Coronary heart disease (CHD) and stroke rank among the leading causes of death in the industrialized world 1 and a significant genetic component underlies both outcomes. These clinical events are often preceded by the development of subclinical atherosclerosis, typically a thickening of the artery wall due to deposition of cholesterol rich material in the arteries that supply blood to major organs.2 Generalized atherosclerosis results from endothelial dysfunction, inflammation, abnormalities in lipoprotein metabolism 3, coagulation and fibrinolysis. 4
Measures of subclinical atherosclerosis, disease that occurs before symptoms are noted, are predictive of incident clinical events and can be detected non-invasively and with reasonable precision in population samples using high resolution ultrasound techniques. Both cIMT and plaque, reflecting a thickening of the carotid artery wall or the presence of large irregular arterial wall deposits, respectively, are established measures of subclinical atherosclerotic disease. While there may be variation in carotid ultrasound measurement techniques, multiple independent studies have established consistent association of carotid phenotypes with coronary events and stroke in prospective studies of young, middle-aged, and older adults 5,6 and recent consensus prevention guidelines cite cIMT as a potentially useful measure for prediction.7 While there is a correlation between common cIMT and carotid plaque, common cIMT reflects carotid artery wall thickening that may result from multiple vascular etiologies including hypertension and atherosclerosis, whereas carotid plaque is an indicator of the discrete occurrence of carotid atherosclerosis. Several recent studies provide evidence that carotid plaque is a better predictor of future cardiovascular disease risk than common cIMT. 8–10
Numerous family studies established consistent evidence for moderate heritabilities for common cIMT, internal cIMT and carotid plaque (Supplementary Table 1). However, candidate gene studies have not found consistent associations between single nucleotide polymorphisms (SNPs) and cIMT,11 and genome-wide linkage scans completed to date have revealed only suggestive regions for common cIMT.12,13 We performed a GWAS of three measures of subclinical carotid atherosclerosis – common cIMT, internal cIMT, and plaque– in a sample of up to 31,211 participants from nine population-based studies that performed genome-wide genotyping with commercial SNP arrays and imputed to the approximately 2.5 million autosomal SNPs in the Phase II HapMap CEU reference panel. In addition, we followed-up our discovery findings in a second stage that included 11,273 participants from 7 independent studies.
Results
The cross-sectional discovery genome-wide analysis of carotid artery phenotypes included 31,211 participants from nine community-based studies whose mean age ranged from 44 to 76 years. Characteristics of the samples are presented in the Supplementary Note. In the studies in which all three carotid measures were available, the correlations between common cIMT and plaque ranged from 0.27 to 0.39, and between common cIMT and internal cIMT, from 0.36 to 0.67 (Supplementary Table 2).
The a priori threshold for genome-wide significance was 5×10−8, and a p-value > 5×10−8 but <4×10−7, corresponding to not more than one expected false positive finding over 2.5 million tests, was considered suggestive evidence for association in our analyses.
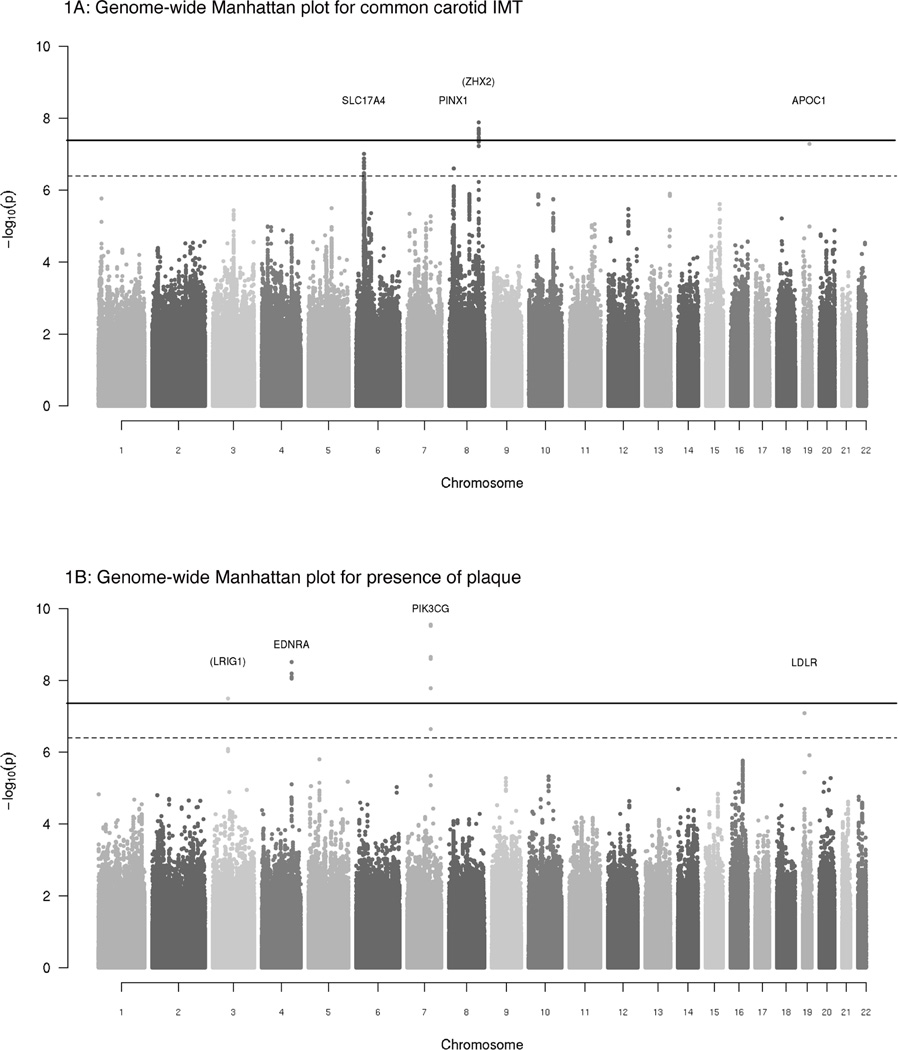
Figure 1A provides a plot of −log10 (p-values) for the associations of the approximately 2.5 million SNPs with common cIMT by chromosome and position for the meta-analysis of the nine discovery studies. P-values from the meta-analysis of plaque (n=25,179 participants) and internal cIMT (n=10,962) are presented according to their genomic positions in Figure 1B and Supplementary Figure 1, respectively. Overall, from the discovery meta-analysis of common cIMT and plaque, we carried forward 3 genome-wide significant SNPs and 5 suggestive SNPs to the second stage. Our second stage included 11,273 participants from seven community-based studies, six of which provided results for common cIMT (total N=10,403) and three of which provided results for plaque (N=6,013). Characteristics of the participants in these studies are shown in the Supplementary Note.
