Abstract
The objective of this study is to establish the efficacy of adherence therapy (AT) compared with treatment as usual (TAU) in reducing blood pressure (BP) in non-compliant hypertensive patients. This study was designed as a parallel-group single-blind randomised controlled trial. The study was carried out at three general hospital outpatient clinics in Jordan. A total of 136 non-compliant hypertensive patients with a mean baseline BP of 164.5 mm Hg (s.d. 10.0) over 102.2 mm Hg (s.d. 7.0) participated in the study. 7 weekly 20-min sessions of AT in addition to TAU. The main outcome of this study is systolic blood pressure (SBP) at 11-weeks follow-up. In all, 68 patients received TAU and 68 AT. Intention-to-treat analysis included all participants randomised. AT lowered SBP by −23.11 mm Hg (95% CI: −25.85, −20.36) and diastolic BP (DBP) by −15.18 mm Hg (95% CI: −17.55, −12.80) at 11 weeks compared with TAU. Adherence (measured by pill counting) was also improved in the AT group by 37% at 11 weeks compared with TAU. No significant adverse events were reported. AT increases adherence to medication for hypertension which then leads to a clinically important reduction in BP.
Keywords: randomised controlled trial, adherence, therapy, compliance, beliefs about medication, ISRCTN: 99494659
Introduction
Hypertension is defined as a blood pressure (BP) (systolic/diastolic) ⩾140/90 mm Hg.1 Hypertension is one of the major cardiovascular diseases worldwide; in 2000 26% of the adult population had hypertension.2 Hypertension has been estimated to be responsible for 4% of the global burden of disease, that is, 64 million disability adjusted life years.3 In both developing and developed regions it is a major cause of morbidity and mortality, particularly cardiovascular and renal diseases.
Reductions in BP are associated with reductions in the incidence of stroke and cardiovascular heart disease.4, 5 However, systolic blood pressure (SBP) has the stronger association (compared with diastolic blood pressure (DBP)) with the global disease burden attributable to hypertension.4, 6, 7 The risk of cardiovascular disease and stroke rises continuously as SBP increases above 115 mm Hg.8, 9
Worldwide successful control of hypertension is poor with only 5–58% of people taking antihypertensive medications managing to achieve a BP of less than 140/90 mm Hg.10 One of the major factors in this poor control is lack of patient adherence to treatment.11 Adherence behaviours are complex, ranging from completely ceasing to take prescribed medications, to taking only some, to taking them in a way that differs from instructions for example, not after food. Overall hypertensive patients are estimated to take only 53–70% of the medication prescribed for them.12, 13, 14 Factors that affect adherence are complex but may include beliefs about illness and treatment, side effects and complexity of treatment regimes.1, 15, 16
The National Institute for Health and Clinical Excellence17 and the WHO1 in their respective guideline and review strongly advocate the adoption of an individualised consultation style that recognises the importance of involving patients in treatment decisions as key to enabling clinicians to enhancing adherence. A focus on exploring patients' beliefs about illness and treatment and providing specific information about the disease (hypertension) and its treatments (medication and life-style modification) are also strongly advocated for example, by using face-to-face education sessions or information leaflets. This guidance is based largely on observational research and to date there has been little experimental evidence to establish the clinical benefit of such a consultation style. Reviews of adherence interventions in general18 and in hypertension specifically19 have reported that much of the experimental work to date has generally taken a pragmatic one size fits all (for example, information, reminders and self monitoring) approach to enhancing adherence; most have been shown to be ineffective.
The intervention under investigation in this trial, adherence therapy (AT), is rooted in the observation that patient' beliefs impact on their treatment adherence.20 For example, patients who do not believe that their health depends on medicines, worry about having to take medicine or are concerned about the side effects of medicine are less likely to adhere to treatment than patients with more positive treatment attitudes for example, ‘my medicines protect me from becoming more sick'.21
AT was developed from work with non-adherent patients with mental health problems and was described initially as compliance therapy.22 It is a patient-centred approach normally delivered by trained clinicians over a series of 7 weekly 20-min consultations each with a different theme. Building on a structured adherence assessment key therapy techniques include, generating discrepancy; medication problem solving; exchanging information; exploring ambivalence; and checking beliefs. Theoretically, these techniques amplify the personally relevant benefits of treatment, modify illness and treatment beliefs and resolve ambivalence towards taking medication.
