Abstract
It has previously been shown that messenger activity for a protein of Mr = ca. 30k exists in RNA fractions extracted from particles of either native or alkali stripped U1 TMV, or from cowpea strain TMV, that are smaller than full genomic length. Analysis of sucrose gradient fractions containing this activity reveals a number of slightly smaller template activities directing synthesis of proteins between 18.5k and 29k in size. All of these messenger activities, including that for the 30k protein, respond to cap analogues in anomalous ways. Discrete RNA species that include active mRNAs for these proteins can be demonstrated in the same fractions by labelling with preparations of vaccinia capping enzyme and [alpha-32P] GTP without prior beta-elimination. Detailed analysis of three of these proteins (of Mr's ca. 30k, 29k and 23k) by peptide mapping and translation of purified vaccinia-labelled RNA demonstrates that all three are unrelated to the large early TMV proteins, but are related to each other in such a way as to form a nested set with staggered N termini and identical C termini. mRNAs of chain lengths ca. 1900 and 1500 bases direct synthesis of the 30k and 23k proteins respectively, an mRNA of about 1850 bases directs both 29k and (perhaps because of cross-contamination) 30k synthesis. Initiation codons for the 29k and 23k proteins have been mapped at positions 4960-4962 and 5191-5193 respectively on TMV RNA. Since all three encapsidated templates have similar properties we conclude that either there is a family of 30k-related proteins with unusual mRNAs, or that none of these in vitro translation products are directed by physiological templates.
Full text
PDF









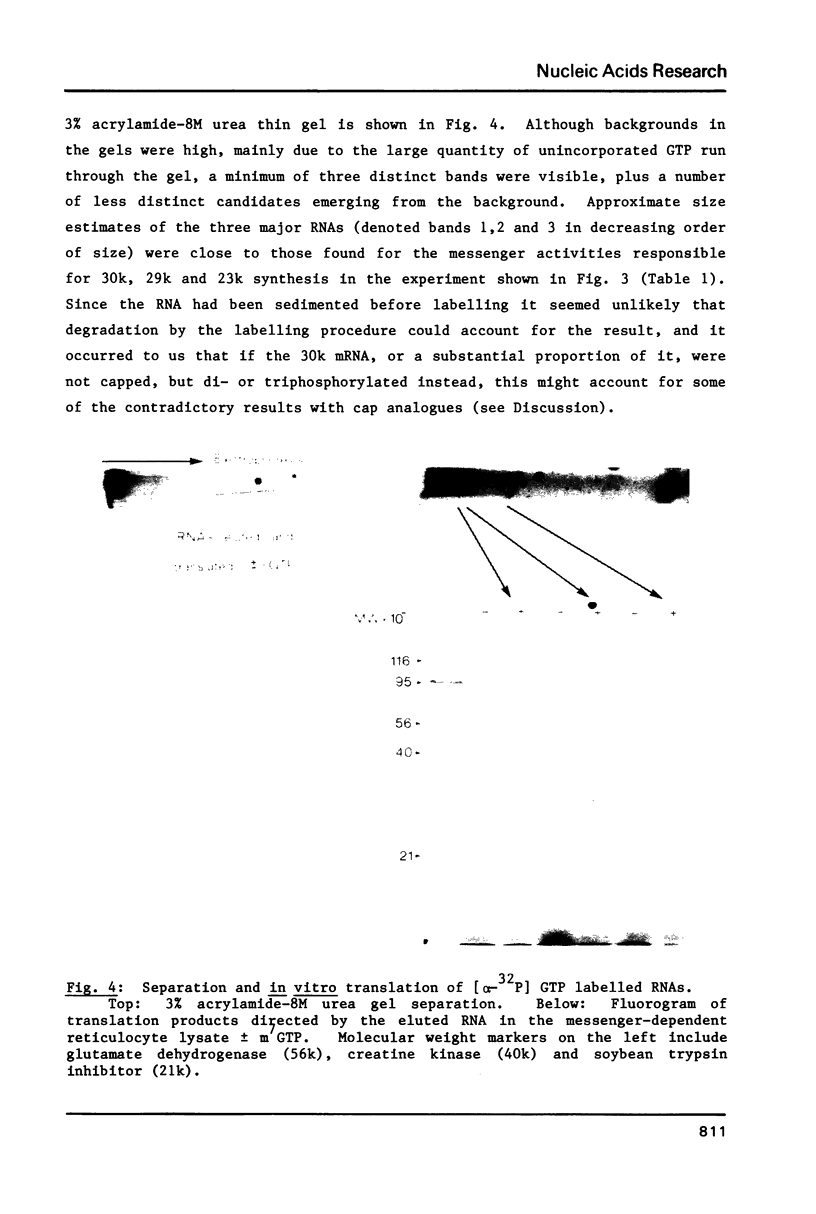
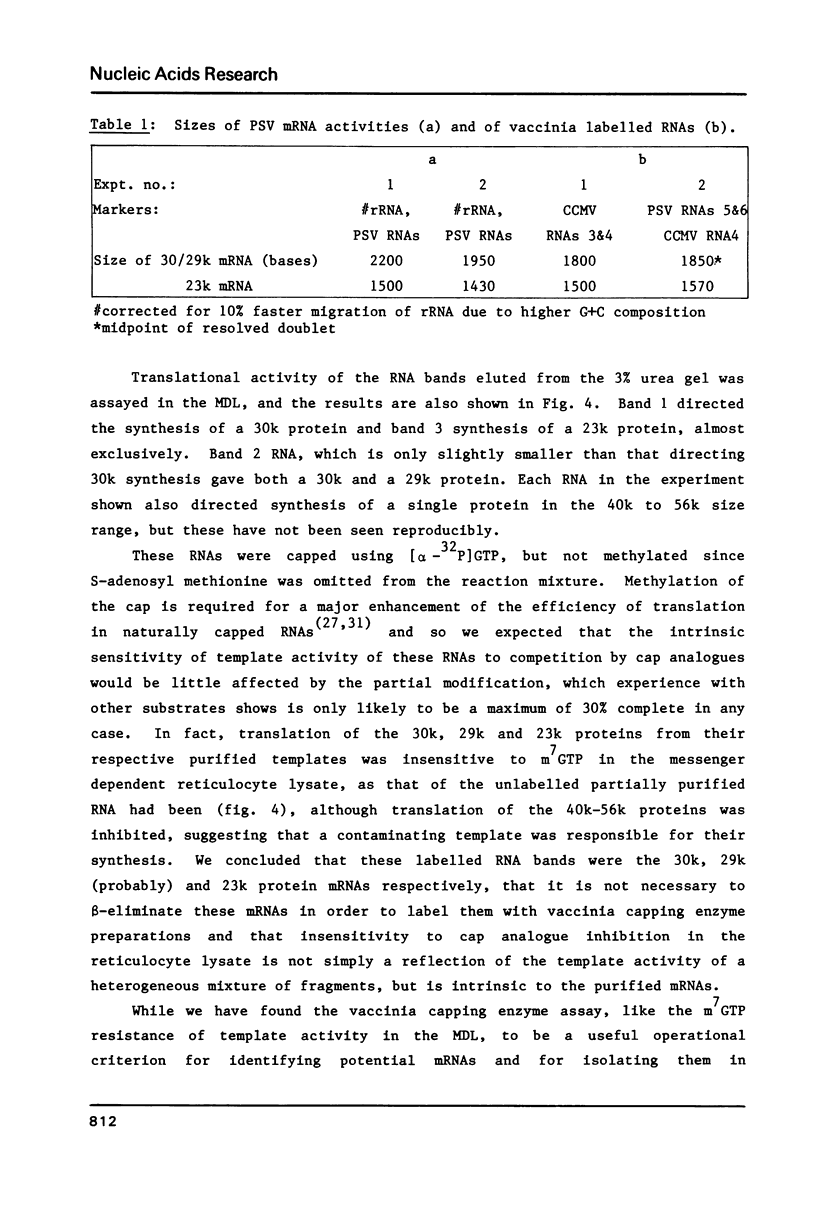









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Shih D. S., Zimmern D., Kaesberg P. Two-step binding of eukaryotic ribosomes to brome mosaic virus RNA3. Nature. 1979 Sep 27;281(5729):277–282. doi: 10.1038/281277a0. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M., Bruening G., Israel H. W. A genetic map for the cowpea strain on TMV. Virology. 1976 Sep;73(2):498–507. doi: 10.1016/0042-6822(76)90411-6. [DOI] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M. Characterization and in vitro translation of the RNAs from less-than-full-length, virus-related, nucleoprotein rods present in tobacco mosaic virus preparations. Virology. 