Figure 1. A–B: Genome-wide Manhattan plots for common cIMT and plaque.
Plots show the individual p-values (based on discovery meta-analysis) against their genomic position for common carotid IMT (Figure 1A), the presence of plaque (Figure 1B). Within each chromosome, shown on the x-axis, the results are plotted left to right from the p-terminal end. The dashed line indicates the threshold for follow-up, p<4 ×10−7 and the solid line indicates the threshold for genome-wide significance, p<4 ×10−8. The nearest genes are indicated above points that surpassed our genome-wide significance threshold; genes that are greater than 100 kb from the signal SNP are indicated in parentheses.
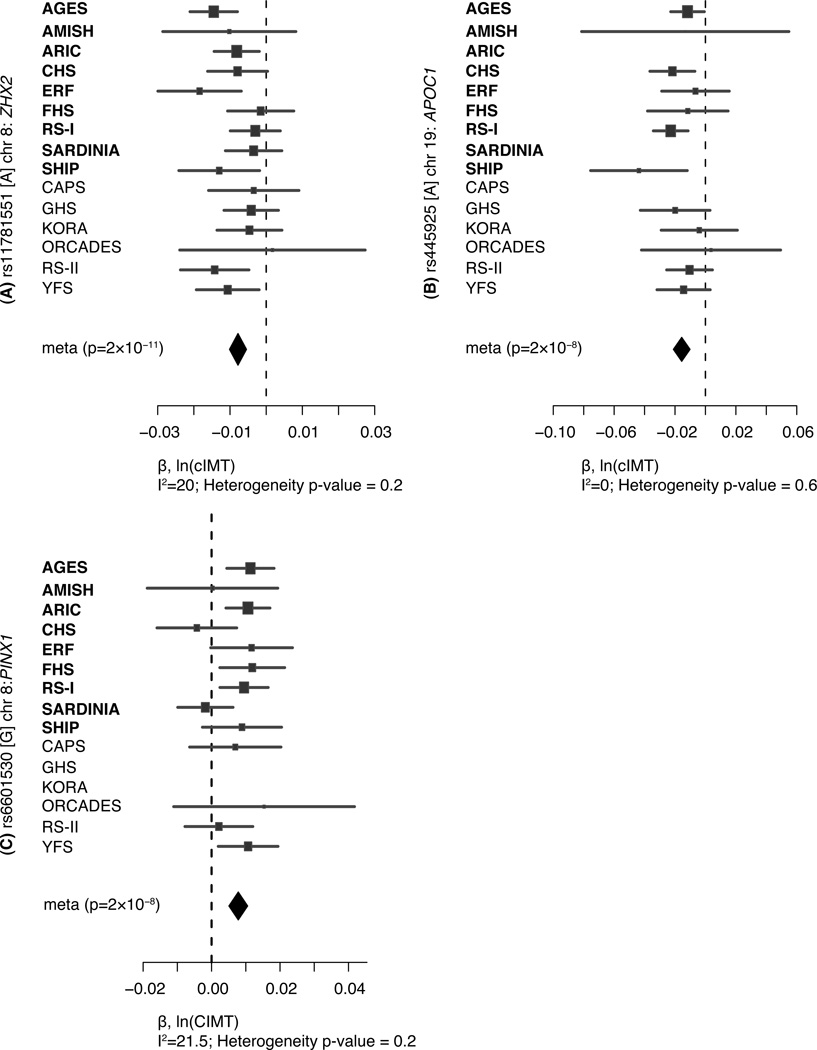
Table 1 presents the genome-wide significant association results for the discovery, second stage, and combined meta-analyses for common cIMT and plaque, respectively. We show the discovery GWAS results for the 100 kb region surrounding the signal SNPs for common cIMT and plaque along with the recombination rates and the known genes in that region in Figures 2 and 3. Figures 4 and 5 show the study-specific findings from the combined meta-analyses of common cIMT and plaque, respectively. Results for the suggestive loci in the meta-analyses of common cIMT and plaque are shown in the Supplementary Table 3 and Supplementary Figures 2–5.
