ankle Flexion | P = 0.13 | P = 0.86 | P = 0.41 | ||
| Movement | R = −0.19 | R = −0.61 | R = −0.13 | R = 0.09 | |
| Time | P = 0.52 | P = 0.02 | P = 0.67 | P = 0.75 | |
| Peak Hip | R = 0.34 | R = 0.47 | R = 0.09 | R = 0.05 | R = −0.71 |
| Flexion | P = 0.23 | P = 0.09 | P = 0.76 | P = 0.86 | P = 0.004 |
When examining changes in sit-to-stand behavior between the NoPain1 and NoPain2 conditions by stepwise regression, the relative time of the participants’ maximum hip extension provided the only significant independent predictor of CNV amplitudes at the Cz electrode, and the participants’ maximum ankle dorsiflexion provided the only significant independent predictor of CNV amplitudes at the C4 electrode (Table 2).
Discussion
Experimentally induced LBP elicited altered postural coordination during and after the experience of pain, and this altered coordination associated with CNV amplitudes. Specifically, the Pain condition elicited smaller peak forces, longer movement times, and altered hip motion during the sit-to-stand movement, similar to previous reports on people with chronic, recurrent LBP (Gioftsos and Grieve 1996; Simmonds et al. 1998; Shum et al. 2005a). These movement modifications exhibited in the Pain condition concomitantly occurred with increased self-reported anxiety to perform the sit-to-stand movement. A post-hoc exploratory analysis revealed that amplitudes of CNV potentials at the C4 electrode increased during the Pain condition compared to the NoPain1 condition, but no significant effects of condition on CNV amplitudes were evident when analyzed by the omnibus statistic. As expected, the group exhibited high inter-individual variability in both motor and neurophysiologic responses to the induced LBP. Amidst the high inter-individual variability that prevented a significant pain-related change in the CNV response across the group, however, the participants’ CNV amplitudes in the Pain condition exhibited moderate to very high coefficients of determination with multiple pain-related changes in movement parameters. The CNV amplitudes exhibited in the Pain condition also moderately associated with the extent that movement parameters were modified from before to after the pain experience. The CNV potential, therefore, provides a neurophysiologic correlate for changes in postural coordination associated with an acute LBP experience.
The pain-related increase in CNV amplitude at the C4 electrode (located on the dorsolateral convexity overlying the sensory-motor and premotor cortex) was not as we predicted; we predicted changes to occur at the Cz electrode, where the CNV potential exhibited its maximal amplitude and is somatotopically localized over the task-relevant leg and trunk region of the sensory-motor cortex. Asymmetric pain-related increases in CNV amplitude, however, have been previously reported at the C4 electrode, but the previous study examined a model of induced pain and motor responses at the finger (Babiloni et al. 2004), rendering it unclear whether the lateral asymmetry reflected the motor characteristics of the task and the somatotopic organization of the sensory-motor cortex rather than somatotopically non-specific pain-associated activity. The increase at the C4 electrode in this study, however, would not likely reflect activation of the arm or hand region, or be asymmetric, because this study’s sit-to-stand task primarily involved bilateral function of the legs and trunk and utilized a bilateral pain stimulus to the low back.
Although speculative and requiring further study for confirmation, the pain-related increase at C4 may reflect activity similar to other reports of right-hemisphere lateralized cortical activity, such as (1) non-motoric right-hemisphere lateralizations of the CNV potential that represent covert shifts in attention (Van ’t Ent and Apkarian 1998), (2) right-hemisphere cerebral hemodynamic responses to induced pain stimuli that depend on the individual’s attention to the painful stimulus (Peyron et al. 1999), and/or (3) the right-hemisphere cerebral hemodynamic responses of the lateral premotor and dorsolateral prefrontal cortex during the inter-stimulus interval between a warning cue and a pain stimulus (similar to a CNV paradigm; López-Solà et al. 2010). In addition, the pain-related changes in pre-movement EMG activity or movement patterns associated with CNV amplitudes may also function to alter the perception of pain during the sit-to-stand movement (Le Pera et al. 2007). Whether this study’s observed changes in CNV potentials at the C4 electrode during the Pain condition reflect these mechanisms, however, remains uncertain.
