Abstract
The exogenous administration of tetrahydrobiopterin (BH4), an essential cofactor of nitric oxide synthase (NOS), has been shown to reduce left ventricular hypertrophy, fibrosis, and cardiac dysfunction in mice with pre-established heart disease induced by pressure-overload. In this setting, BH4 re-coupled endothelial NOS (eNOS), with subsequent reduction of NOS-dependent oxidative stress and reversal of maladaptive remodeling. However, recent studies suggest the effective BH4 dosing may be narrower than previously thought, potentially due to its oxidation upon oral consumption. Accordingly, we assessed the dose response of daily oral synthetic sapropterin dihydrochloride (6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, 6R-BH4) on pre-established pressure-overload cardiac disease. Mice (n=64) were administered 0-400 mg/kg/d BH4 by ingesting small pre-made pellets (consumed over 15-30 min). In a dose range of 36-200 mg/kg/d, 6R-BH4 suppressed cardiac chamber remodeling, hypertrophy, fibrosis, and oxidative stress with pressure-overload. However, at both lower and higher doses, BH4 had less or no ameliorative effects. The effective doses correlated with a higher myocardial BH4/BH2 ratio. However, BH2 rose linearly with dose, and at the 400 mg/kg/d, this lowered the BH4/BH2 ratio back towards control. These results expose a potential limitation for the clinical use of BH4, as variability of cellular redox and perhaps heart disease could produce a variable therapeutic window among individuals.
Keywords: Nitric oxide synthase, tetrahydrobiopterin, NOS- uncoupling, Oxidative Stress, pressure-overload, mouse models
Introduction
Tetrahydrobiopterin (BH4) is an endogenously synthesized essential co-factor for aromatic amino acid hydroxylases, glyceryl-ether mono-oxygenase, and all three isoforms of nitric oxide synthase (NOS) [1]. It was first discovered as an obligate cofactor of phenylalanine hydroxylase (PAH) and is clinically used to treat phenylketonuria (PKU) which results from loss-of-function mutations in this enzyme[2, 3]. BH4 was subsequently discovered to be an essential cofactor for all three nitric oxide synthases (NOS) [4], where it plays a key role in cardiovascular, neural, and other cell regulation [5] .
BH4 facilitates electron transfer in the NOS enzyme from NADPH in its reductase domain to heme in its oxidase domain, and it is required for the conversion of arginine to citrulline to generate nitric oxide (NO). Decreased BH4 bioavailability leads to conformational changes in the NOS homodimer, leading to the phenomenon known as NOS-uncoupling, where less NO is generated and instead the enzyme produces superoxide [6]. Studies in the heart and vasculature have shown BH4 deficiency or its oxidation to BH2 increases NOS-derived reactive oxygen species (ROS) generation. This contributes to the pathophysiology of systemic and pulmonary hypertension [7, 8], diabetes [9-11], hypercholesterolemia [12], atherosclerosis [13], aging [14], acute cardiac transplant rejection [15], and cardiac hypertrophy/remodeling [16]. Studies enhancing BH4 levels either by exogenous administration [17-21] or by genetic uregulation of its rate-limiting synthetic enzyme, GTP cyclohydrolase (GCH-1) [22, 23], have shown improvement in each of these disease conditions. Importantly, benefit has been observed when BH4 is provided after disease is established, as in hearts with pre-existing ventricular hypertrophy, fibrosis, and dilation [20], or diastolic dysfunction and hypertrophy[24]. Intriguingly, these effects were not duplicated by administering a less targeted anti-oxidant (tempol), despite its ability to reduce overall myocardial superoxide levels, nor did they appear in hearts treated with BH4 at an earlier stage of hypertrophy when NOS uncoupling was not yet present. These results support the conclusion that the efficacy of exogenous BH4 stems from its selective targeting of uncoupled NOS.
