Abstract
Cadherin-based intercellular adhesions are important determinants of proper tissue architecture. These adhesions must be both stable and dynamic to maintain tissue integrity as cells undergo morphogenetic movements during development. The role of α-catenin in this process has been vigorously debated due to conflicting in vitro and in vivo evidence regarding its molecular mechanism of action. Recent data supports the classical view that α-catenin facilitates actin attachments at adherens junctions, but also suggests that α-catenin may act as a force transducer, and may have additional roles in the cytoplasm. These multiple functions for α-catenin converge on the regulation of adhesion and may help to explain its stable yet dynamic nature.
Introduction
The challenge of morphogenesis: Balancing adhesive stability and pliancy.
During morphogenesis in animal embryos, changes in tissue architecture are supported by stable adhesive interactions between neighboring cells. At the same time, however, local changes in cell attachments and position require these adhesive interactions to be highly dynamic (Gumbiner, 1996). How cells are able to measure and respond to morphogenetic forces and change the strength of cell–cell adhesions accordingly is a critical question in developmental biology. Intercellular adhesions also support other aspects of tissue formation, including cell sorting and recognition, and the acquisition of apicobasal polarity in epithelial cells (Gumbiner, 1996; Takeichi, 1991). Adhesion is likewise important in maintaining mature tissues, as disruption of proteins involved in intercellular adhesions can lead to a variety of pathologies, including heart dysfunction (Radice et al., 1997; Luo et al., 2001; Kostetskii et al., 2005; Sheikh et al., 2006), tumorigenesis and metastasis (Benjamin and Nelson, 2008), and hyperproliferation related to up-regulated cell signaling (Vasioukhin et al., 2001; Lien et al., 2006).
The cadherin–catenin complex: Deceptively simple?
A key cell–cell adhesion structure is the adherens junction (AJ), which features the highly conserved cadherin–catenin complex (CCC; Fig. 1 A). Three decades ago, cadherins were identified as calcium-dependent transmembrane glycoproteins that mediate adhesion through homophilic interactions of their extracellular domain with cadherins on adjacent cells (for historical review, see Franke, 2009). Not long after the discovery of cadherins, three distinct proteins termed catenins were found to be associated with their cytoplasmic tails (Ozawa et al., 1989; Reynolds et al., 1994). β-Catenin, which was soon realized to be a member of a highly conserved family of proteins that includes Drosophila Armadillo (Peifer et al., 1992), contains 12 α-helical armadillo (arm) repeats and binds directly to the tail of cadherin (Stappert and Kemler, 1994; Huber et al., 1997a). β-Catenin also binds directly to α-catenin (Aberle et al., 1994; Huber et al., 1997b), and in addition to its role in cell–cell adhesion, has functions in Wnt signaling (Nelson and Nusse, 2004). A fourth conserved member of the CCC is p120-catenin, an arm repeat protein that binds a juxtamembrane region of the cadherin cytoplasmic tail and is known to be an effector of Rho GTPases and to be involved in regulating cadherin stability (Anastasiadis, 2007).
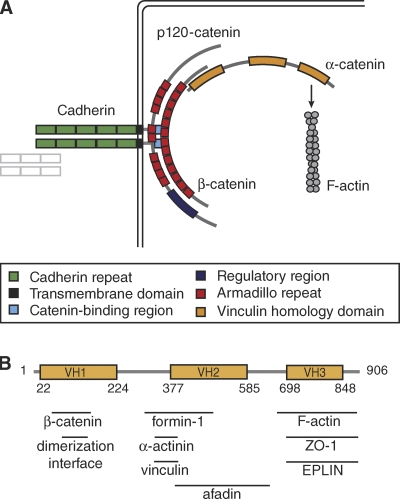
Figure 1.
The role of α-catenin in cell–cell adhesion. (A) The cadherin–catenin complex (CCC). The transmembrane cadherin mediates cell–cell adhesion through calcium-dependent homophilic binding of an adjacent cadherin. Intracellularly, cadherin interacts directly with p120-catenin and β-catenin. α-Catenin joins the complex by binding to β-catenin through its N terminus, while the C-terminal actin-binding domain recruits the actin cytoskeleton. (B) Key features of α-catenin, including the three vinculin homology (VH) domains and the binding sites of several proteins discussed in this review.
α-Catenin (Fig. 1 B) is an actin-binding and -bundling protein (Rimm et al., 1995) that contains three domains (VH domains) homologous to another actin-binding and -bundling protein, vinculin (Herrenknecht et al., 1991; Nagafuchi et al., 1991). The N terminus of α-catenin contains a β-catenin–binding site (Aberle et al., 1994; Huber et al., 1997b; Koslov et al., 1997; Nieset et al., 1997; Pokutta and Weis, 2000), whereas the C terminus contains the actin-binding domain (Nagafuchi et al., 1994; Imamura et al., 1999; Pokutta et al., 2002). Other binding partners include an assortment of actin-related proteins, including vinculin itself (Watabe-Uchida et al., 1998; Weiss et al., 1998; Imamura et al., 1999), α-actinin (Knudsen et al., 1995; Nieset et al., 1997), ZO-1 (Itoh et al., 1997; Imamura et al., 1999), afadin (Pokutta et al., 2002), formin-1 (Kobielak et al., 2004), and Rho (Magie et al., 2002). These features made α-catenin a logical choice for linking the CCC directly to the actin cytoskeleton. A simple model predicted that cadherin, β-catenin, and α-catenin bind one another as a ternary complex with 1:1:1 stoichiometry, which in turns binds F-actin (Rimm et al., 1995; Pokutta et al., 2002; Pappas and Rimm, 2006).
This simple and appealing model for α-catenin function left several unanswered questions. First, how is it that a quaternary complex of CCC + F-actin can mediate dynamic adhesions that are still capable of resisting mechanical tension, as must be true in developing embryos? Second, are there roles for additional actin-binding proteins that connect to the adherens junction through α-catenin? Third, are there any roles for α-catenin beyond its function as a simple linker between the CCC and F-actin? Collaborative studies from Nelson, Weis, and colleagues on the vertebrate epithelial isoform of α-catenin, αE-catenin, have challenged the earlier, simple models for α-catenin function. These studies indicated that the binding affinities of αE-catenin appear unexpectedly complex: purified αE-catenin could bind E-cadherin–β-catenin or F-actin in vitro, but these binding events appeared to be mutually exclusive. Moreover, quaternary CCC + F-actin complexes could not be reconstituted on membrane patches (Yamada et al., 2005). Other structural studies further suggested that the N terminus of α-catenin contains overlapping and mutually exclusive binding sites for itself and β-catenin that form a conformational “switch” (Pokutta and Weis, 2000). Moreover, the affinity for F-actin was higher for αE-catenin homodimers compared with αE-catenin–β-catenin heterodimers, and these homodimers could, at fairly high concentrations, inhibit Arp2/3-dependent actin polymerization in vitro (Drees et al., 2005). Taken together, these studies suggested that αE-catenin homodimers might regulate actin dynamics independently of direct physical association with the CCC (Drees et al., 2005; Yamada et al., 2005; for a more detailed analysis of this work, see Gates and Peifer, 2005; Pokutta et al., 2008).