Considering two of the AT techniques, generating discrepancy and checking beliefs, in more detail. The therapist can amplify the personally relevant benefits of medication by generating discrepancy between what the patients says is important to them (for example, being able to work, not visiting hospital so frequently, not worrying family) and what they actually do that is, not taking medication. The therapist might respond to a patient who says that their medication is important but repeatedly misses doses by saying: ‘Im a bit confused, because you say that you need to keep your blood pressure under control so that you are able to work and yet you keep missing doses of medication, can you help me understand that a bit better?'.
Beliefs identified from the assessment that may negatively affect adherence are explored using a three-step process. Step 1, the patient is asked to rate the conviction (as a percentage), with which they hold a belief (for example, the patient says they are 60% sure most medication is addictive). Step 2, they are then asked to generate evidence that supports and refutes the belief. Step 3, the therapist rechecks the conviction, with which the belief is held. This process provides patients an opportunity to ‘test out' their beliefs. Direct confrontation or challenge of the belief is avoided.
Each consultation follows a standard structure (review of previous meeting and homework, set agenda, task (for example, exploring ambivalence), feedback and setting homework). A copy of the AT manual is available from the authors on request. The concept that we are seeking to test in this trial is that AT will modify patient's unhelpful beliefs about taking their medication, which will in turn improve compliance, which will result in a reduction in patients' BP.
Our randomised controlled trial will therefore test the following hypotheses:
AT leads to a reduction in BP in people with hypertension at 11 weeks, compared with treatment as usual (TAU).
AT modifies patient's attitudes and beliefs in a favourable direction towards taking their medication compared with TAU, as measured by the Beliefs about Medications Questionnaire.
AT improves adherence to antihypertensive medication compared with TAU.
Methods
A parallel-group randomised controlled trial of adherence therapy. AT was compared with TAU. The assessors of BP and adherence and the data analyst were blinded to the group assignment.
Study participants
All hypertensive patients that met our selection criteria who attended the outpatient clinics in three government run hospitals, which serve 70% of the Jordanian population, were invited to participate. The trial was conducted between August 2009 and January 2010. Jordan has a population of over 6 million people, of whom it is estimated that 26% are hypertensive.23 Treatment non-adherence in this population is estimated to be 57%.24
Eligible patients were adults (⩾18 years) with a diagnosis of hypertension, currently hypertensive that is, BP ⩾140/90 mm Hg (information extracted from case notes), and on monthly follow-up schedules at the participating clinics. Records were selected for assessment on the basis that the patients had been seen recently in the clinics. Records were assessed in reverse date order of last clinic appointment, and assessment of potential participants was stopped when sufficient numbers had been recruited to the study. Potential participants were screened with the Morisky Medication Adherence Scale25 (MMAS) before the trial. Those assessed as non-adherent were invited to participate. Patients were excluded if they had:
complications of hypertension, had diabetes, congestive heart failure and renal impairment. These patients were excluded because their treatment regime was more likely to be complex, introducing more potential confounders that might not be adequately dealt with by randomisation.
mental illness or any other long-term health conditions for example, asthma, Parkinson's disease, epilepsy, cancer and chronic obstructive pulmonary disease. These patients were excluded because these comorbid conditions may also introduce confounding factors that might limit the effectiveness of the therapy
pregnant patients were excluded as the aetiology and duration of their hypertension would likely differ from essential hypertension.
We identified 181 eligible non-adherent patients, 45 of whom refused to participate, the remaining 136 agreed to participate in the study and provided written informed consent.
Ethical approval
Appropriate permissions and ethical approvals were given by the Ministry of Health in Jordan and the University of East Anglia's ethics committee.
Sample size
We expected to observe a difference of 3 mm Hg in SBP between the groups.26, 27 Assuming a s.d. of 5.7, (see refs 28, 29) an alpha of 0.05, a 2-tailed test of significance, and 80% power, we required 60 patients in each arm of the trial, that is, a sample size of 120. We estimated from previous studies of adherence interventions in hypertensives30 that at most 12% (that is, 16) of patients would withdraw from the study, so we aimed to recruit 136 patients.