Upon oral administration, BH4 is largely oxidized to BH2, and then must be re-reduced once taken up by a cell [25]. However, only BH4 is a functional NOS cofactor, and recent in vitro data suggests that relative increases in BH2 can compete with BH4 to impair NOS coupling [26, 27]. This poses potential limitations to oral dosing as one might hypothesize that higher doses of BH4 might tip the balance favoring BH2, offsetting physiologic benefits present at lower doses. To date, no study has examined such dose-response characteristics in vivo. Here, we present results from an oral dose-response experiment in which mice were subjected to sustained pressure-overload to establish pathophysiological remodeling and activation of oxidative stress, and then subsequently treated with daily BH4 at varying doses. We reveal a bi-model dose response that supports the importance of BH4/BH2 ratio in vivo to treatment efficacy.
Methods
Experimental Model
Mice (C57Bl/6) were first subjected to trans-aortic constriction (TAC) as described [28] and allowed to develop LV hypertrophy and dysfunction over a period of 5 weeks. Only mice with at least a 30% decline in ejection fraction were then entered into the treatment/placebo arm of the study. Sixty-four mice (aged 8-9 weeks) met this criteria and were randomized to receive placebo, or 12, 36, 200 or 400mg/kg/d of BH4 (sapropterin dihydrochloride (6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, 6R-BH4, BioMarin Pharmaceutical Inc., Novato, CA) for 5 additional weeks. Pressure-overload was sustained during this period as well. Drug was pre-mixed into 4mm diameter pellets (Rodent Treats, Bio-serv, Frenchtown, NJ) flavored to be attractive to mice. Individual animals were placed in shoe-box sized plastic containers each containing a small dish with the pellets (drug or placebo), and mice ate them fairly quickly (15-30 minutes) once acclimated to the procedure. This provided accurate daily oral dosing without requiring gavage. Mice were studied by serial echocardiography, and the study terminated at week 10, when hearts were harvested for biochemical and histological analysis. The protocol was approved by the Animal Care and Use Committee at the Johns Hopkins University.
Cardiac Function and Geometry
In vivo cardiac function and morphometry was assessed in conscious mice as previously described[16], and assessed at pre-TAC baseline, after 5 weeks of TAC (prior to initiating any treatment) and at terminal study (10 weeks TAC + 5wks treatment). Function was assessed by echocardiography (Acuson Sequoia C256, 13-MHz transducer, Siemens Medical Systems, Malvern, PA) measured in conscious animals from which left ventricular end-diastolic and end systolic dimension, wall thickness (mean of septal and posterior wall measurements) and fractional shortening were determined. LV mass (cylindrical model) and ejection fraction were also calculated as described[16].
Histology
Myocardium was fixed in 10% formalin and stained with hematoxylin and eosin and Masson trichrome to assess interstitial fibrosis. Fibrosis was scored on a qualitative scale (0 to 3) assigned by pathologist blinded as to source of the tissue [20].
Analysis of myocardial superoxide
Myocardial superoxide generation was assessed using dihydroethidine (DHE) fluorescent microtopography and lucigenin-enhanced chemiluminescence. Fresh-frozen LV tissue was sliced (8μm thickness), mounted onto glass slides and incubated with 2μM DHE (Invitrogen, Carlsbad, CA) for 1 hour at 37°C. Fluorescent imaging was performed by confocal microscopy done as previously described [16]. Lucigenin analysis was performed using snap-frozen myocardial samples as described, with 5μM lucigenin and NADPH (100μM) added to the homogenate, and chemiluminescence measured by scintillation counter (LS6000IC, Beckman Instruments, Fullerton, CA) at 37°C. Data are reported as counts/min/mg of tissue after background subtraction [20].
Myocardial BH4/BH2 Analysis
BH4 and BH2 were analyzed by HPLC assay [23, 29] in myocardium obtained from a separate group of mice treated with varying doses of BH4 but without prior surgery. Comparison at one dose (200 mg/kg/day) was made to another group of mice that had been subjected to TAC and treated with the same BH4 dose for 5 weeks. Fresh frozen myocardial samples (20-30 mg) were homogenized for 30 seconds in 400μL of ice cold re-suspension buffer (50mM phosphate buffered saline, pH 7.4; 1mM dithioerythritol; 1 mM EDTA) using a pre-cooled Polytron® probe (PT2100 Kinematika®, Switzerland). After two sequential centrifugations at 13,000 rpm (15 minutes at 4°C), supernatants from both spins were combined and mixed with 20μL of ice cold 10x precipitation buffer (1M phosphoric acid, 2M trichloroacetic acid, 1mM dithioerythritol). Samples were re-spun in the same manner, and supernatant (150μL) transferred to a 96-well HPLC plate for analysis, which was performed blinded as to tissue source.