From simplicity to complexity: Re-evaluating the role of α-catenin during morphogenesis.
In light of the new evidence on αE-catenin function, as well as the remaining questions regarding how adhesions are regulated during development, efforts have been made to reevaluate the role of α-catenin at AJs and to define its exact molecular activities. In this review, we discuss new and classical experiments that provide evidence for α-catenin–dependent actin attachment to the AJ; we also present emerging evidence that α-catenin can act as a force transducer and that it may have additional roles in the cytoplasm. These seemingly disparate functions may better explain how α-catenin is able to modulate the stability of the AJ and how it participates in morphogenetic processes.
Direct or indirect: Probing the linkage of the CCC to actin
Testing direct linkage through cadherin–α-catenin chimeras.
Once α- and β-catenin were identified as intracellular binding partners of cadherin, it was soon realized that cadherins lacking the cytoplasmic tail no longer bind to catenins (Nagafuchi and Takeichi, 1989; Ozawa et al., 1989, 1990), are nonfunctional in adhesion, and fail to interact with the actin cytoskeleton (Nagafuchi and Takeichi, 1988). If α-catenin provides a direct linkage between cadherins and F-actin, a straightforward prediction is that a cadherin–α-catenin chimera should confer cell–cell adhesion abilities on cells that lack cadherin function. To test this hypothesis, chimeric fusions that directly linked portions of αE-catenin to a nonfunctional, C-terminally deleted E-cadherin construct (Fig. 2 A) were expressed in fibroblasts lacking cadherin activity. In this assay, both the full-length αE-catenin fusion (nEα) and a fusion with only the C terminus of αE-catenin (nEαC) were sufficient to confer strong adhesion and recruit F-actin, but a fusion containing only the N terminus of αE-catenin (nEαN) was not (Nagafuchi et al., 1994; Imamura et al., 1999). Although there is some concern about the levels of endogenous α-catenin in these assays (possibly dimerizing with the fusions; see the discussion at the end of this section), these studies suggested that cadherin–α-catenin chimeras are sufficient for rescue in cultured cells, and that this rescue requires the C terminus of αE-catenin.
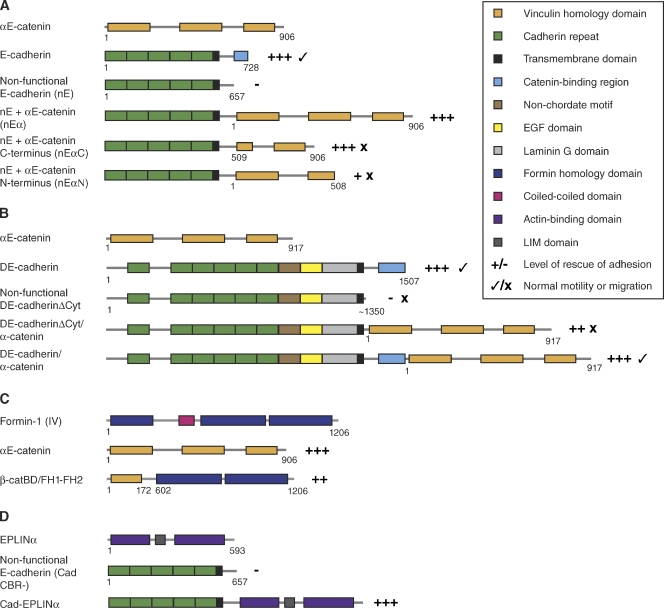
Figure 2.
Structure and function of chimeric adhesion constructs. Fusions between the cytoplasmic tail of cadherins and α-catenin, or between cadherin and actin-binding proteins, have been used to probe the connection between the CCC and the actin cytoskeleton. Note: Not all of the chimeras used in each experiment are depicted in this schematic; only those discussed in this review are shown. (A) Chimeric fusion constructs of mammalian E-cadherin and αE-catenin (Nagafuchi et al., 1994; Imamura et al., 1999). (B) Chimeric fusions of Drosophila DE-cadherin and α-catenin (Pacquelet and Rørth, 2005). (C) Chimeric fusion of mammalian αE-catenin and formin-1(IV) (Kobielak et al., 2004). (D) Chimeric fusion of mammalian E-cadherin and EPLINα (Abe and Takeichi, 2008).
In vivo, adhesions must be dynamically regulated. To test whether cadherin–α-catenin chimeras are sufficient in vivo, a similar experiment has been conducted in Drosophila (Pacquelet and Rørth, 2005) using both a nonfunctional DE-cadherin lacking the cytoplasmic tail (ΔCyt) and a full-length DE-cadherin, each fused to α-catenin (Fig. 2 B). The DE-cadherinΔCyt–α-catenin fusion was able to rescue adhesion defects seen in DE-cadherin null mutants during oogenesis, including follicle cell sorting, loss of epithelial integrity, and oocyte mispositioning, but not defects in border cell migration. In contrast, the full-length DE-cadherin–α-catenin fusion was able to rescue all defects, including border cell migration. This latter result was independent of β-catenin because the construct was still able to rescue the same defects in arm null mutants (Pacquelet and Rørth, 2005). Interestingly, Nagafuchi et al. (1994) saw defects in intercellular motility in their original cell culture fusion experiments where they also used a nonfunctional cadherin lacking the cytoplasmic domain (Fig. 2 A). They concluded this effect might be due to a lack of β-catenin at the junction (Nagafuchi et al., 1994), but unfortunately, no follow up experiments were conducted to test this theory. It would be interesting to see if a full-length cadherin fused to α-catenin is able to rescue this intercellular motility defect in cells lacking endogenous β-catenin, which would correlate with the D. melanogaster study.