Table 1.
A: Discovery, Second Stage, and Combined meta-analysis for common cIMT and plaque
| Discovery GWAS (cIMT) |
Second Stage Meta-analysis (cIMT) |
Combined Meta-analysis (cIMT) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr | Nearest gene |
Alleles | AF | β | SE | N | p-value | AF | β | SE | N | p-value | β | SE | p-value |
| rs11781551 | 8 | ZHX2 | A/G | 0.48 | −0.0081 | 0.0014 | 30,894 | 1.3×10−8 | 0.47 | −0.0072 | 0.0020 | 10,401 | 0.0004 | −0.0078 | 0.0012 | 2.4×10−11 |
| rs445925 | 19 | APOC1 | A/G | 0.11 | −0.0179 | 0.0033 | 12,395 | 5.2×10−8 | 0.10 | −0.0116 | 0.0047 | 4,790 | 0.01 | −0.0156 | 0.0028 | 1.7×10−8 |
| rs6601530 | 8 | PINX1 | G/A | 0.45 | 0.0078 | 0.0015 | 28,124 | 2.5×10−7 | 0.46 | 0.0073 | 0.0029 | 4,507 | 0.01 | 0.0078 | 0.0014 | 1.7×10−8 |
| Discovery GWAS (plaque) |
Second Stage Meta-analysis (plaque) |
Combined Meta-analysis (plaque) |
||||||||||||||
| SNP | Chr | Nearest gene |
Alleles | AF | OR (95% CI) | N | p-value | AF | OR (95% CI) | N | p-value | OR (95% CI) | p-value | |||
| rs17398575 | 7 | PIK3CG | A/G | 0.25 | 1.17 (1.12 – 1.23) | 23,520 | 2.8×10−10 | 0.25 | 1.20 (1.07 – 1.35) | 5,735 | 0.002 | 1.18 (1.12 – 1.23) | 2.3×10−12 | |||
| rs1878406 | 4 | EDNRA | T/C | 0.13 | 1.21 (1.13 – 1.28) | 24,089 | 3.1×10−9 | 0.13 | 1.31 (1.13 – 1.52) | 5,738 | 0.0003 | 1.22 (1.15 – 1.29) | 6.9×10−12 | |||
Alleles indicates the coded (named first) & non-coded allele; AF indicates allele frequency for the coded allele, an average weighted by study size; OR indicates odds ratio, CI, confidence interval; N indicates effective sample size, calculated by taking the sum of each study’s sample size multiplied by the SNP’s imputation quality. When more than one SNP at a locus surpassed our p-value threshold, we presented the SNP with the lowest p-value.
Figure 2. Regional plots for common carotid IMT SNPs.
Plots are centered on the most significant SNP at locus along with the meta-analysis results for SNPs in the 100kb region surrounding it. All SNPs are plotted with their discovery meta-analysis p-values against their genomic position, with the most significant SNP in the region indicated as a diamond and other SNPs shaded according to their pairwise correlation (r2) with the signal SNP. The light blue line represents the estimated recombination rates. Gene annotations are shown as dark green lines.
Figure 3. Regional plots for plaque SNPs.
Plots are centered on the most significant SNP at each locus along with the meta-analysis results for SNPs in the 100kb region surrounding it. All SNPs are plotted with their discovery meta-analysis p-values against their genomic position, with the most significant SNP in the region indicated as a diamond and other SNPs shaded according to their pairwise correlation (r2) with the signal SNP. The light blue line represents the estimated recombination rates. Gene annotations are shown as dark green lines.
Figure 4. Forest plots for common carotid IMT SNP associations.
Plots show the study-specific association estimates (β) and 95% confidence intervals for the nine discovery and second stage studies, presented as bars. The scale is ln(cIMT). The association estimate and confidence interval for the meta-analysis combining discovery and second stage results is presented as a diamond. Blank spaces indicate occasions in which a particular study was not able to provide results for a given SNP.
Figure 5. Forest plots for plaque SNP associations.
Plots show the study-specific association estimates (OR) and 95% confidence intervals for the nine discovery and second stage studies, presented as bars. The association estimate and confidence interval for the meta-analysis combining discovery and second stage results is presented as a diamond. Blank spaces indicate occasions in which a particular study was not able to provide results for a given SNP.
Common cIMT
For common cIMT, 3 independent loci achieved our genome-wide significance threshold (p<5×10−8) in the combined meta-analysis.
The strongest association was for rs11781551, found on 8q24 approximately 385 kb from ZHX2, where the A allele (allele frequency [AF]=0.48), was associated with lower common cIMT (β=−0.0078, p= 2.4×10−11), i.e. a 0.8% lower mean common cIMT per copy of the A allele. The second association was for rs445925, located 2.3 kb from APOC1 on 19q13, a region that also includes APOE, APOC2, and APOC4. The G allele (AF=0.11) was associated with lower common cIMT (β=-0.0156, p= 1.7×10−8). The third association was for rs6601530, located within the PINX1 gene on 8q23.1. Each copy of the G allele (AF = 0.45) was associated with higher common cIMT (β=0.0078, p= 1.7×10−8). We also identified a suggestive locus, marked by rs4712972 near the SLC17A4 gene on 6p22, where the A allele was associated with higher common cIMT (β=0.0099, p= 7.8×10−8).