Statistics
Serial echocardiography data were analyzed using a 2-way ANOVA with repeated measures. Terminal study data were analyzed using a 1-way ANOVA. Statistical significance was reported at the p<0.05 level.
Results
BH4 improves cardiac function in a dose dependent manner
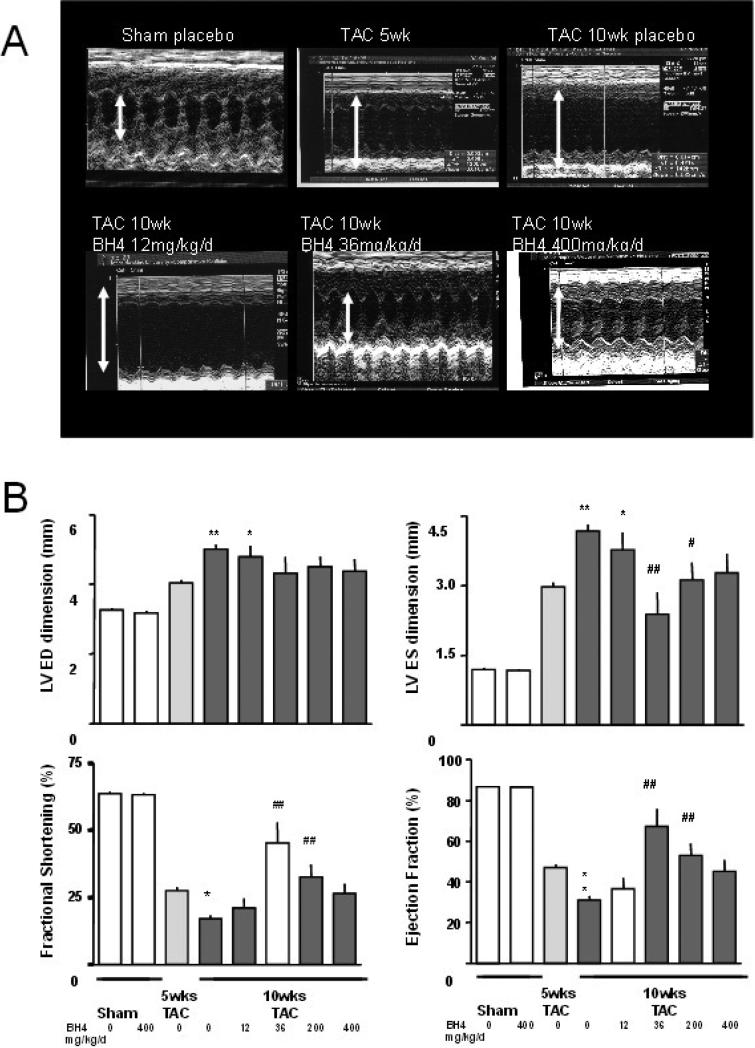
Figure 1A shows example M-mode echocardiograms for the study, and summary data are displayed in panel B. After 5-wks of TAC, hearts had reduced fractional shortening, with a mean ejection fraction of 46.9±1.7% compared to 86.7±0.6% at baseline (p<0.001), and left ventricular dilation (end-diastolic dimension: 4.0±0.1 vs. 3.3±0.1 mm, p<0.0001; end-systolic dimension 3.0±0.1 vs. 1.2 ±0.04 mm, p<0.001). In mice that subsequently received 6R-BH4 during weeks 5-10, chamber size diminished and fractional shortening increased despite persistent pressure-overload; however, this response was dose dependent in a bi-modal manner. The peak effective dose was 36 mg/kg/d, which reversed pre-existing maladaptive remodeling and improved ejection fraction (67.2±8.9%, p<0.001 compared to 5-wk pre-treatment). However, both the lowest tested dose (≤ 12 mg/kg/d) and highest dose (400 mg/kg/d) conferred minimal to no significant benefit on cardiac function or reverse remodeling.