Both the cell culture and D. melanogaster chimeric fusion results support the idea that the predominant function of β-catenin is to link cadherin to α-catenin and thus the actin cytoskeleton, and that modulation of this link is unnecessary. This finding was not as surprising in a simple cell culture system as it was in D. melanogaster, where the chimeric fusions not only rescued basic adhesions but also complex morphogenetic movements in the absence of β-catenin. There are, however, caveats to these experiments. In both the fibroblasts and Drosophila embryos used in these experiments, there was at least some endogenous α-catenin expression, which could complicate the interpretations because mammalian αE-catenin is known to homodimerize. Moreover, although the chimeric constructs were sufficient to rescue adhesion, full-length α-catenin may be necessary for other activities that were not assayed in these experiments, or were masked because of residual endogenous expression. Conclusive corroboration of these results awaits testing in true α-catenin null mutants.
α-Catenin as an intermediary.
In part because of the recent doubts cast on the simple, direct linkage model, other studies have used the same basic chimeric construct approach to examine the possibility that other junctional actin-associated proteins provide an indirect link to the actin cytoskeleton through α-catenin. Formin-1, which nucleates unbranched actin filaments, was identified as a binding partner of α-catenin in a yeast two-hybrid screen of cDNAs from newborn mouse epithelia (Kobielak et al., 2004). Formin-1 localizes to nascent AJs and coimmunoprecipitates with α-catenin in wild-type keratinocytes. In contrast, formin-1 fails to localize to AJs in cells lacking endogenous α-catenin, which lack the radial actin cables typical in wild-type cells. To determine if formin-1 is able to modulate actin organization at the junction independent of most of α-catenin, chimeric fusions were created that link the β-catenin binding site of α-catenin to the actin polymerization domains (FH1-FH2) of formin-1 (Fig. 2 C). In α-catenin null keratinocytes expressing this construct, cells were able to form nascent AJs with properly localized radial actin cables (Kobielak et al., 2004). Similar results were subsequently obtained for EPLIN, an actin-binding protein that stabilizes actin filaments by suppressing depolymerization. Like formin-1, EPLIN could be coimmunoprecipitated from mouse tissue lysates with CCC components. EPLIN colocalizes with the CCC in multiple epithelial cell lines, and this association is perturbed when adhesion is disrupted by α-catenin depletion (Abe and Takeichi, 2008). A chimeric fusion of a nonfunctional cadherin with full-length EPLIN (Fig. 2 D) expressed in the fibroblast cells used in the original studies of Nagafuchi et al. (1994) rescued nascent AJ formation and recruitment of radial actin cables similar to cadherin–α-catenin fusions (Abe and Takeichi, 2008). Taken together, these experiments support a role for other actin-binding proteins in linking the CCC to F-actin in an α-catenin–dependent fashion. To what extent such indirect linkages contribute to actin recruitment to the CCC under normal circumstances is unclear, as is whether such linkages are mutually exclusive of direct binding of α-catenin to F-actin.
α-Catenin under stress: Roles as a dynamic mechanical modulator
α-Catenin during actomyosin-mediated contractile events.
However α-catenin may mediate actin attachment to the CCC, recent studies in model organisms implicate α-catenin in conferring the ability of the CCC to dynamically associate with the F-actin cytoskeleton in cells under tension. During Caenorhabditis elegans embryogenesis, contractile stress transmitted through circumferential actin filament bundles that are anchored to the adherens junctions in the outer epithelial cells mediates elongation of the embryo. Mutations in the C-terminal actin-binding domain of hmp-1, the worm homologue of α-catenin, result in defects in the organization of circumferential actin filament bundles during this morphogenetic process. These defects include large gaps and bunching between the normally equally spaced parallel actin filaments, as well as an absence of F-actin at AJs (Costa et al., 1998; Pettitt et al., 2003; Kwiatkowski et al., 2010), which leads to physical ripping of AJs as epidermal cells generate myosin-dependent tensile forces (Costa et al., 1998). In embryos lacking maternal hmp-1 mRNA, an earlier defect occurs, as the initial attachment of epidermal cells at the ventral midline fails. In this case, nascent adhesions cannot withstand tension generated within the epithelium during ventral enclosure (Raich et al., 1999).
Roles for α-catenin in junctions under stress have also been identified in Drosophila. In early embryonic epithelia of D. melanogaster, E-cadherin and α-catenin localize to extremely stable microdomains called spot AJs. Some of the F-actin contained in these microdomains is resistant to latrunculin A, a known inhibitor of actin polymerization. The localization of stable F-actin to these spot AJs is unperturbed after knockdown of α-catenin via RNAi, indicating that α-catenin is not required to maintain stable F-actin at these spots. However, α-catenin knockdown prevents the lateral diffusion of spot AJs after ablating the stable F-actin using nanojoule pulses of a near-infrared laser (nano-scissors), indicating a role for α-catenin in mediating the more dynamic, junctional pool of F-actin with respect to spot AJ mobility (Cavey et al., 2008). More recent work suggests that myosin-dependent events at the junction may be affected by a dynamic, nonjunctional actomyosin network (Rauzi et al., 2010). Taken together, these studies indicate that in vivo, α-catenin is crucial for dynamic actin recruitment, and that at least some of these functions are mediated by the same region of α-catenin that normally enables it to bind F-actin in vitro. Whether these functions are mediated directly via F-actin binding, or indirectly through other actin-binding proteins such as formin-1 and EPLIN (Kobielak et al., 2004; Abe and Takeichi, 2008; Cavey et al., 2008), remains unclear. Dissecting these possibilities awaits further analysis of amino acids in the C terminus of α-catenin that mediate one or both functions in vivo.
α-Catenin: Potential roles in mechanotransduction.
Because cells and tissues during morphogenesis often experience contractile forces, it seems logical that a pathway should exist to measure and respond to these forces appropriately to maintain tissue integrity. Proteins involved in adhesive interactions have been likely candidates because they are directly responsible for maintaining stable tissues during morphogenetic movements. Integrins have been studied for many years as mechanosensors in cell–matrix adhesions. The size of integrin-containing focal adhesions depends not only on the amount of tension on specific adhesions, but also on the rigidity of the substrate, with larger focal adhesions forming under more tension and higher rigidity (Bershadsky et al., 2003). The downstream effectors of this pathway include talin, which connects integrins to the actin cytoskeleton, and vinculin. Increased association of vinculin with focal adhesions is force dependent. Talin contains cryptic vinculin-binding sites that are exposed in response to force exerted on talin through its binding to the actin cytoskeleton (del Rio et al., 2009). Tension on vinculin itself has recently been measured using Förster resonance energy transfer (FRET), and highest tension is associated with expanding focal contacts at the leading edges of migrating cells (Grashoff et al., 2010).