While our genome-wide significant and suggestive SNPs from combined meta-analyses for common cIMT explained a small proportion of the trait variance (up to 1.1%), we further constructed an additive genetic risk score (0–8 alleles) comprised of the number of common cIMT risk alleles at the four loci. In the discovery samples, the additive risk score showed graded increasing association with common cIMT across all studies with an average increase of 9.5% in common cIMT from the lowest (0–2) to the highest (6–8) risk category (Supplementary Figure 6).
Plaque
In analysis of carotid artery plaque, 2 independent loci achieved the genome-wide significance threshold (p<5×10−8) in the combined meta-analysis.
The most significant signal was observed for rs17398575, situated 96.5 kb from the PIK3CG gene on 7q22. Per copy of the T allele (AF=0.25), we observed an 18% increased odds of presence of plaque (p=2.3×10−12). The second signal was centered at rs1878406, located 8.5 kb from EDNRA on 4q31. Each copy of the T allele (AF=0.13) was associated with a 22% increased odds of the presence of plaque (p= 6.9×10−12). Furthermore, two SNPs showed suggestive evidence for association in our combined meta-analysis. The first suggestive locus was rs17045031 on 3p13 where each copy of the A allele was associated with decreased odds of the presence of plaque (p= 1.0×10−7). Our second suggestive locus was rs6511720, near LDLR on 19p13. Per copy of the T allele we observed a decreased odds of the presence of plaque (P=3.8×10−7).
For both cIMT and plaque, secondary discovery genome-wide meta-analyses conditioned on the genome-wide significant and suggestive SNPs from the combined meta-analyses did not reveal any additional associations.
Internal cIMT
No SNP achieved our significance threshold for follow up in the discovery analyses of internal cIMT. Results for internal cIMT SNPs with p <1.0 × 10−5 are shown in Supplementary Table 4.
Cross-phenotype comparisons
Supplementary Table 5 shows the results for the genome-wide significant and suggestive SNPs from our combined meta-analyses for common cIMT and plaque across the three carotid phenotypes. The directions of association were generally consistent and three SNPs, rs445925 (APOC1) from the common cIMT analysis and rs17398575 (PIK3CG) and rsrs1878406 (EDNRA) from the plaque analysis, were associated with all three phenotypes (p < 0.05/8/2 = 0.003) in cross-phenotype comparisons.
Associations with coronary artery disease
We investigated the genome-wide significant and suggestive SNPs from our combined meta-analyses for common cIMT and plaque for their potential associations with coronary artery disease (CAD) in the CARDIoGRAM Consortium (Table 2). Two SNPs from our plaque analysis had a p-value for association with CAD less than 0.006 (0.05/8 tests). The first, rs6511720, near LDLR, where the G allele was associated with both higher plaque risk in our study and higher CAD risk (p=0.0002); and rs1878406, near EDNRA where the C allele was associated with lower risk of plaque and lower risk of CAD (p=2×10−6). One SNP from common cIMT analysis, rs445925 near APOC1, showed a suggestive association with CAD with the same allele (A) being associated with higher common cIMT and higher CAD risk (p=0.02). Another SNP identified in the plaque analysis, rs17045031 near LRIG1, showed a suggestive association with CAD, with the G allele associated with both lower odds of plaque and lower risk of CAD (p=0.04).
Table 2.
Association of genome-wide significant and suggestive common cIMT and plaque SNPs with CAD in the CARDIoGRAM Consortium
| source | SNP | Chr | Nearest Gene |
Allele | AF | OR | N | p-value |
|---|---|---|---|---|---|---|---|---|
| Comm. cIMT |
rs11781551 | 8 | ZHX2 | G | 0.53 | 1.02 (0.99 – 1.05) | 83,379 | 0.2 |
| rs445925 | 19 | APOC1 | G | 0.91 | 1.11 (1.02 – 1.20) | 34,216 | 0.02 | |
| rs6601530 | 8 | PINX1 | G | 0.40 | 1.02 (0.99 – 1.05) | 79,512 | 0.1 | |
| rs4712972 | 6 | SLC17A4 | G | 0.86 | 1.02 (0.97 – 1.06) | 84,001 | 0.5 | |
| Plaque | rs17398575 | 7 | PIK3CG | G | 0.73 | 0.98 (0.95 – 1.01) | 83,028 | 0.2 |
| rs1878406 | 4 | EDNRA | C | 0.86 | 0.91 (0.87 – 0.95) | 81,804 | 2×10-6 | |
| rs6511720 | 19 | LDLR | G | 0.90 | 1.13 (1.06 – 1.21) | 56,420 | 0.0002 | |
| rs17045031 | 3 | LRIG1 | G | 0.94 | 1.09 (1.00 – 1.18) | 80,655 | 0.04 | |
Allele indicates the coded allele in the CARDIoGRAM Consortium meta-analysis; AF indicates allele frequency for the coded allele; OR indicates odds ratio, CI, confidence interval; N indicates sample size.
Conversely, none of SNPs reported to be associated with coronary artery disease in the CARDIoGRAM consortium 14 had a significant association (i.e., a p-value less than 0.00072, a conservative Bonferroni correction for 23 tests across three phenotypes) in our discovery meta-analyses of common cIMT, internal cIMT, or plaque (Supplementary Table 6).