Figure 1.
Effects of exogenous BH4 on pressure overload induced myocardial dysfunction. A) Example M-mode echocardiograms are shown (clockwise from top left) at baseline, after 5-wks transaortic constriction (TAC) with placebo, and at 10 weeks TAC with placebo, 12, 36, and 400 mg/kg/d 6R-BH4 treatment. B) Summary data for echocardiographic analysis (mean ± SEM). Administration of BH4 at 36 and 200 mg/kg/d significantly suppressed cardiac dilation and dysfunction. However responses with the lower and highest tested doses were much less or ineffective. *: p<0.05 (versus 5wks); **: p<0.001 (versus 5wks); #: p<0.05 (versus 10wks placebo); ##: p<0.001 (versus 10wks placebo). One-way ANOVA with posthoc Tukey test on 4 groups.
Dose dependent effects of BH4 on myocardial hypertrophy and fibrosis
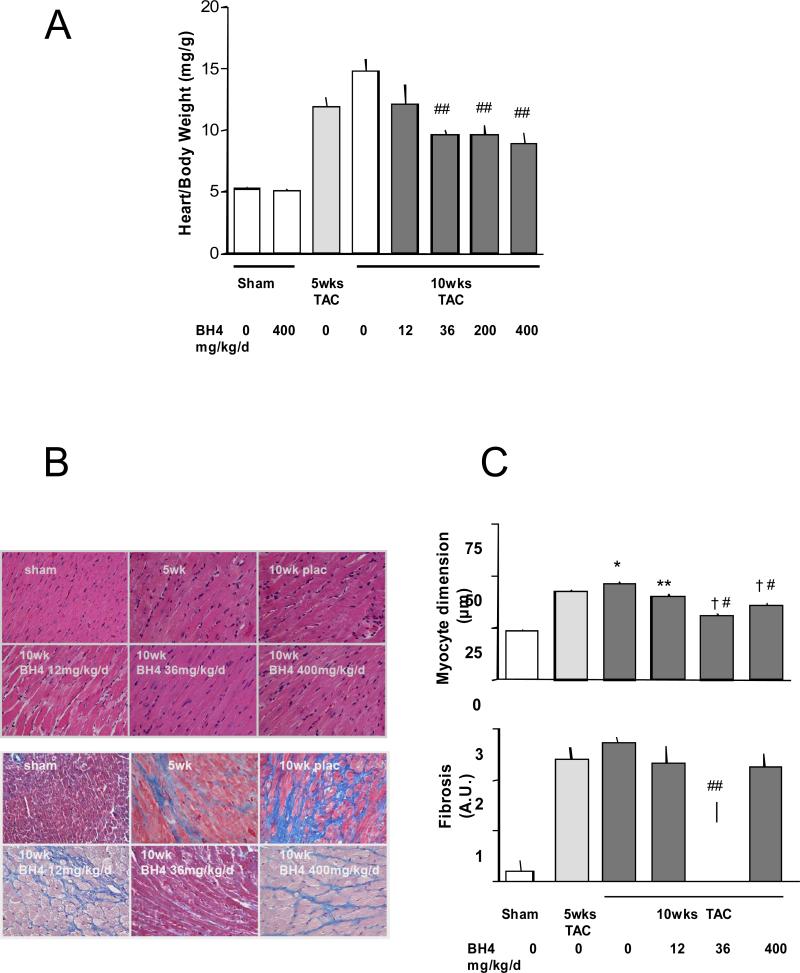
Left ventricular mass more than doubled after 5-wks TAC (heart/body weight ratio: 11.9±0.8 vs. baseline: 5.2± 0.2 mg/g, p<0.0001, Figure 2A). This was accompanied by increased myocyte size (short axis dimension: 42.6±0.7 vs. baseline: 23.6±0.5 μm, p<0.0001) and fibrosis (2.4±0.25 vs. baseline 0.2±0.2 (qualitative score units, p=0.0001, Figure 2B and C). As with LV function, the optimal BH4 dose that suppressed hypertrophy and fibrosis was 36 mg/kg/d (myocyte dimension 31.2±0.5 μm, p< 0.001, fibrosis score 1.17±0.4 AU, p=0.03), whereas the lowest dose only slightly reduced myocyte size and had no impact on fibrosis. At 400 mg/kg/d, chamber and myocyte hypertrophy declined, but there was no significant reduction in fibrosis.