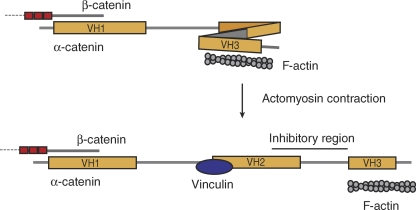
Like cell–matrix contacts, cell–cell adhesions must presumably have tension-sensing capabilities. Several studies have shown that vinculin localizes to AJs through binding to residues 325–402 of α-catenin (Watabe-Uchida et al., 1998; Weiss et al., 1998; Imamura et al., 1999; Yonemura et al., 2010). Given its pivotal role in recruitment of actin to AJs and its ability to bind vinculin, α-catenin is a sensible candidate for mediating such mechanotransduction at AJs. Two recent studies suggest that α-catenin can indeed function in this manner. Treatment of epithelial cells with a myosin II inhibitor abrogates vinculin localization at AJs, suggesting that vinculin recruitment is tension dependent (Yonemura et al., 2010). Analysis of αE-catenin deletion constructs further suggests that α-catenin contains a conformation-dependent inhibitory region that prevents vinculin binding under low-stress conditions. Release of this inhibition requires both applied tension and the C-terminal actin-binding domain of αE-catenin (Fig. 3). Interestingly, a monoclonal antibody to αE-catenin also localizes to AJs in a force-dependent manner; the epitope has been mapped to the region between the vinculin-binding site and the putative inhibitory region (Yonemura et al., 2010). A second study, this time using magnetic twisting cytometry, also identified the CCC as a mechanosensor that acts via force-dependent vinculin localization. Applying force to beads coated with Fc-E-cadherin attached to the dorsal surface of cultured cells causes decreased bead displacement over time, implying a stiffening response to prolonged loading (le Duc et al., 2010). This stiffening response decreases when cells are treated with inhibitors of either actin or myosin II, as well as in vinculin knockout cells. Although le Duc et al. (2010) did not address the role of α-catenin in mechanosensing directly, their results are consistent with a model of force-dependent conformational change in α-catenin’s binding affinities that allows additional adaptor proteins to bind to AJs under conditions of heightened tension.
Figure 3.
A model for tension-induced conformational changes in αE-catenin. αE-catenin may act as a mechanosensor to transduce changes in force to changes in the strength of cell–cell adhesions by recruiting additional adaptor proteins. See the text for further discussion. When αE-catenin is not under tension, its conformation may allow an inhibitory region to block its vinculin binding site. Upon the application of force, such as actomyosin-mediated contraction transduced through is C-terminal F-actin binding domain, αE-catenin may undergo a conformational change that displaces the inhibitory region from the vinculin binding site, allowing vinculin to bind. Vinculin, which has its own F-actin binding site, may in turn recruit additional F-actin to the CCC (Yonemura et al., 2010).
These in vitro studies provide evidence that α-catenin may serve as a tension sensor that can modulate the strength of actin attachments at AJs. But is the C-terminal actin-binding activity of α-catenin itself regulated via tension? Although such a role has not been shown, studies of the C. elegans homologue of α-catenin, HMP-1, are suggestive (Kwiatkowski et al., 2010). HMP-1 may be useful for examining vinculin-independent mechanotransduction mediated via α-catenin because the vinculin homologue DEB-1 has not been shown to be expressed in epithelial cells in worms (Barstead and Waterston, 1989). Somewhat surprisingly, unlike vertebrate αE-catenin, even under the most favorable conditions recombinant HMP-1 cannot homodimerize. Nevertheless, HMP-1 appears to undergo regulated binding to F-actin. In an actin cosedimentation assay, full-length recombinant HMP-1 does not pellet significantly with F-actin. Sequential addition of recombinant HMR-1/cadherin, HMP-2/β-catenin, and full-length HMP-1/α-catenin to form the ternary complex did not increase avidity for F-actin. In contrast, however, C-terminal fragments of HMP-1 that contain the actin-binding domain cosediment at levels comparable to the same region of mammalian αE-catenin. These data suggest that binding of F-actin to HMP-1 is regulated by intramolecular inhibition that is neither alleviated by homodimerization nor formation of the ternary complex (Kwiatkowski et al., 2010). The precise basis for this intramolecular regulation is unknown, as is how widespread such regulation is among α-catenin family members. However, it is appealing to speculate that α-catenin is tightly regulated, both in terms of its ability to recruit secondary sites for F-actin attachment via other actin-binding proteins and in terms of its own ability to bind F-actin. In the case of C. elegans HMP-1, such regulation would have a clear physiological role. As the embryo elongates, tension is exerted along circumferential actin filament bundles, which in turn must be stably anchored to the plasma membrane through the CCC (Priess and Hirsh, 1986). Tension-dependent enhancement of the stability of the connection between HMP-1 and actin could thereby promote the reliability of this actin-dependent morphogenetic process. Recent work by Zhang et al. (2011) has further implicated fibrous organelles, hemidesmosome-like, intermediate filament–based attachment structures in the C. elegans epidermis, as part of a multi-tissue, mechanosensory feedback loop during elongation (Zhang et al., 2011). How this feedback loop relates to the CCC during elongation is an interesting question for future study.
More than a linker: Additional roles of α-catenin
α-Catenin in the cytosol: Potential new roles for a versatile protein.
Cell–cell interactions are crucial not only for allowing cells to adhere to one another, but for regulating cell proliferation and down-regulating excess motility as cells form stable adhesions in epithelia. Indeed, cell adhesion molecules are well known as tumor suppressors (Hanahan and Weinberg, 2011). Within the CCC, the prototype in this regard is β-catenin, which is well known to serve double duty as a downstream component in Wnt signaling (Cadigan and Peifer, 2009; Heuberger and Birchmeier, 2010). Recent experiments suggest that α-catenin also lies at the intersection of cell–cell adhesion and cell signaling. Although an irenic synthesis of α-catenin’s nonjunctional roles awaits future experiments, here we review some of these functions, and suggest potential connections to the “canonical” functions of α-catenin at junctions.
Several early studies suggested that a significant fraction of the total cellular pool of α-catenin may exist in the cytosol. In Xenopus embryo extracts, both immunodepletion of extracts using anti-cadherin antibodies and sucrose gradient fractionation suggested that a large fraction of total α-catenin, like β-catenin, is nonjunctional (Schneider et al., 1993). In cultured cells, not all α-catenin cosediments with cadherin-containing fractions, consistent with the results from Xenopus (Gottardi and Gumbiner, 2004). Overexpression of full-length α-catenin attenuates Wnt signaling in Xenopus (Sehgal et al., 1997; Gottardi and Gumbiner, 2004); although its relevance under normal conditions is unclear, these results raised the possibility that cytosolic α-catenin plays roles distinct from α-catenin at junctions, possibly via interaction with cytosolic β-catenin.