Discussion
In this meta-analysis of G WAS data from nine studies of common cIMT and seven studies of plaque, we identified genome-wide significant associations between 3 regions and common cIMT and between 2 regions and the presence of carotid plaque in over 40,000 participants of European ancestry. Interestingly, EDNRA one of our genome-wide significant regions in the combined meta-analysis of plaque was related to multiple carotid phenotypes and was also associated with coronary artery diseases in the recent large meta-analysis by the CARDIoGRAM Consortium.
Three SNPs emerged as genome-wide significant from our combined meta-analysis of common cIMT. The strongest association, on chromosome 8 (rs11781551), is an intergenic SNP located 385 kb from the ZHX2 gene. Members of this gene family are nuclear homodimeric transcriptional repressors that interact with the A subunit of nuclear factor-Y (NF-YA) and contain two C2H2-type zinc fingers and five homeobox DNA-binding domains. Little information about these proteins exists regarding cardiovascular disease or population studies.
A second association, on 19q13 (rs445925), fell upstream of the APOC1 gene. While this region has been of interest for its role in neurological genetics because of the APOE gene, it is also been frequent candidate gene for cardiovascular disease traits. 15 Although some previous studies have found associations of variation at the APOE locus and common cIMT,16 among 4 of our discovery studies that had independently measured the APOE epsilon variants, the correlation between rs445925 and the e4 allele was less than 0.05. Further, models that included both the APOE e4 and the APOC1 variant indicated that the APOE gene was not associated with common cIMT in these studies (Supplementary Table 7), while the APOC1 variant still showed a significant association with common cIMT. While APOE variants have been implicated in cases of familial dyslipidemia and premature atherosclerosis and in recent genome-wide association studies with variation in multiple lipoprotein measures,17 our results suggest that APOC1 is the primary variant of interest for carotid traits.
The third association (rs6601530) was located in an intron of the Pin2-interacting protein 1 (PINX1) gene. The protein, a telomerase inhibitor 18 that plays a role in chromosomal segregation in mitosis,19 has been investigated in relation to cancers, but was not considered a candidate gene for cardiovascular phenotypes.
The region on chromosome 6 marked by rs4712972, which includes the SLC17A4, SLC17A1, and SLC17A3 genes showed suggestive evidence for association with common cIMT in our combined meta-analysis. This region may merit further investigation as recent genome-wide association studies have implicated this region with uric acid levels.20,21 Although high uric acid levels have been associated with cardiovascular disease and all-cause mortality,22 the contribution to atherosclerotic vascular disease remains controversial.23
Plaque associations
For plaque, two regions were genome-wide significant in our combined meta-analysis. The first region was within 100kb of the PIK3CG gene, which encodes one of the pi3/pi4-kinase family of proteins. These proteins are important modulators of extracellular signals, including those elicited by E-cadherin-mediated cell-cell adhesion, which plays an important role of endothelin in maintenance of the structural and functional integrity of epithelia. The fact that this region was reported as a top hit in a recent GWAS of both platelet volume 24 and aggregation 25 suggests pleiotropy and highlights the interconnectedness of multiple cardiometabolic traits.
The second genome-wide significant region was near the EDNRA gene. Because of the role of endothelin as a potent vasoconstrictor, the endothelin receptor, type A is a target for pharmacologic treatments to reduce blood pressure.26 In addition, variation in the gene was associated with blood pressure 27, atherosclerosis 28 and cardiovascular disease endpoints 29 in candidate gene studies.
Two more regions showed suggestive evidence for association in our combined meta-analysis for plaque. The first region, near the LDLR gene is a particularly interesting candidate for subclinical atherosclerosis because of its role in familial hypercholesterolemia and its appearance in recent genome-wide association studies for lipid traits 30–33 and myocardial infarction. 14,34 Notably, the LDLR SNP recently reported to be associated with MI (rs1122608) is located 38 kb away and is in modest LD (r2=0.2 in HapMap CEU) with the signal SNP (rs6511720) in our analysis that also showed an association with CAD in the CARDIoGRAM consortium. The second was in the vicinity of LRIG1, which negatively regulates growth factor signaling and is involved in the regulation of epidermal stem cell quiescence.
Interestingly, we found three loci (APOC1, PIK3CG, and EDNRA) that were associated with all three carotid phenotypes. Among these, the EDNRA locus was also significantly associated with coronary artery disease in the recent large meta-analysis by the CARDIoGRAM Consortium. These associations may provide important insights into the pathophysiological mechanisms relating the genes to atherosclerosis and subsequent coronary artery disease. In particular, the concordance of association with SNPs in EDNRA with both carotid plaque and CHD suggests a common etiology for subclinical and clinically apparent disease that warrants further investigation.
The strengths of the current study include the large sample size, the population-based designs, the collaboratively designed pre-specified analysis plan, and the high quality of both genotyping and phenotyping. Further, our ability to relate our findings to the outcome of CAD in a large independent meta analysis provides important additional context to our results. These associations are unlikely to be due to population stratification since the discovery sample was restricted to whites of European origin and was also investigated for global latent population substructure.
The study also has limitations. A single cross-sectional IMT assessment was used in all studies and ultrasound protocols varied across participating studies. For example, plaque definition included the presence of any plaque in most studies and stenosis greater than 25% in others. The heterogeneity of measurement techniques may have compromised our ability to detect small associations. Despite this heterogeneity, the ability to detect consistent genetic associations for several carotid measures suggests that additional signals may be discovered in future studies utilizing a larger sample size or a higher resolution technique such as magnetic resonance imaging. Further, few studies had internal cIMT measures since these are more difficult to obtain than common cIMT measurements and thus limited our ability to discover associations with this phenotype. Although our sample size was reasonably large, we still had limited power to detect associations with small effect sizes. Genome-wide association studies are known for revealing associations with common variants and may miss rare variants not covered by the commercial genotyping arrays. For instance, the sparse coverage of the APOC1 and LDLR gene regions resulted in varying imputation quality and a lower effective sample size for the analysis of these two regions.