It is important to recognize that the C4 electrode’s increased CNV amplitudes during the Pain condition were identified by a post-hoc paired-t test and not by the omnibus statistic. This result must, therefore, be considered exploratory due to the increased chance of a Type I error. Because, however, the pain-related changes in sit-to-stand behavior predicted these CNV amplitudes to a coefficient of determination equal to 0.95, we believe it is unlikely that the pain-related increase in CNV amplitudes at the C4 electrode represent a chance phenomenon.
As noted in the introduction, the CNV potential relates to the anticipation of the stimuli and motor task, sensory-motor status prior to the imperative stimulus, and motor preparation for the response (Kok 1978; Haagh and Brunia 1985; van Boxtel and Brunia 1994; Pfeuty et al. 2008), representing activation of a widespread cortical network (Lamarche et al. 1995; Hamano et al. 1997; Bares et al. 2007). The stepwise regression analyses identified both pre-movement changes in muscle activation as well as sit-to-stand kinematic and kinetic response characteristics as significant independent predictors of CNV amplitudes, suggesting any or all functional aspects of the CNV potential may have been affected in the Pain condition. Thus, further research is warranted to disentangle the functional role of the CNV potential in relation to the modification of movement patterns associated with acute LBP.
To account for methodological considerations, it is possible the changes in CNV amplitudes in the Pain condition simply reflect the slowed velocity of the sit-to-stand movements, rather than the more complex motor modifications known to accompany LBP. We believe it is highly unlikely the pain-related changes in CNV amplitudes solely reflect a slower pace of movement, however, because: (1) previous comparisons of CNV amplitudes between movement conditions of slow and fast velocity demonstrated decreased CNV amplitudes with decreased velocity (Grünewald et al. 1979), whereas this study demonstrated increased CNV amplitudes in the Pain condition that elicited longer movement times, and (2) only one of the stepwise regression models identified movement time as a significant predictor of CNV amplitudes and all models included other variables of force output or joint motion as significant predictors of CNV amplitudes independent of movement time.
In addition, the generalizability of this study’s results should be tempered on the basis that electrocutaneous stimulations might not accurately model the pain experience of chronic, recurrent LBP. We argue, however, that this model of painful electrocutaneous stimulation to the low back has previously elicited changes in postural coordination that parallel those demonstrated by people with chronic, recurrent LBP (Hodges and Richardson 1999; Moseley and Hodges 2005; Moseley and Hodges 2006; Jacobs et al. 2009), and this study’s results likewise elicited changes in coordination during the sit-to-stand task exhibited by people with a history of chronic, recurrent LBP (Gioftsos and Grieve 1996; Simmonds et al. 1998; Shum et al. 2005a). In addition, experimentally induced LBP and clinical pain populations exhibit changes in cerebral activation within many shared regions (Peyron et al. 2000). Despite these arguments, some differences appear to exist between the cerebral hemodynamic responses associated with the experience of spontaneous pain in a chronic LBP population and those associated with experimentally induced LBP (Baliki et al. 2006). Further, although induced pain elicits changes in CNV amplitudes prior to movement (Stude et al. 2003; Babiloni et al. 2004; Babiloni et al. 2005), people with chronic LBP do not exhibit consistent differences in pre-movement cortical potentials such as the CNV or bereitschaftspotential (although, similar to this study, inter-individual differences in the bereitschaftspotential correlated with differences in postural coordination) (Tandon and Kumar 1996; Jacobs et al. 2010). Nevertheless, the results provide insight into how an acute LBP experience could elicit changes in movement patterns that are also exhibited by people with a history of chronic, recurrent LBP.
In summary, the present study demonstrates that acute, experimentally induced LBP elicits modifications in sit-to-stand movement patterns similar to those reported for people with chronic, recurrent LBP, thereby further supporting the face validity of this induced-pain model for study on LBP. In addition, this study identified the CNV potential as a neurophysiologic correlate for modified sit-to-stand behavior associated with acutely induced LBP. Thus, future studies investigating the effects of acute LBP on the CNV potential and motor coordination have promise to improve our understanding of the neurophysiologic mechanisms that govern the long-term behavioral changes in movement patterns associated with LBP.