α-Catenin and proliferative signaling.
The first indication from loss-of-function experiments that α-catenin may have nonjunctional roles came from studies using Cre-mediated recombination to conditionally ablate αE-catenin in the mouse epidermis (Vasioukhin et al., 2001). Several defects mimicking human squamous cell carcinoma were seen, including defects in cell polarity, internalized cell masses, and hyperproliferation. The hyperproliferation was found to be independent of defects in cell adhesion or Wnt signaling and instead due to increased levels of both activated Ras and MAPK. The increased levels of Ras/MAPK signaling appeared to be due to an increased sensitivity of αE-catenin knockout cells to insulin and the insulin-like growth factor, IGF-1. Further follow-up showed that IRS-1, a protein that interacts with activated insulin and IGF-1 receptors to promote downstream signaling, associates with E-cadherin in αE-catenin null cells (Vasioukhin et al., 2001). How αE-catenin normally prevents the association of IRS-1 with E-cadherin and how this aberrant association causes a robust increase in downstream signaling is unclear.
This surprising result was followed by other studies suggesting nonjunctional roles for αE-catenin. Using microarray analysis and in situ hybridization, target genes of the Hedgehog (Hh) pathway were found to be up-regulated specifically in the cerebral cortex in conditional knockout mice lacking α-catenin in the brain. Cyclopamine, a specific inhibitor of the Hh coreceptor Smoothened, rescued the hyperproliferation defects (Lien et al., 2006). The up-regulated Hh signaling and subsequent hyperproliferation were attributed to a loss of “crowd control” in αE-catenin null brains via a mechanism akin to contact inhibition. There have been no follow-up experiments to test whether αE-catenin can directly interact with members of the Hh pathway, or, as is more likely, if this interaction is indirect. In either case, αE-catenin’s involvement in both Ras/MAPK and Hh signaling suggests an intimate relationship between the formation of cell–cell adhesions and regulation of the cell cycle.
Recent experiments further support the role of α-catenin in proliferation control through the Hippo signaling pathway (Kim et al., 2011; Schlegelmilch et al., 2011; Silvis et al., 2011). In nonconfluent wild-type or α-catenin null cells, the transcriptional coactivator Yap1/Yorkie localizes to the nucleus to up-regulate proliferative genes. When wild-type cells become confluent, Yap1 is found in the cytoplasm; however, in α-catenin null cells, Yap1 remains nuclear (Schlegelmilch et al., 2011; Silvis et al., 2011). Although biochemical assays have identified α-catenin as a novel physical interactor with Yap1 (Silvis et al., 2011; Schlegelmilch et al., 2011), there is no consensus on how α-catenin may be controlling Yap1 localization and activity. Another study found that E-cadherin homophilic ligation independent of other intercellular connections could control the localization of YAP in a density-dependent manner. This was shown by incubating sparse or isolated cells with E-cadherin–coated coverslips or beads. This same effect was seen in cadherin-deficient cells expressing either E-cadherin alone or an E-cadherin–α-catenin fusion (like nEα in Fig. 2 A; Kim et al., 2011). Taken together, these results support a model whereby adherens junctions transduce information concerning cell density to regulators of cell proliferation through α-catenin.
α-Catenin as a cytoskeletal modulator.
Suppression of cell proliferation in coherent tissue sheets is only one of many potential nonjunctional roles for α-catenin. In addition, cell–cell junction formation has profound effects on the state of the microtubule- (Stehbens et al., 2009) and actin-based cytoskeleton (Cavey and Lecuit, 2009), which in turns allows cells to integrate into coherent tissue sheets. Recent evidence suggests that α-catenin is involved in such regulation. Based on the observation that cadherin-mediated cell adhesions can stabilize noncentrosomal microtubules (MTs) throughout the cytoplasm, not just near cell–cell contacts (Chausovsky et al., 2000), Shtutman et al. (2008) tested whether or not α-catenin may act in this pathway. Using chimeric fusions, p120-catenin, β-catenin, and α-catenin were all targeted to the membrane independent of cadherin. Only the expression of an α-catenin fusion was able to stabilize MTs in the noncentrosome cytoplasts of fibroblast-like cells that contain low levels of endogenous cadherin (Shtutman et al., 2008). What downstream effector(s) might mediate this effect on cortical MTs remains unclear. The authors suggest formins may play a role because both mDia1 and formin-1 were able to positively affect microtubule polymerization in a similar assay (Shtutman et al., 2008), and at least formin-1 is able to bind αE-catenin (Kobielak et al., 2004). Future experiments will be required to explore these interesting possibilities.
Another link to MTs comes from work analyzing dynactin-mediated intercellular traffic (Lien et al., 2008). In a yeast two-hybrid screen using the VH2-VH3 domains of αE-catenin as bait, dynamitin, a member of the dynactin complex, was identified as a novel αE-catenin binding partner. In wild-type keratinocytes, MTs and small amounts of dynamitin–αE-catenin complexes can be found at AJs and the cell periphery, and these are lost in αE-catenin null cells. Using the movement of lysosomes as a readout of intracellular trafficking, Lien et al. (2008) found that lysosomes in null cells traveled significantly farther than those in wild-type cells, and this could be rescued by expression of epitope-tagged αE-catenin. Significantly, loss of AJs in low-calcium media did not affect the distance traveled by lysosomes, supporting an adhesion-independent role of αE-catenin in dynactin-mediated intercellular traffic. Interestingly, the actin cytoskeleton also appears to be involved in this process. Treatment of wild-type cells with latrunculin also caused lysosomes to travel farther than controls. The same treatment in αE-catenin null cells had no additional effect on movement, and unlike addition of full-length αE-catenin, a construct lacking the actin-binding domain was unable to suppress the increased trafficking (Lien et al., 2008), suggesting an αE-catenin–actin interaction is necessary for these effects. It is not clear whether F-actin interacts directly with dynactin complexes to control intracellular trafficking, or if its removal simply causes less impedance of cargo movement through the cell. Although the experiments of Shtutman et al. (2008) imply adhesion-dependent effects on the MT cytoskeleton, it would be interesting to know if dynactin complexes colocalize with the α-catenin fusion construct used in those assays as well.