Because we did not conduct follow-up fine mapping of the results, and because some SNPs were distant from known genes, it is likely that the identified SNPs are not causal variants, but, instead, may be in linkage disequilibrium with variants that were not analyzed. Because some of our associations attained genome-wide significant p-values only in the combined meta-analysis, confirmation of our findings in other populations and further exploration of these genomic regions with dense genotyping, expression, and translational studies will be required to better understand the role of these genes in subclinical atherosclerotic disease.
In summary, our meta-analysis of GWAS data from nine community-based studies has revealed 5 new loci for common cIMT and plaque. These loci implicate LDL metabolism (APOC1), endothelial dysfunction (EDNRA), platelet biology (PIK3CG), and telomere maintenance (PINX1). Two of our identified loci are also associated with coronary artery disease in the recent large meta-analysis by the CARDIoGRAM Consortium. Exploring the molecular, cellular and clinical consequences of genetic variation at these loci may yield novel insights into the pathophysiology of clinical and subclinical cardiovascular disease.
Supplementary Material
Funding/Acknowledgments
AGES: The Age, Gene/Environment Susceptibility Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament).
Amish: The Amish studies are supported by grants and contracts from the NIH including R01 AG18728 (Amish Longevity Study), R01 HL088119 (Amish Calcification Study), U01 GM074518-04 (PAPI Study), and U01 HL072515-06 (HAPI Study), the University of Maryland General Clinical Research Center, grant M01 RR 16500, the Baltimore Veterans Administration Medical Center Geriatrics Research and Education Clinical Center and the Paul Beeson Physician Faculty Scholars in Aging Program. We thank our Amish research volunteers for their long-standing partnership in research, and the research staff at the Amish Research Clinic for their hard work and dedication.
ARIC: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
ERF: The ERF study was supported by grants from The Netherlands Organisation for Scientific Research, Erasmus MC and the Centre for Medical Systems Biology (CMSB). We are grateful to all study participants and their relatives, general practitioners and neurologists for their contributions and to P. Veraart for her help in genealogy, J. Vergeer for the supervision of the laboratory work and P. Snijders for his help in data collection.
CHS: The CHS research reported in this article was supported by NHLBI contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grants HL080295, HL075366, HL087652, HL105756 with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm. DNA handling and genotyping was supported in part by National Center for Research Resources grant M01RR00069 to the Cedars-Sinai General Clinical Research Center Genotyping core and National Institute of Diabetes and Digestive and Kidney Diseases grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
FHS: From the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195) and its contract with Affymetrix, Inc for genotyping services (Contract No. N02-HL-6-4278), and by grants from the National Institute of Neurological Disorders and Stroke (NS17950; PAW) and the National Institute of Aging (AG08122, AG16495; PAW and AG033193; SS). A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. Analyses reflect intellectual input and resource development from the Framingham Heart Study investigators participating in the SNP Health Association Resource (SHARe) project.
Rotterdam Study I and II (RS I and RS II): The Rotterdam GWA study was funded by the Netherlands Organisation of Scientific Research NWO Investments (nr. 175.010.2005.011, 911-03-012), the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI)/Netherlands Consortium for Healthy Aging (NCHA) project nr. 050-060-810. This study is further supported by NWO grant (vici, 918-76-619). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. The authors are very grateful to the participants and staff from the Rotterdam Study, the participating general practioners and the pharmacists. We thank Pascal Arp, Mila Jhamai, Dr Michael Moorhouse, Marijn Verkerk, and Sander Bervoets for their help in creating the GWAS database. We would like to thank Dr. Tobias A. Knoch, Luc V. de Zeeuw, Anis Abuseiris, and Rob de Graaf as well as their institutions the Erasmus Computing Grid, Rotterdam, The Netherlands, and especially the national German MediGRID and Services@MediGRID part of the German D-Grid, both funded by the German Bundesministeriumfuer Forschung und Technology under grants #01 AK 803 A-H and # 01 IG 07015 G, for access to their grid resources.
SardiNIA: This work was supported by the Intramural Research Program of the National Institute on Aging, NIH. The SardiNIA (‘‘Progenia’’) team was supported by Contract NO1-AG-1–2109 from the National Institute on Aging. The efforts of JBG and GRA, were supported in part by contract 263-MA-410953 from the National Institute on Aging to the University of Michigan and by research grants HG005581 and HL084729 from the National Institutes of Health (to GRA).
We warmly thank Monsignore Piseddu, Bishop of Ogliastra; Mayor Enrico Lai and his administration in Lanusei for providing and furnishing the clinic site; the mayors of Ilbono, Arzana, and Elini; the head of the local Public Health Unit Ar1; and the residents of the towns for their volunteerism and cooperation. We also thank Harold Spurgeon and Paul Pullen for invaluable help with equipment and readings, and Michele Evans and Dan Longo for helpful discussions.