Figure 2.
Exogenous BH4 administration suppresses pre-established cardiac hypertrophy and fibrosis, but the latter is less effective at low and higher doses. A) Summary data for left ventricular mass (terminal study) normalized to body weight shows reduced hypertrophy with BH4 treatment at doses > 36 mg/kg/d, including the high dose. B) Example histologic sections stained with H/E (upper panels) or Masson trichrome (lower panels) for assessing myocyte dimension and interstitial fibrosis. C) Summary data for histologic analysis. Myocyte hypertrophy was reduced at all BH4 doses, whereas fibrosis was only reduced at the 36 mg/kg/d dose (200 mg/kg/d was not tested in this analysis). *: p<0.05 (versus 5wks); †: p<0.001 (versus 5wks); **: p<0.05 (versus 10wks placebo); #: p<0.001 (versus 10wks placebo). One-way ANOVA with posthoc Bonferoni test on 4 groups.
Interaction between BH4 and ROS
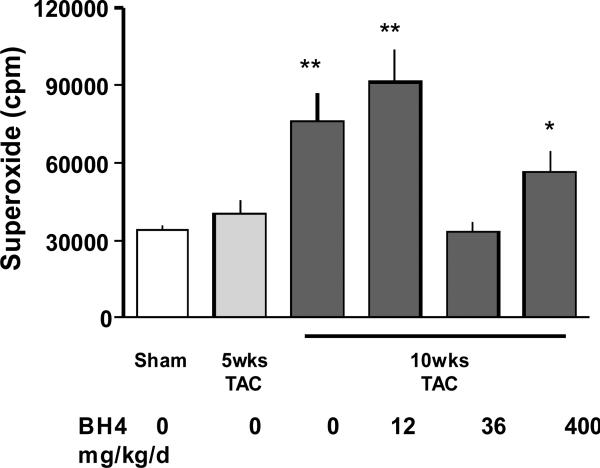
Effective doses of BH4 resulted in a marked decline in myocardial superoxide formation, detected by lucigenin assay (Figure 3). This was observed at the lower 36 mg/kg/d dose, but intriguingly, not at either the lowest or highest doses (TAC 10wk: 76186 ±10987cpm, TAC 10wk + BH4 12mg/kg/d 91028±12998cpm, TAC 10wk+ BH4 36mg/kg/d 33403±3949cpm, TAC 10wk+ BH4 400mg/kg/d 56353±7959cpm). The lack of anti-oxidant changes paralleled the loss of anti-fibrotic effects or functional improvement at these doses.
Figure 3.
Effect of BH4 administration on pressure-overload induced superoxide generation. BH4 administered at 36 mg/kg/d reduced lucigenin luminescence, but this effect was absent at both lowest and highest doses of BH4. *: p <0.05 vs. sham; **: p<0.001 vs. sham; #:p<0.001 vs. 10wks TAC placebo treatment.