In addition to its potential roles in modulating MTs, a recent study (Benjamin et al., 2010) further implicates αE-catenin in nonjunctional regulation of the actin cytoskeleton. Previous work from the Nelson and Weis groups had shown that αE-catenin homodimers have a higher affinity for F-actin than do αE-catenin–β-catenin heterodimers, and that homodimers can inhibit Arp2/3-dependent actin polymerization, albeit at a high overall concentration (Drees et al., 2005). These data led to the suggestion that association of αE-catenin with E-cadherin–β-catenin may act to raise the local concentration of αE-catenin once it dissociates from the CCC, promoting homodimer formation, which would in turn inhibit the Arp2/3 complex (Drees et al., 2005; Yamada et al., 2005). In a follow-up paper, Benjamin et al. (2010) attempted to specifically deplete only the cytosolic pool of αE-catenin in epithelial cells, which would presumably contain the homodimeric fraction, and assess changes in the morphology of the actin cytoskeleton. They used a chimeric fusion containing the αE-catenin binding site of β-catenin and a mitochondrial targeting sequence to sequester nonjunctional αE-catenin without affecting AJs. Expression of this construct caused cells to exhibit increased lamellipodial dynamics, increased migration in a wound-healing assay, and a broader zone of F-actin containing components of the Arp2/3 complex. Conversely, targeting cytosolic αE-catenin to the plasma membrane independent of E-cadherin caused decreased lamellipodial dynamics compared with control cells (Benjamin et al., 2010). Collectively, these data support the existence of two functional populations of αE-catenin: an E-cadherin–β-catenin associated population that acts to attach F-actin to AJs and a nonjunctional, cytosolic pool that regulates actin organization to control cell migration (Fig. 4). In vivo functions for these two pools have not been determined. One potential place in which such α-catenin–dependent dampening of cell motility may come into play is during maturation of cell–cell junctions during epithelial sealing events in embryos. Evidence in several systems indicates that lamellipodial-driven cell migration, which results in the formation of nascent adhesions, is followed by a reorganization of the actin cytoskeleton and a dampening of membrane dynamics (Raich et al., 1999; Vasioukhin et al., 2000; Ehrlich et al., 2002; Sheffield et al., 2007).
Figure 4.
Non-junctional, homodimeric αE-catenin regulates membrane dynamics. In this model, there are two distinct populations of αE-catenin. At the AJ, αE-catenin associates with the CCC and mediates attachment to the actin cytoskeleton, either directly or indirectly. Near the membrane, a cytosolic pool of αE-catenin is created through dissociation from the CCC. This cytosolic pool has a high local concentration and can therefore form αE-catenin homodimers that are able to inhibit the Arp2/3 complex and thereby dampen membrane dynamics by preventing the formation of branched F-actin (Benjamin et al., 2010).
Junctional and nonjunctional α-catenin: Potential interactions.
Even though the precise mechanism by which nonjunctional α-catenin acts is still unclear, there are several questions raised by these recent studies. First, a particularly intriguing question is how a functionally distinct pool of nonjunctional α-catenin might interrelate with pools of α-catenin performing its other known functions. In their mechanotransduction experiments, Yonemura et al. (2010) tested whether full-length α-catenin mechanically linked to a nonfunctional E-cadherin could still participate in force transduction. In cells lacking endogenous cadherin, this fusion construct rescued cell–cell adhesion and recruited vinculin to AJs in a force-dependent manner, indicating that a nonjunctional pool of α-catenin is not required for this pathway (Yonemura et al., 2010). A second question is whether the ratio of junctional to nonjunctional α-catenin is important, or instead its absolute level in the two pools. A nonjunctional pool of αE-catenin acting to inhibit the Arp2/3 complex would, at first glance, appear to correlate with the decreased motility and migration noted in the earlier cadherin–α-catenin fusion studies (Nagafuchi et al., 1994; Pacquelet and Rørth, 2005); however, because the chimeric constructs are assumed to be strictly junctional and endogenous αE-catenin was present in both cases, a cytosolic pool should not have been affected. Alternatively, chimeras that retain the dimerization domain of α-catenin may still be able to homodimerize with endogenous α-catenin monomer and inhibit Arp2/3. Therefore, overexpression of these chimeras may lead to increased suppression of membrane dynamics, explaining the motility defects (Weis and Nelson, 2006). This explanation would not seem to apply to the chimera lacking the α-catenin N terminus, which exhibits the same decrease in intercellular motility as the full-length α-catenin fusion (nEαC and nEα, respectively; Fig. 2 A; Nagafuchi et al., 1994). It should also be noted that with respect to DE-cadherinΔCyt/α-catenin (Fig. 2 B), addition of DE-cadherin’s cytoplasmic tail (DE-cadherin/α-catenin, Fig. 2 B), rather than removing α-catenin’s dimerization domain, rescued migration defects in Drosophila (Fig. 2 B) (Pacquelet and Rørth, 2005).
Another hypothesis is that increased cell–cell adhesion due to overexpression of the chimeric constructs ultimately interferes with the creation of other cell surface complexes that aid migration, either by sequestering common proteins or due to downstream signaling from the CCC. This is in part supported by experiments using micropatterned surfaces containing stripes of either collagen IV or E-cadherin-Fc to mimic focal adhesions or cell–cell adhesions, respectively (Borghi et al., 2010). The results show that in a concentration-dependent manner, cadherin-based adhesions can suppress membrane dynamics and guide the direction of migration along ECM surfaces without affecting migration rate, supporting a cross talk model between the CCC and focal adhesions. Clearly there are many unresolved issues regarding how α-catenin acts beyond its role in recruiting actin to the CCC.
Conclusions
Cells in animal embryos place high demands on cell–cell adhesions, which must be dynamically regulated to yield connections that are simultaneously stable yet flexible. α-Catenin lies at the heart of this regulation. In addition to its role in attaching F-actin to the AJ, α-catenin has also emerged as a force transducer and regulator of cell signaling and motility. Although the exact molecular mechanisms by which α-catenin functions likely depend on the organism or the cellular event, the challenge for the future is to devise ways to cleanly delineate its multiple functions in complicated in vivo contexts.
Acknowledgments
We apologize to colleagues whose work, due to space limitations, we were unable to discuss in this review. We thank Bill Bement and Angela Kita for helpful discussion.
This work was supported by National Institutes of Health grant GM058038 (awarded to J. Hardin) and an American Heart Association predoctoral fellowship (awarded to S.L. Maiden).