SHIP: SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. Genome-wide data have been supported by the Federal Ministry of Education and Research (grant no. 03ZIK012) and a joint grant from Siemens Healthcare, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania. The SHIP authors are grateful to the contribution of Alexander Teumer, Anja Hoffmann, and Astrid Petersmann in generating the SNP data. The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of the Siemens AG.
ASPS: The research reported in this article was funded by the Austrian Science Fond (FWF) grant number P20545-P05 and P13180. The Medical University of Graz supports the databank of the ASPS.
CAPS: The research leading these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 201668; AtheroRemo.
GHS: The Gutenberg Heart Study is funded through the government of Rheinland-Pfalz (“Stiftung Rheinland Pfalzfür Innovation,” contract number AZ 961-386261/733), the research programs “Wissenschafft Zukunft” and “Schwerpunkt Vaskuläre Prävention” of the Johannes Gutenberg-University of Mainz and its contract with BoehringerIngelheim and PHILIPS Medical Systems including an unrestricted grant for the Gutenberg Heart Study. Specifically, the research reported in this article was supported by the National Genome Network “NGFNplus” (contract number project A3 01GS0833) by the Federal Ministry of Education and Research, Germany.
KORA: The MONICA/KORA Augsburg studies were financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg, Germany and supported by grants from the German Federal Ministry of Education and Research (BMBF). Part of this work was financed by the German National Genome Research Network (NGFNplus, project number 01GS0834) and through additional funds from the University of Ulm. Furthermore, the research was supported within the Munich Center of Health Sciences (MC Health) as part of LMU innovative. IMT measurement of the KORA cohort was funded by a grant of the Karl-Wilder Foundation. Finally, part of this work was financed by the German Diabetes Center, which is funded by the German Federal Ministry of Health and the Ministry of Innovation, Science, Research and Technology of the State of North Rhine Westphalia.
ORCADES: ORCADES was supported by the Chief Scientist Office of the Scottish Government, the Royal Society and the European Union framework program 6 EUROSPAN project (contract no. LSHG-CT-2006-018947). DNA extractions were performed at the Wellcome Trust Clinical Research Facility in Edinburgh. We would like to acknowledge the invaluable contributions of Lorraine Anderson and the research nurses in Orkney, the administrative team in Edinburgh and the people of Orkney.
YFS: The Cardiovascular Risk in Young Finns study (YFS) is supported by the Academy of Finland (grant no. 117797, 121584 and 126925), the Social Insurance Institution of Finland, University Hospital Medical funds to Tampere, and Turku University Hospitals, the Finnish Foundation of Cardiovascular Research.
CARDIoGRAM: We acknowledge the contributions of all of the authors of the CARDIoGRAM report, as listed in their primary analysis publication. 14
Genome-wide Association Studies of Carotid Intima Media Thickness and Plaque: Meta-analysis from the CHARGE Consortium
Carotid intima media thickness (cIMT) and plaque determined by ultrasonography are established measures of subclinical atherosclerosis that each predict future cardiovascular disease events. We conducted a meta-analysis of genome-wide association data in 31,211 participants of European ancestry from nine large studies in the setting of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. We then sought additional evidence to support our findings among 11,273 individuals using data from 7 additional studies. In the combined meta-analysis, we identified three genomic regions associated with common cIMT and two different regions associated with the presence of carotid plaque (p <5×10−8). The associated SNPs mapped in, or near, genes related to cellular-signaling, lipid metabolism, and blood pressure homeostasis and two of the regions were associated with coronary artery disease (p <0.006) in the CARDIoGRAM consortium. Our findings may provide new insight into pathways leading to subclinical atherosclerosis and subsequent cardiovascular events.
Footnotes
Contributions:
Study concept and design: J.C.B., M.K., N.F., G.R.A., L.A.C., T.L., S.R.H., K.N., C.H., O.R., C.S.F., V.N., R.B.S., T.A., M.S., M.K., P.S.W., A.R.S., J.I.R., J.S., D.H.O., E.G.L., B.M.P., M.U., E.B., J.V., W.K., S.B., A.B.N., J.W., C.v.D., A.Scu.V.G.., C.J.O.
Acquisition of the data: J.C.B., A.I., G.R.A., U.S., W.P., H.S.M., R.S., T.L., B.O., S.B., E.S., O.R., C.M., H.V., B.T., F.R., K.E.P., H.E.W., R.B.S., M.D., A.P., T.A., S.K., M.P.R., K.T., M.S., M.K., T.I., P.S.W., M.O., J.L., A.R.S., G.E., J.I.R., A.H., J.S., T.Z., G.U., F.E., L.J.L., R.B.D., D.H.O., J.T., T.B.H., P.A.W., B.M.P., J.F.P., W.R., E.B., N.K., H.S., J.F.W., J.V., W.K., S.B., A.B.N., G.H., C.v.D., A.Scu.G.H., B.D.M., V.G., C.J.O.
Statistical analysis and interpretation of the data: J.C.B., M.K., N.F., A.I., G.R.A., A.V.S., L.A.C., J.E.H., T.L., J.B., S.R.H., A.D., K.N., C.H., O.R., A.Sch.S.S., Y.C., K.R., V.N., E.H., K.E.P., T.A., S.D., S.K., P.S.W., C.C.W., R.B.D., A.Z., R.K.C., H.S., C.J.O.
Drafting of the manuscript: J.C.B., M.K., N.F., A.I., K.N., O.R., M.K., J.F.P., J.V., C.J.O.