Myocardial BH4/BH2 ratio correlates with dose efficacy
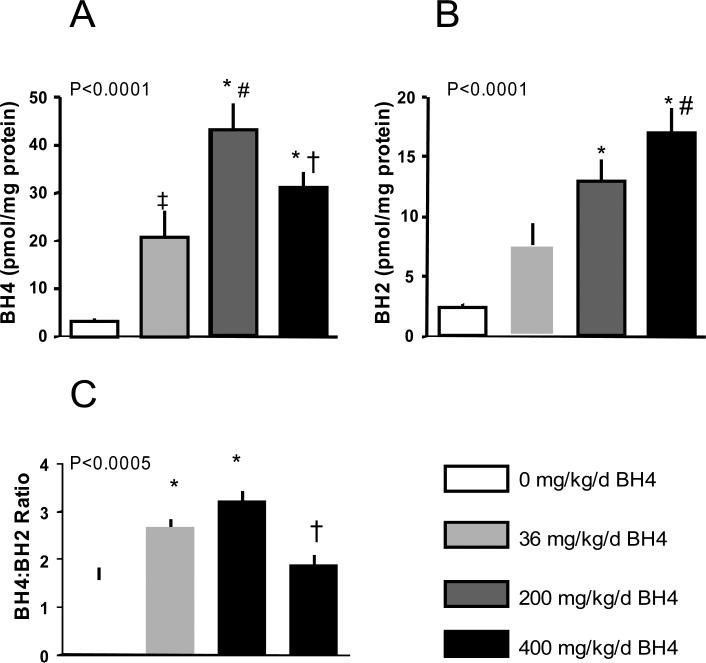
A potential mechanism for the loss of BH4 efficacy at higher doses was an accumulation of the oxidized form, BH2, that might not be effectively re-converted to BH4. To test this, we examined BH4/BH2 ratio in mice administered BH4 for 5 days. We observed a linear dose response in BH2 levels with increasing BH4, whereas BH4 levels peaked at a lower dose and then declined (Figure 4). As a result, BH4/BH2 ratio first rose but then fell again towards baseline (i.e. no treatment) at the highest dose tested. We further compared the results obtained in these mice, that were not subjected to TAC or administered BH4 for a longer period to animals in which the treatment protocol followed the primary TAC study. This was done for the 200 mg/kg/d dose, and the results were comparable (BH4: 46.6±7.6 – 5 days, no TAC; 40.5±7.8 – 5 weeks+TAC, p=0.6; BH4/BH2 ratio: 3.2±0.2 versus 3.6±0.25, p=0.3).
Figure 4.
Myocardial BH4 and BH2 levels after exogenous BH4 administration. BH2 increased in a dose-dependent manner, but there as a biomodal response in BH4, with an initial rise but later decline at the high dose. As a result, the BH4:BH2 ratio increased at lower but not the high dose. P-values in each graph are for 1-way ANOVA. Post hoc testing: A) * p<0.0001 vs no drug; # p<0.005 vs 36 mg/kg/d; † p<0.05 vs 200 mg/kg/d; ‡ p<0.03 vs no drug. B) * p<0.001 versus no drug; # p<0.02 versus 36 mg/kg/d. C) * p<0.02 versus no drug; † p<0.005 versus 200 mg/kg/d.
Discussion
The current results provide important new insights into the pharmacology and therapeutic efficacy of BH4 for the treatment of hypertrophic heart disease. As in our prior study [20], we observed a reduction of LV hypertrophy, fibrosis, and cardiac dysfunction by BH4 (using a synthetic formulation in this case), in hearts with pre-established disease induced by pressure-overload (Figs 1 and 2). However, the dose response was bi-modal, first rising and then declining at higher doses. This pertained to chamber remodeling, function, fibrosis, and oxidative stress (Fig. 3), and when observed, benefits correlated with an enhanced ratio of BH4/BH2 (Fig. 4). The latter first rose with increasing BH4 dose, but declined at the higher dose. These results expose a potential limitation for the clinical use of BH4 in diseases characterized by oxidative stress, as defining the oral dose that would optimally provide BH4 rather than BH2 maybe difficult and vary from individual to individual.
In its reduced form, BH4 serves as a critical co-factor enabling NOS to generate NO. However, its role is impeded if BH4 is oxidized to BH2. BH2 binds eNOS with an affinity equal to that of BH4 in murine endothelial cells [27]. However, as BH2 cannot function as a NOS cofactor, this competitive binding [30] effectively replacing eNOS-bound BH4 [27] and enhances uncoupling [31]. Sugiyama et al. [26] compared effects of gene silencing of GCH-1 (also known as GTPCH), the rate-limiting enzyme for BH4 biosynthesis, and dihydrofolate reductase (DHFR), a key enzyme restoring BH4 from BH2, in studies performed in isolated endothelial cells. Knockdown of DHFR but not GCH-1 increased ROS, and the former but not latter reduced the relative BH4/BH2 ratio. Crabtree et al. [27] observed similar behavior, and showed that in cells expressing eNOS but low biopterin levels, DHFR inhibition/gene silencing reduced BH4/BH2 ratio further, exacerbating eNOS uncoupling. Thus, the relative BH4/BH2 ratio and BH2 level, rather than BH4 levels per se, appears to be central for perturbing NOS signaling. Plasma BH4/BH2 ratio also correlates with depressed endothelial function in patients with more than two cardiovascular risk factors[32]. The present data indicate that at higher doses of exogenous BH4 (and thus BH2), the intrinsic capacity to maintain BH4/BH2 ratios may become compromised, limiting net efficacy.