Footnotes
Abbreviations used in this paper:
- AJ
- adherens junction
- arm
- Armadillo repeat
- CCC
- cadherin–catenin complex
- MT
- microtubule
- VH
- vinculin homology
References
- Abe K., Takeichi M. 2008. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc. Natl. Acad. Sci. USA. 105:13–19 10.1073/pnas.0710504105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107:3655–3663 [DOI] [PubMed] [Google Scholar]
- Anastasiadis P.Z. 2007. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim. Biophys. Acta. 1773:34–46 10.1016/j.bbamcr.2006.08.040 [DOI] [PubMed] [Google Scholar]
- Barstead R.J., Waterston R.H. 1989. The basal component of the nematode dense-body is vinculin. J. Biol. Chem. 264:10177–10185 [PubMed] [Google Scholar]
- Benjamin J.M., Nelson W.J. 2008. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin. Cancer Biol. 18:53–64 10.1016/j.semcancer.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin J.M., Kwiatkowski A.V., Yang C., Korobova F., Pokutta S., Svitkina T., Weis W.I., Nelson W.J. 2010. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J. Cell Biol. 189:339–352 10.1083/jcb.200910041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A.D., Balaban N.Q., Geiger B. 2003. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19:677–695 10.1146/annurev.cellbio.19.111301.153011 [DOI] [PubMed] [Google Scholar]
- Borghi N., Lowndes M., Maruthamuthu V., Gardel M.L., Nelson W.J. 2010. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc. Natl. Acad. Sci. USA. 107:13324–13329 10.1073/pnas.1002662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan K.M., Peifer M. 2009. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb. Perspect. Biol. 1:a002881 10.1101/cshperspect.a002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M., Lecuit T. 2009. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1:a002998 10.1101/cshperspect.a002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M., Rauzi M., Lenne P.F., Lecuit T. 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 453:751–756 10.1038/nature06953 [DOI] [PubMed] [Google Scholar]
- Chausovsky A., Bershadsky A.D., Borisy G.G. 2000. Cadherin-mediated regulation of microtubule dynamics. Nat. Cell Biol. 2:797–804 10.1038/35041037 [DOI] [PubMed] [Google Scholar]
- Costa M., Raich W., Agbunag C., Leung B., Hardin J., Priess J.R. 1998. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 141:297–308 10.1083/jcb.141.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J.M., Sheetz M.P. 2009. Stretching single talin rod molecules activates vinculin binding. Science. 323:638–641 10.1126/science.1162912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F., Pokutta S., Yamada S., Nelson W.J., Weis W.I. 2005. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 123:903–915 10.1016/j.cell.2005.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich J.S., Hansen M.D., Nelson W.J. 2002. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell. 3:259–270 10.1016/S1534-5807(02)00216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W.W. 2009. Discovering the molecular components of intercellular junctions—a historical view. Cold Spring Harb. Perspect. Biol. 1:a003061 10.1101/cshperspect.a003061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J., Peifer M. 2005. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 123:769–772 10.1016/j.cell.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Gottardi C.J., Gumbiner B.M. 2004. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J. Cell Biol. 167:339–349 10.1083/jcb.200402153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C., Hoffman B.D., Brenner M.D., Zhou R., Parsons M., Yang M.T., McLean M.A., Sligar S.G., Chen C.S., Ha T., Schwartz M.A. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 466:263–266 10.1038/nature09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B.M. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 84:345–357 10.1016/S0092-8674(00)81279-9 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. 2011. Hallmarks of cancer: the next generation. Cell. 144:646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Herrenknecht K., Ozawa M., Eckerskorn C., Lottspeich F., Lenter M., Kemler R. 1991. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc. Natl. Acad. Sci. USA. 88:9156–9160 10.1073/pnas.88.20.9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger J., Birchmeier W. 2010. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2:a002915 10.1101/cshperspect.a002915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A.H., Nelson W.J., Weis W.I. 1997a. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 90:871–882 10.1016/S0092-8674(00)80352-9 [DOI] [PubMed] [Google Scholar]
- Huber O., Krohn M., Kemler R. 1997b. A specific domain in alpha-catenin mediates binding to beta-catenin or plakoglobin. J. Cell Sci. 110:1759–1765 [DOI] [PubMed] [Google Scholar]
- Imamura Y., Itoh M., Maeno Y., Tsukita S., Nagafuchi A. 1999. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 144:1311–1322 10.1083/jcb.144.6.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Moroi S., Tsukita S. 1997. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 138:181–192 10.1083/jcb.138.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.G., Koh E., Chen X., Gumbiner B.M. 2011. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA. 108:11930–11935 10.1073/pnas.1103345108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K.A., Soler A.P., Johnson K.R., Wheelock M.J. 1995. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J. Cell Biol. 130:67–77 10.1083/jcb.130.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A., Pasolli H.A., Fuchs E. 2004. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 6:21–30 10.1038/ncb1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslov E.R., Maupin P., Pradhan D., Morrow J.S., Rimm D.L. 1997. Alpha-catenin can form asymmetric homodimeric complexes and/or heterodimeric complexes with beta-catenin. J. Biol. Chem. 272:27301–27306 10.1074/jbc.272.43.27301 [DOI] [PubMed] [Google Scholar]
- Kostetskii I., Li J., Xiong Y., Zhou R., Ferrari V.A., Patel V.V., Molkentin J.D., Radice G.L. 2005. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 96:346–354 10.1161/01.RES.0000156274.72390.2c [DOI] [PubMed] [Google Scholar]
- Kwiatkowski A.V., Maiden S.L., Pokutta S., Choi H.J., Benjamin J.M., Lynch A.M., Nelson W.J., Weis W.I., Hardin J. 2010. In vitro and in vivo reconstitution of the cadherin-catenin-actin complex from Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 107:14591–14596 10.1073/pnas.1007349107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Duc Q., Shi Q., Blonk I., Sonnenberg A., Wang N., Leckband D., de Rooij J. 2010. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 189:1107–1115 10.1083/jcb.201001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien W.H., Klezovitch O., Fernandez T.E., Delrow J., Vasioukhin V. 2006. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 311:1609–1612 10.1126/science.1121449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien W.H., Gelfand V.I., Vasioukhin V. 2008. Alpha-E-catenin binds to dynamitin and regulates dynactin-mediated intracellular traffic. J. Cell Biol. 183:989–997 10.1083/jcb.200805041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Ferreira-Cornwell M., Baldwin H., Kostetskii I., Lenox J., Lieberman M., Radice G. 2001. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development. 128:459–469 [DOI] [PubMed] [Google Scholar]