1977 Aug;81(1):160–169. doi: 10.1016/0042-6822(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Mundry K. W., Issinger O. G. In vivo and in vitro translation of the RNAs of four tobamoviruses. Intervirology. 1980;14(5-6):292–299. doi: 10.1159/000149199. [DOI] [PubMed] [Google Scholar]
- Browning K. S., Clark J. M., Jr Translation initiation site of the coat protein messenger ribonucleic acid of the cowpea strain of tobacco mosaic virus. Biochemistry. 1980 Dec 9;19(25):5922–5926. doi: 10.1021/bi00566a040. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Karn J. Tobacco mosaic virus induces the synthesis of a family of 3' coterminal messenger RNAs and their complements. J Mol Biol. 1982 Jan 25;154(3):541–550. doi: 10.1016/s0022-2836(82)80013-2. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth L., Richards K. E. Tobacco mosaic virus: model for structure and function of a simple virus. Adv Virus Res. 1981;26:145–199. doi: 10.1016/s0065-3527(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Horst J., Fraenkel-Conrat H., Mandeles S. Terminal heterogeneity at both ends of the satellite tobacco necrosis virus ribonucleic acid. Biochemistry. 1971 Dec 7;10(25):4748–4752. doi: 10.1021/bi00801a022. [DOI] [PubMed] [Google Scholar]
- Hunter A. R., Farrell P. J., Jackson R. J., Hunt T. The role of polyamines in cell-free protein synthesis in the wheat-germ system. Eur J Biochem. 1977 May 2;75(1):149–157. doi: 10.1111/j.1432-1033.1977.tb11512.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Gibson W. Characterization of the mRNA's for the polyoma virus capsid proteins VP1, VP2, and VP3. J Virol. 1978 Oct;28(1):240–253. doi: 10.1128/jvi.28.1.240-253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W., Friedmann T., Esty A., LaPorte P., Deininger P. Regions of the polyoma genome coding for T antigens. Nucleic Acids Res. 1979 Dec 20;7(8):2275–2288. doi: 10.1093/nar/7.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonard G., Richards K. E., Guilley H., Hirth L. Sequence from the assembly nucleation region of TMV RNA. Cell. 1977 Jul;11(3):483–493. doi: 10.1016/0092-8674(77)90066-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Meshi T., Ohno T., Okada Y. Nucleotide sequence and its character of cistron coding for the 30 K protein of tobacco mosaic virus (OM strain). J Biochem. 