Despite the intriguing promise of BH4 treatment for cardiovascular and other disorders involving NOS uncoupling and associated oxidative stress, clinical trials targeting these diseases have so far been disappointing [33]. In particular, 6R-BH4 has been recently tested in studies targeting hypertension, cardio-renal syndrome with proteineuria, sickle cell disease, and pulmonary hypertension. None of these studies achieved their primary endpoint to demonstrate clinical efficacy, and all were discontinued. The current findings may provide an explanation. If the efficacy of exogenous BH4 to reverse NOS uncoupling critically depends upon the BH2/BH4 balance, and this in turn varies with BH4 dose and redox milieu in a particular situation, the dose-response window may be relatively narrow, and identifying the optimal dose for a given patient difficult. Efforts aimed at reducing the oxidation of BH4 to BH2 after oral consumption, perhaps by encapsulation to promote more distal gut absorption, stimulating DHFR, or by enhancing a reducing environment, may be important so as to maintain efficacy.
Our study has several limitations. First, while we previously reported that BH4 therapy restores NOS3 coupling and NO generation, we did not examine this in the current dose-response study, and thus cannot confirm that that re-appearance of ROS at a high BH4 dose was indeed due to the re-emergence of NOS3 uncoupling. The study sponsor, Biomarin, who provided 6R-BH4 in the pellets has suspended their cardiovascular research program in this area, and could not provide material for these additional studies. While future studies using this method would not be identical to what we employed, BH4 (or 6R-BH4) is commercially available, and can be processed by Bio-serv to generate the rodent treats used to administer drug. Alternative dosing options would be available for other animal models (e.g. gavage or oral tablets for larger mammals).
In summary, we have demonstrated a bi-modal dose-response relation to exogenous BH4 treatment in mice with pre-established hypertrophy and cardiac dysfunction due to sustained pressure-overload. Beneficial effects on hypertrophy, LV function, fibrosis, and oxidative stress in the myocardium correlate with the ratio of BH4/BH2 achieved by the administered dose. Inadequate conversion of BH2 to BH4 may pose a limitation to its therapeutic use, and co-administration of agents that stimulate such conversion may be valuable to enhance its therapeutic benefits in vivo.
Highlights.
NOS generates oxidative stress in pressure-overload cardiac hypertrophy.
This contributes to pathological hypertrophy, fibrosis, and cardiac dysfunction.
Lower range doses of oral tetrahydrobiopterin (BH4) reverse these changes.
Higher doses fail. BH4 is oxidized to BH2, fibrosis, and hypertrophy re-appear.
Defining the therapeutic window for BH4 is key and depends on BH4/BH2 ratio.
Acknowledgments
This work was supported by a grant from Biomarin, NIH grants HL-89297, HL-59480, The Peter Belfer Laboratory, and Fondation Leducq (DAK), and research grants from the American Heart Association, Dutch Heart Foundation (NHS-subsidieronde 2009) and Dutch Government (NWO-VIDI/Aspasia) to AM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare that there are no actual or potential conflicts of interest, including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence (bias) their work.