Critical revision of the manuscript: J.C.B., M.K., N.F., A.I., G.R.A., U.S., W.P., L.A.C., H.S.M., R.S., T.L., J.B., T.M., S.R.H., A.D., K.N., O.R., C.M., H.V., B.T., K.R., F.R., V.N., H.E.W., R.B.S., M.D., A.P., S.D., M.P.R., K.T., D.J.C., M.S., M.K., T.I., J.L., G.E., J.I.R., A.H., J.S., F.E., L.J.L., R.B.D., D.H.O., C.B., A.Z., E.G.L., R.K.C., T.B.H., B.M.P., J.F.P., X.L., W.R., E.B., J.V., W.K., S.B., J.W., C.v.D., A.Scu.G.H., B.D.M., V.G., C.J.O.
Obtained funding: G.R.A., U.S., H.S.M., R.S., T.L., O.R., H.V., L.P., M.D., S.K., M.S., M.K., P.S.W., A.R.S., J.I.R., A.H., J.S., A.Z., P.A.W., B.M.P., J.F.P., W.R., M.U., E.B., H.S., J.F.W., J.V., W.K., A.B.N., G.H., C.v.D., G.H., B.D.M., V.G., C.J.O.
Disclosures:
V.N. indicated that he has a non-financial research collaboration with General Electric and Medipattern Inc.
References
- 1.Lloyd-Jones D, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 3.Insull W., Jr The Pathology of Atherosclerosis: Plaque Development and Plaque Responses to Medical Treatment. The American Journal of Medicine. 2009;122:S3–S14. doi: 10.1016/j.amjmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 6.Stein JH, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189-90. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 8.Johnsen SH, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 9.Mathiesen EB, et al. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the tromso study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 10.Nambi V, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolio TA, Boerwinkle E, O'Donnell CJ, Wilson AF. Genetics of ultrasonographic carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1567–1577. doi: 10.1161/01.ATV.0000138789.11433.c1. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, et al. A genome-wide scan for carotid artery intima-media thickness: the Mexican-American Coronary Artery Disease family study. Stroke. 2005;36:540–545. doi: 10.1161/01.STR.0000155746.65185.4e. [DOI] [PubMed] [Google Scholar]
- 13.Fox CS, et al. Genomewide linkage analysis for internal carotid artery intimal medial thickness: evidence for linkage to chromosome 12. Am J Hum Genet. 2004;74:253–261. doi: 10.1086/381559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennet AM, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. Jama. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 16.Paternoster L, et al. Genetic effects on carotid intima-media thickness: systematic assessment and meta-analyses of candidate gene polymorphisms studied in more than 5000 subjects. Circ Cardiovasc Genet. 2010;3:15–21. doi: 10.1161/CIRCGENETICS.108.834366. [DOI] [PubMed] [Google Scholar]
- 17.Chasman DI, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009;5:e1000730. doi: 10.1371/journal.pgen.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou XZ, Lu KP. The Pin2/TRF1-interacting protein PinX1 is a potent telomerase inhibitor. Cell. 2001;107:347–359. doi: 10.1016/s0092-8674(01)00538-4. [DOI] [PubMed] [Google Scholar]
- 19.Yuan K, et al. PinX1 is a novel microtubule-binding protein essential for accurate chromosome segregation. J Biol Chem. 2009;284:23072–23082. doi: 10.1074/jbc.M109.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolz M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehghan A, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meisinger C, Koenig W, Baumert J, Doring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 2008;28:1186–1192. doi: 10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 23.Stark K, et al. Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS One. 2009;4:e7729. doi: 10.1371/journal.pone.0007729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soranzo N, et al. A novel variant on chromosome 7q22.3 associated with mean platelet volume, counts, and function. Blood. 2009;113:3831–3837. doi: 10.1182/blood-2008-10-184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson AD, et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat Genet. 2010;42:608–613. doi: 10.1038/ng.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakov R, Pfarr E, Eberle S. Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am J Hypertens. 2002;15:583–589. doi: 10.1016/s0895-7061(02)02933-3. [DOI] [PubMed] [Google Scholar]
- 27.Rahman T, Baker M, Hall DH, Avery PJ, Keavney B. Common genetic variation in the type A endothelin-1 receptor is associated with ambulatory blood pressure: a family study. J Hum Hypertens. 2008;22:282–288. doi: 10.1038/sj.jhh.1002322. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda H, et al. Association of single nucleotide polymorphisms in endothelin family genes with the progression of atherosclerosis in patients with essential hypertension. J Hum Hypertens. 2007;21:883–892. doi: 10.1038/sj.jhh.1002234. [DOI] [PubMed] [Google Scholar]
- 29.Oguri M, et al. Association of genetic variants with myocardial infarction in Japanese individuals with metabolic syndrome. Atherosclerosis. 2009;206:486–493. doi: 10.1016/j.atherosclerosis.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Chasman DI, et al. Genetic loci associated with plasma concentration of low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, apolipoprotein A1, and Apolipoprotein B among 6382 white women in genome-wide analysis with replication. Circ Cardiovasc Genet. 2008;1:21–30. doi: 10.1161/CIRCGENETICS.108.773168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatti C, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kathiresan S, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aulchenko YS, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kathiresan S, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Psaty BM, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of Prospective Meta-Analyses of Genome-Wide Association Studies From 5 Cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AD, O'Donnell CJ. An open access database of genome-wide association results. BMC Med Genet. 2009;10:6. doi: 10.1186/1471-2350-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.