1982 Apr;91(4):1441–1444. doi: 10.1093/oxfordjournals.jbchem.a133833. [DOI] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Both G. W., Furuichi Y., Shatkin A. J. 5'-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975 May 1;255(5503):33–37. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Moss B., Cooper J. A., Maxwell E. S. Influence of 5'-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J Biol Chem. 1978 Mar 10;253(5):1710–1715. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of fragmented viral RNA in vitro: initiation at multiple sites. FEBS Lett. 1979 Apr 1;100(1):195–199. doi: 10.1016/0014-5793(79)81162-x. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Wilson T. M. The polarity of stripping of coat protein subunits from the RNA in tobacco mosaic virus under alkaline conditions. FEBS Lett. 1976 Feb 1;62(1):11–15. doi: 10.1016/0014-5793(76)80005-1. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Sollner-Webb B., Wahn H. L. Sites of transcription initiation in vivo on Xenopus laevis ribosomal DNA. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5402–5406. doi: 10.1073/pnas.74.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. R., Higgins T. J. Occurrence of short particles in beans infected with the cowpea strain of TMV. I. Purification and characterization of short particles. Virology. 1976 Jun;71(2):471–485. doi: 10.1016/0042-6822(76)90375-5. [DOI] [PubMed] [Google Scholar]
- Wimmer E., Chang A. Y., Clark J. M., Jr, Reichmann M. E. Sequence studies of satellite tobacco necrosis virus RNA. Isolation and characterization of a 5'-terminal trinucleotide. J Mol Biol. 1968 Nov 28;38(1):59–73. doi: 10.1016/0022-2836(68)90128-9. [DOI] [PubMed] [Google Scholar]
- Zimmern D. The 5' end group of tobacco mosaic virus RNA is m7G5' ppp5' Gp. Nucleic Acids Res. 1975 Jul;2(7):1189–1201. doi: 10.1093/nar/2.7.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D. The nucleotide sequence at the origin for assembly on tobacco mosaic virus RNA. Cell. 1977 Jul;11(3):463–482. doi: 10.1016/0092-8674(77)90065-4. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Wilson T. M. Location of the origin for viral reassembly on tobacco mosaic virus RNA and its relation to stable fragment. FEBS Lett. 1976 Dec 1;71(2):294–298. doi: 10.1016/0014-5793(76)80954-4. [DOI] [PubMed] [Google Scholar]