- Magie C.R., Pinto-Santini D., Parkhurst S.M. 2002. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 129:3771–3782 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 7:3679–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. 1989. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M., Tsukita S. 1991. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 65:849–857 10.1016/0092-8674(91)90392-C [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Ishihara S., Tsukita S. 1994. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J. Cell Biol. 127:235–245 10.1083/jcb.127.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W.J., Nusse R. 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 303:1483–1487 10.1126/science.1094291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset J.E., Redfield A.R., Jin F., Knudsen K.A., Johnson K.R., Wheelock M.J. 1997. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J. Cell Sci. 110:1013–1022 [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. 1989. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 8:1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. 1990. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc. Natl. Acad. Sci. USA. 87:4246–4250 10.1073/pnas.87.11.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquelet A., Rørth P. 2005. Regulatory mechanisms required for DE-cadherin function in cell migration and other types of adhesion. J. Cell Biol. 170:803–812 10.1083/jcb.200506131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas D.J., Rimm D.L. 2006. Direct interaction of the C-terminal domain of alpha-catenin and F-actin is necessary for stabilized cell-cell adhesion. Cell Commun. Adhes. 13:151–170 10.1080/15419060600726142 [DOI] [PubMed] [Google Scholar]
- Peifer M., McCrea P.D., Green K.J., Wieschaus E., Gumbiner B.M. 1992. The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J. Cell Biol. 118:681–691 10.1083/jcb.118.3.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J., Cox E.A., Broadbent I.D., Flett A., Hardin J. 2003. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J. Cell Biol. 162:15–22 10.1083/jcb.200212136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Weis W.I. 2000. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol. Cell. 5:533–543 10.1016/S1097-2765(00)80447-5 [DOI] [PubMed] [Google Scholar]
- Pokutta S., Drees F., Takai Y., Nelson W.J., Weis W.I. 2002. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J. Biol. Chem. 277:18868–18874 10.1074/jbc.M201463200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Drees F., Yamada S., Nelson W.J., Weis W.I. 2008. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem. Soc. Trans. 36:141–147 10.1042/BST0360141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess J.R., Hirsh D.I. 1986. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117:156–173 10.1016/0012-1606(86)90358-1 [DOI] [PubMed] [Google Scholar]
- Radice G.L., Rayburn H., Matsunami H., Knudsen K.A., Takeichi M., Hynes R.O. 1997. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181:64–78 10.1006/dbio.1996.8443 [DOI] [PubMed] [Google Scholar]
- Raich W.B., Agbunag C., Hardin J. 1999. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr. Biol. 9:1139–1146 10.1016/S0960-9822(00)80015-9 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Lenne P.F., Lecuit T. 2010. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 468:1110–1114 10.1038/nature09566 [DOI] [PubMed] [Google Scholar]
- Reynolds A.B., Daniel J., McCrea P.D., Wheelock M.J., Wu J., Zhang Z. 1994. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell. Biol. 14:8333–8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D.L., Koslov E.R., Kebriaei P., Cianci C.D., Morrow J.S. 1995. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA. 92:8813–8817 10.1073/pnas.92.19.8813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J.R., Zhou D., Kreger B.T., Vasioukhin V., Avruch J., Brummelkamp T.R., Camargo F.D. 2011. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 144:782–795 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Herrenknecht K., Butz S., Kemler R., Hausen P. 1993. Catenins in Xenopus embryogenesis and their relation to the cadherin-mediated cell-cell adhesion system. Development. 118:629–640 [DOI] [PubMed] [Google Scholar]
- Sehgal R.N., Gumbiner B.M., Reichardt L.F. 1997. Antagonism of cell adhesion by an alpha-catenin mutant, and of the Wnt-signaling pathway by alpha-catenin in Xenopus embryos. J. Cell Biol. 139:1033–1046 10.1083/jcb.139.4.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield M., Loveless T., Hardin J., Pettitt J. 2007. C. elegans Enabled exhibits novel interactions with N-WASP, Abl, and cell-cell junctions. Curr. Biol. 17:1791–1796 10.1016/j.cub.2007.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh F., Chen Y., Liang X., Hirschy A., Stenbit A.E., Gu Y., Dalton N.D., Yajima T., Lu Y., Knowlton K.U., et al. 2006. alpha-E-catenin inactivation disrupts the cardiomyocyte adherens junction, resulting in cardiomyopathy and susceptibility to wall rupture. Circulation. 114:1046–1055 10.1161/CIRCULATIONAHA.106.634469 [DOI] [PubMed] [Google Scholar]
- Shtutman M., Chausovsky A., Prager-Khoutorsky M., Schiefermeier N., Boguslavsky S., Kam Z., Fuchs E., Geiger B., Borisy G.G., Bershadsky A.D. 2008. Signaling function of alpha-catenin in microtubule regulation. Cell Cycle. 7:2377–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis M.R., Kreger B.T., Lien W.H., Klezovitch O., Rudakova G.M., Camargo F.D., Lantz D.M., Seykora J.T., Vasioukhin V. 2011. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 4:ra33 10.1126/scisignal.2001823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappert J., Kemler R. 1994. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes. Commun. 2:319–327 10.3109/15419069409014207 [DOI] [PubMed] [Google Scholar]
- Stehbens S.J., Akhmanova A., Yap A.S. 2009. Microtubules and cadherins: a neglected partnership. Front. Biosci. 14:3159–3167 10.2741/3442 [DOI] [PubMed] [Google Scholar]
- Takeichi M. 1991. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 251:1451–1455 10.1126/science.2006419 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer C., Yin M., Fuchs E. 2000. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 100:209–219 10.1016/S0092-8674(00)81559-7 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer C., Degenstein L., Wise B., Fuchs E. 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 104:605–617 10.1016/S0092-8674(01)00246-X [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M., Uchida N., Imamura Y., Nagafuchi A., Fujimoto K., Uemura T., Vermeulen S., van Roy F., Adamson E.D., Takeichi M. 1998. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 142:847–857 10.1083/jcb.142.3.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W.I., Nelson W.J. 2006. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 281:35593–35597 10.1074/jbc.R600027200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E.E., Kroemker M., Rüdiger A.H., Jockusch B.M., Rüdiger M. 1998. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J. Cell Biol. 141:755–764 10.1083/jcb.141.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Pokutta S., Drees F., Weis W.I., Nelson W.J. 2005. Deconstructing the cadherin-catenin-actin complex. Cell. 123:889–901 10.1016/j.cell.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Wada Y., Watanabe T., Nagafuchi A., Shibata M. 2010. alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12:533–542 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- Zhang H., Landmann F., Zahreddine H., Rodriguez D., Koch M., Labouesse M. 2011. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature. 471:99–103 10.1038/nature09765 [DOI] [PubMed] [Google Scholar]