Randomisation
FA checked patient eligibility and consent against a checklist. FA was allocated a personal 6-digit ‘PIN' identifier that allowed her access to the online independent randomisation service at the Clinical Research Trials Unit (CRTU) at the University of East Anglia (UEA). On entry of a valid PIN, the system generated a unique Study Code and randomly allocate the patients to either the AT or TAU arm of the trial. The study code and allocation was sent in an email to FA and the trial database manager and stored in the trial database on the secure CRTU server at UEA.
A computer generated randomisation list allocated the 136 patients in a 1:1 ratio to AT or TAU. To ensure a reasonably even distribution of patients in the two arms throughout the course of the trial, patients were allocated in randomly permuted blocks of four and six.
Intervention
Control group: patients received TAU, which consisted of monthly outpatient clinics where BP was measured, medication reviewed and laboratory investigations and other care was delivered depending on individual needs. We did not provide any guidelines about what constituted usual care. TAU was provided by the patients usual clinical care team. This consisted of a clinician-led team of medical and nursing staff based in the outpatient clinic.
Experimental group: In addition to TAU, patients in the experimental group received seven one-to-one sessions of AT lasting 20 min over a 7-weeks period. AT was delivered by FA who received one-to-one training in AT over seven sessions each lasting 1 h from RG who developed the intervention. AT sessions were delivered in the hospital outpatient clinics (∼25% of all sessions) or at the patient's home (∼75% of all sessions) depending on patient preference. Treatment fidelity was checked by an independent rater (AHN) against an AT scale using audio tapes of a random selection of sessions.
Translations
The translations to Arabic language for AT, Morisky Medication Adherence Scale (MMAS) and Beliefs about Medicines Questionnaire (BMQ,) was based on The Eight Steps Translation Process that combined the recommendations of WHO31 and Brislin et al.32, 33, 34
Demographical information and patient characteristics
We recorded the following demographical information and patient charcateristics at the baseline assessment: age, gender, living arrangements, marital status, smoking status, self-reported levels of physical activity, self-reported adherence to a low-salt, low-fat diet, employment status, presence of medical insurance and levels of education. These were assessed using a questionnaire developed by the study's authors.
Outcomes
The primary outcome, for this study, was the difference in SBP from baseline to follow-up (11 weeks after randomisation). If the results of this study are positive, subsequent trials should then test the durability of the intervention. Our secondary measures were the difference in DBP, medication adherence rates and BMQ from baseline to follow-up (11 weeks after randomisation). BP was measured by a nurse blinded to the group allocation and time of intervention at baseline and 11 weeks. The BP was measured using a stethoscope with mercury sphygmomanometer twice from the right upper arm of a seated patient who had been resting for more than 10 min. The average of the two measurements was used. Patients were asked to not smoke or drink coffee during the examination and it was recorded if they had any alcohol, coffee or cigarettes in the 30 min before the examination.35, 36
At 11 weeks, percentage adherence was calculated by dividing the number of pills not taken in the preceding month by the total number prescribed. Pill counting was carried out by a nurse blinded to group allocation.
The BMQ was used to measure patients' attitudes and beliefs toward medication.21 We considered using the specific version of the BMQ, which relates to patients attitudes to a single medication but as hypertension patients tend to be prescribed multiple drugs, we considered the general version of the measure to be more appropriate. Each item is rated on a five-point Likert-type scale ranging from ‘strongly disagree' (1) to ‘strongly agree' (5). The BMQ has established validity and is routinely used as an outcome measure in adherence research. The questionnaire has four sections that evaluate attitudes about:
General harm (G-H) that is, the intrinsically harmful properties of medications (four questions such as ‘most medicines are addictive').
General overuse (G-O) of medications by healthcare professionals (four questions such as ‘Doctors use too many medicines').
General sensitivity (G-S) to adverse events from medications (five questions such as ‘my body is very sensitive to medicines').
General benefit (G-B) that is, the intrinsically beneficial properties of medications (four questions such as ‘in most cases the benefit of medicines outweigh the risks').
Finally a note was made of the amount of time taken to deliver the AT, so that an estimate of the costs of delivery could be made.