REFERENCES
- 1.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol. 2007 Sep;50(3):238–46. doi: 10.1097/FJC.0b013e318123f854. [DOI] [PubMed] [Google Scholar]
- 2.Danks DM, Cotton RG, Schlesinger P. Letter: Tetrahydrobiopterin treatment of variant form of phenylketonuria. Lancet. 1975 Nov 22;2(7943):1043. doi: 10.1016/s0140-6736(75)90335-9. [DOI] [PubMed] [Google Scholar]
- 3.Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007 Aug 11;370(9586):504–10. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 4.Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–501. [PubMed] [Google Scholar]
- 5.Vasquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic Biol Med. 2009 Oct 15;47(8):1108–19. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotsonis P, Frohlich LG, Shutenko ZV, Horejsi R, Pfleiderer W, Schmidt HH. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J. 2000 Mar 15;346(Pt 3):767–76. [PMC free article] [PubMed] [Google Scholar]
- 7.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000 May 16; doi: 10.1161/01.cir.101.19.2264. Sect. 2264-70. [DOI] [PubMed] [Google Scholar]
- 8.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008 Jun;10(6):1115–26. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 9.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, et al. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes. 1999 Dec;48(12):2437–45. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki N, Yamashita T, Takaya T, Shinohara M, Shiraki R, Takeda M, et al. Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arterioscler Thromb Vasc Biol. 2008 Jun;28(6):1068–76. doi: 10.1161/ATVBAHA.107.160754. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors. 2009 Jan-Feb;35(1):21–7. doi: 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino F, Hurlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart. 2008 Apr;94(4):487–92. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 13.Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation. 2000 Oct 31;102(18):2172–9. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- 14.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1829–36. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieper GM, Ionova IA, Cooley BC, Migrino RQ, Khanna AK, Whitsett J, et al. Sepiapterin decreases acute rejection and apoptosis in cardiac transplants independently of changes in nitric oxide and inducible nitric-oxide synthase dimerization. J Pharmacol Exp Ther. 2009 Jun;329(3):890–9. doi: 10.1124/jpet.108.148569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005 May;115(5):1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis. 2009 May;204(1):73–8. doi: 10.1016/j.atherosclerosis.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, et al. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci U S A. 2007 Sep 18;104(38):15081–6. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Worthley MI, Kanani RS, Sun YH, Sun Y, Goodhart DM, Curtis MJ, et al. Effects of tetrahydrobiopterin on coronary vascular reactivity in atherosclerotic human coronary arteries. Cardiovasc Res. 2007 Dec 1;76(3):539–46. doi: 10.1016/j.cardiores.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008 May 20;117(20):2626–36. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L, Pung YF, Zhang J, Chen P, Wang T, Li M, et al. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H331–9. doi: 10.1152/ajpheart.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation. 2008 Feb 26;117(8):1045–54. doi: 10.1161/CIRCULATIONAHA.107.748236. [DOI] [PubMed] [Google Scholar]
- 23.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003 Sep;112(5):725–35. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010 Feb 2;121(4):519–28. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiege B, Ballhausen D, Kierat L, Leimbacher W, Goriounov D, Schircks B, et al. Plasma tetrahydrobiopterin and its pharmacokinetic following oral administration. Mol Genet Metab. 2004 Jan;81(1):45–51. doi: 10.1016/j.ymgme.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009 May 8;284(19):12691–700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009 Oct 9;284(41):28128–36. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005 Feb;11(2):214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 29.Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007 Nov;117(11):3198–210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorren AC, Bec N, Schrammel A, Werner ER, Lange R, Mayer B. Low-temperature optical absorption spectra suggest a redox role for tetrahydrobiopterin in both steps of nitric oxide synthase catalysis. Biochemistry. 2000 Sep 26;39(38):11763–70. doi: 10.1021/bi0007775. [DOI] [PubMed] [Google Scholar]
- 31.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1530–40. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda M, Yamashita T, Shinohara M, Sasaki N, Takaya T, Nakajima K, et al. Plasma tetrahydrobiopterin/dihydrobiopterin ratio: a possible marker of endothelial dysfunction. Circ J. 2009 May;73(5):955–62. doi: 10.1253/circj.cj-08-0850. [DOI] [PubMed] [Google Scholar]
- 33.Porkert M, Sher S, Reddy U, Cheema F, Niessner C, Kolm P, et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. 2008 Jun;22(6):401–7. doi: 10.1038/sj.jhh.1002329. [DOI] [PubMed] [Google Scholar]