Acknowledgment
The authors wish to thank the members of the Department of Human Movement and Health, Kanazawa University, for their assistance in participant recruitment and data collection. The Japan Society for the Promotion of Science (PE08031; Jacobs, mentored by Fujiwara) and the National Institute for Arthritis and Musculoskeletal and Skin Diseases, National Institutes if Health (T32 AR07568; Jacobs, mentored by Henry) funded this study.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Andersson G. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. doi: S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Arendt-Nielsen L, et al. Cortical sensorimotor interactions during the expectancy of a go/no-go task: effects of painful stimuli. Behav Neurosci. 2004;118:925–935. doi: 10.1037/0735-7044.118.5.925. doi: 2004-19432-006. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Pizzella V, et al. Contingent negative variation in the parasylvian cortex increases during expectancy of painful sensorimotor events: a magnetoencephalographic study. Behav Neurosci. 2005;119:491–502. doi: 10.1037/0735-7044.119.2.491. doi: 2005-03585-013. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. doi: 26/47/12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bares M, Nestrasil I, Rektor I. The effect of response type (motor output versus mental counting) on the intracerebral distribution of the slow cortical potentials in an externally cued (CNV) paradigm. Brain Res Bull. 2007;71:428–435. doi: 10.1016/j.brainresbull.2006.10.012. doi: S0361-9230(06)00313-3. [DOI] [PubMed] [Google Scholar]
- Coghlin SS, McFadyen BJ. Transfer strategies used to rise from a chair in normal and low back pain subjects. Clin Biomech. 1994;9:85–92. doi: 10.1016/0268-0033(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Cui RQ, Egkher A, Huter D, Lang W, Lindinger G, Deecke L. High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clin Neurophysiol. 2000;111:1847–1859. doi: 10.1016/s1388-2457(00)00388-6. doi: S1388-2457(00)00388-6. [DOI] [PubMed] [Google Scholar]
- Drake JD, Callaghan JP. Elimination of electrocardiogram contamination from electromyogram signals: An evaluation of currently used removal techniques. J Electromyogr Kinesiol. 2006;16:175–187. doi: 10.1016/j.jelekin.2005.07.003. doi: S1050-6411(05)00086-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Maeda K, Toyama H. Influences of illusionary position perception on anticipatory postural control associated with arm flexion. J Electromyogr Kinesiol. 2003;13:509–517. doi: 10.1016/s1050-6411(03)00083-x. doi: S105064110300083X. [DOI] [PubMed] [Google Scholar]
- Gioftsos G, Grieve DW. The use of artificial neural networks to identify patients with chronic low-back pain conditions from patterns of sit-to-stand manoeuvres. Clin Biomech (Bristol, Avon) 1996;11:275–280. doi: 10.1016/0268-0033(96)00013-7. doi: 0268003396000137. [DOI] [PubMed] [Google Scholar]
- Goulart FR, Valls-Solé J. Patterned electromyographic activity in the sit-to-stand movement. Clin Neurophysiol. 1999;110:1634–1640. doi: 10.1016/s1388-2457(99)00109-1. doi: S1388245799001091. [DOI] [PubMed] [Google Scholar]
- Grünewald G, Grünewald-Zuberbier E, Netz J, Hömberg V, Sander G. Relationships between the late component of the contingent negative variation and the bereitschaftspotential. Electroencephalogr Clin Neurophysiol. 1979;46:538–545. doi: 10.1016/0013-4694(79)90007-5. [DOI] [PubMed] [Google Scholar]
- Haagh SA, Brunia CH. Anticipatory response-relevant muscle activity, CNV amplitude and simple reaction time. Electroencephalogr Clin Neurophysiol. 1985;61:30–39. doi: 10.1016/0013-4694(85)91070-3. [DOI] [PubMed] [Google Scholar]
- Hamano T, Lüders HO, Ikeda A, Collura TF, Comair YG, Shibasaki H. The cortical generators of the contingent negative variation in humans: a study with subdural electrodes. Electroencephalogr Clin Neurophysiol. 1997;104:257–268. doi: 10.1016/s0168-5597(97)96107-4. [DOI] [PubMed] [Google Scholar]
- Hammill RR, Beazell JR, Hart JM. Neuromuscular consequences of low back pain and core dysfunction. Clin Sports Med. 2008;27:449–462. doi: 10.1016/j.csm.2008.02.005. ix doi: S0278-5919(08)00020-3. [DOI] [PubMed] [Google Scholar]
- Harris-Hayes M, Van Dillen LR, Sahrmann SA. Classification, treatment and outcomes of a patient with lumbar extension syndrome. Physiother Theory Pract. 2005;21:181–196. doi: 10.1080/09593980500212987. [DOI] [PubMed] [Google Scholar]
- Hart L, Deyo R, Cherkin D. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. Phila Pa 1976. [DOI] [PubMed] [Google Scholar]
- Hiebert R, Weiser S, Campello M,MN. Nonspecific low back pain. In: Rom WN, Markowitz SB, editors. Environmental and Occupational Medicine. Lippincott Williams and Wilkins; Philadelphia: 2007a. pp. 924–936. [Google Scholar]
- Hiebert R, Weiser S, Campello M, Nordin M. Nonspecific low back pain. In: Rom WN, Markowitz SB, editors. Environmental and Occupational Medicine. Lippincott Williams and Wilkins; Philadelphia: 2007b. pp. 924–936. [Google Scholar]
- Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80:1005–1012. doi: 10.1016/s0003-9993(99)90052-7. doi: S0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behav Neurosci. 2009;123:455–458. doi: 10.1037/a0014479. doi: 2009-04037-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin Neurophysiol. 2010;121:431–440. doi: 10.1016/j.clinph.2009.11.076. doi: S1388-2457(09)00740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Katz J. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. doi: 88/1_suppl_2/21. [DOI] [PubMed] [Google Scholar]
- Kok A. The effect of warning stimulus novelty on the P300 and components of the contingent negative variation. Biol Psychol. 1978;6:219–233. doi: 10.1016/0301-0511(78)90024-8. doi: 0301-0511(78)90024-8. [DOI] [PubMed] [Google Scholar]
- Kuriyama N, Ito H. Electromyographic functional analysis of the lumbar spinal muscles with low back pain. J Nippon Med Sch. 2005;72:165–173. doi: 10.1272/jnms.72.165. doi: JST.JSTAGE/jnms/72.165. [DOI] [PubMed] [Google Scholar]
- Lamarche M, Louvel J, Buser P, Rektor I. Intracerebral recordings of slow potentials in a contingent negative variation paradigm: an exploration in epileptic patients. Electroencephalogr Clin Neurophysiol. 1995;95:268–276. doi: 10.1016/0013-4694(95)00117-h. doi: 001346949500117H. [DOI] [PubMed] [Google Scholar]
- Langevin H, Sherman K. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses. 2007;68:74–80. doi: 10.1016/j.mehy.2006.06.033. doi: S0306-9877(06)00491-9. [DOI] [PubMed] [Google Scholar]
- Le Pera D, Brancucci A, De Armas L, et al. Inhibitory effect of voluntary movement preparation on cutaneous heat pain and laser-evoked potentials. Eur J Neurosci. 2007;25:1900–1907. doi: 10.1111/j.1460-9568.2007.05389.x. doi: EJN5389. [DOI] [PubMed] [Google Scholar]
- Low MD, McSherry JW. Further observations of psychological factors involved in CNV genesis. Electroencephalogr Clin Neurophysiol. 1968;25:203–207. doi: 10.1016/0013-4694(68)90017-5. [DOI] [PubMed] [Google Scholar]
- López-Solà M, Pujol J, Hernández-Ribas R, et al. Dynamic assessment of the right lateral frontal cortex response to painful stimulation. Neuroimage. 2010;50:1177–1187. doi: 10.1016/j.neuroimage.2010.01.031. doi: S1053-8119(10)00051-0. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Moseley GL, Hodges PW. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain. 2009;142:183–188. doi: 10.1016/j.pain.2008.12.002. doi: S0304-3959(08)00721-5. [DOI] [PubMed] [Google Scholar]
- Mok N, Brauer S, Hodges P. Failure to use movement in postural strategies leads to increased spinal displacement in low back pain. Spine. 2007;32:E537–543. doi: 10.1097/BRS.0b013e31814541a2. Phila Pa 1976. doi: 00007632-200709010-00019. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Hodges PW. Are the changes in postural control associated with low back pain caused by pain interference? Clin J Pain. 2005;21:323–329. doi: 10.1097/01.ajp.0000131414.84596.99. doi: 00002508-200507000-00007. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Hodges PW. Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci. 2006;120:474–476. doi: 10.1037/0735-7044.120.2.474. doi: 2006-05348-023. [DOI] [PubMed] [Google Scholar]
- Peyron R, García-Larrea L, Grégoire MC, et al. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122( Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. doi: S0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pfeuty M, Ragot R, Pouthas V. Brain activity during interval timing depends on sensory structure. Brain Res. 2008;1204:112–117. doi: 10.1016/j.brainres.2008.01.022. doi: S0006-8993(08)00088-7. [DOI] [PubMed] [Google Scholar]
- Rom WN, Markowitz S. Environmental and occupational medicine. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- Sahrmann S. Diagnosis and treatment of movement impairment syndromes. Mosby, St. Louis; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtes SA, Gombatto SP, Van Dillen LR. Differences in lumbopelvic motion between people with and people without low back pain during two lower limb movement tests. Clin Biomech (Bristol, Avon) 2009;24:7–12. doi: 10.1016/j.clinbiomech.2008.09.008. doi: S0268-0033(08)00277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum GL, Crosbie J, Lee RY. Effect of low back pain on the kinematics and joint coordination of the lumbar spine and hip during sit-to-stand and stand-to-sit. Spine. 2005a;30:1998–2004. doi: 10.1097/01.brs.0000176195.16128.27. Phila Pa 1976. doi: 00007632-200509010-00015. [DOI] [PubMed] [Google Scholar]
- Shum GL, Crosbie J, Lee RY. Symptomatic and asymptomatic movement coordination of the lumbar spine and hip during an everyday activity. Spine. 2005b;30:E697–702. doi: 10.1097/01.brs.0000188255.10759.7a. Phila Pa 1976. doi: 00007632-200512010-00021. [DOI] [PubMed] [Google Scholar]
- Shum GL, Crosbie J, Lee RY. Three-dimensional kinetics of the lumbar spine and hips in low back pain patients during sit-to-stand and stand-to-sit. Spine. 2007;32:E211–219. doi: 10.1097/01.brs.0000259204.05598.10. Phila Pa 1976. doi: 00007632-200704010-00026. [DOI] [PubMed] [Google Scholar]
- Shum GL, Crosbie J, Lee RY. Energy transfer across the lumbosacral and lower-extremity joints in patients with low back pain during sit-to-stand. Arch Phys Med Rehabil. 2009;90:127–135. doi: 10.1016/j.apmr.2008.06.028. doi: S0003-9993(08)01538-4. [DOI] [PubMed] [Google Scholar]
- Simmonds MJ, Olson SL, Jones S, Hussein T, Lee CE, Novy D, Radwan H. Psychometric characteristics and clinical usefulness of physical performance tests in patients with low back pain. Spine. 1998;23:2412–2421. doi: 10.1097/00007632-199811150-00011. Phila Pa 1976. [DOI] [PubMed] [Google Scholar]
- Stude P, Wischniewski C, Thümler P, Lehmenkühler A, Richter F, Wiemann M, Bingmann D. Scalp-recorded contingent negative variation (CNV) increases during experimentally induced sustained ischemic pain in humans. Neurosci Lett. 2003;348:9–12. doi: 10.1016/s0304-3940(03)00642-6. doi: S0304394003006426. [DOI] [PubMed] [Google Scholar]
- Tandon OP, Kumar S. Contingent negative variation response in chronic pain patients. Indian J Physiol Pharmacol. 1996;40:257–261. [PubMed] [Google Scholar]
- Tsao H, Galea M, Hodges P. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131:2161–2171. doi: 10.1093/brain/awn154. doi: awn154. [DOI] [PubMed] [Google Scholar]
- Van ’t Ent D, Apkarian P. Inter-hemispheric lateralization of event related potentials; motoric versus non-motoric cortical activity. Electroencephalogr Clin Neurophysiol. 1998;107:263–276. doi: 10.1016/s0013-4694(98)00068-6. doi: S0013469498000686. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, Brunia CH. Motor and non-motor components of the Contingent Negative Variation. Int J Psychophysiol. 1994;17:269–279. doi: 10.1016/0167-8760(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Maluf KS, Sahrmann SA. Further examination of modifying patient-preferred movement and alignment strategies in patients with low back pain during symptomatic tests. Man Ther. 2009;14:52–60. doi: 10.1016/j.math.2007.09.012. doi: S1356-689X(07)00161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. J Orthop Sports Phys Ther. 2003;33:126–142. doi: 10.2519/jospt.2003.33.3.126. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Wagner JM. Classification, intervention, and outcomes for a person with lumbar rotation with flexion syndrome. Phys Ther. 2005;85:336–351. [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]