Data analysis
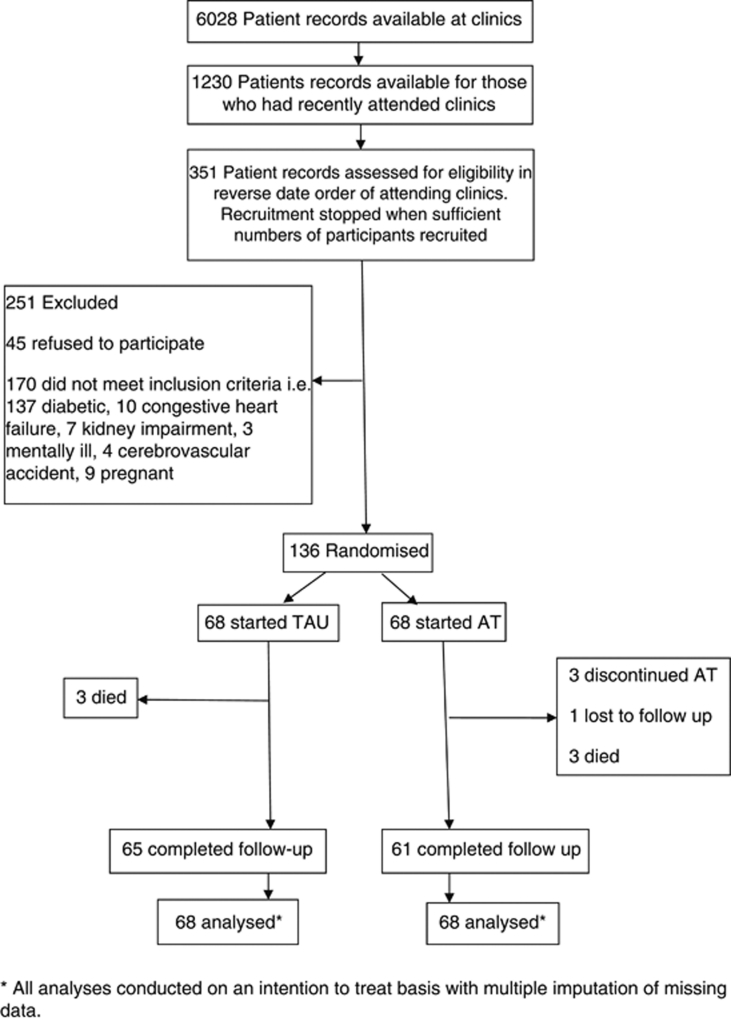
The data analyst (AC) was blinded to group allocation. All analyses were conducted on an intention to treat basis. Although there was very little missing data (six patients died, three discontinued AT and one lost to follow-up, Figure 1) this was dealt with by multiple imputation of missing data being based on iterative chain equation modelling including all available outcome measures and their baseline values and treatment arm. Five imputed data sets were created, which were then analysed and combined using Rubin's equations.37 The differences in change of BP and BMQ were assessed using an unadjusted analysis and after adjusting for possible prognostic factors (gender, education, economic status and medical insurance status) using an analysis of covariance. Adherence rate and medication change were similarly analysed using logistic regression with only treatment arm as a covariate, as an unadjusted analysis and an adjusted analysis was based on included prognostic factors. Subgroup analyses of change in SBP by the use or not of cardiovascular medication and the use of two or more antihypertensive drugs or not at baseline was based on testing for an interaction effect in a regression model at baseline.
Figure 1.
Participant Flow Diagram. *All analyses conducted on an intention to treat basis with multiple imputation of missing data.
Results
We recruited 136 non-adherent patients with hypertension and randomised them equally to the AT and TAU groups. Six patients (three in each group) died before the end of the study, three withdrew from the AT intervention and one patient in the AT group was lost to follow-up (see participant flow diagram). Tables 1 and 2 show the demographical and clinical characteristics of participants in the trial.
Table 1. Baseline demographical characteristics of participants.
| Characteristics | AT Group n=68 n (%) | TAU Group n=68 n (%) |
|---|---|---|
| Age (mean, s.d.) | 53.4 (10.7) | 53.9 (11.7) |
| Female | 43 (63) | 30 (44) |
| Living alone | 4 (6) | 9 (13) |
| Marital status: Married | 56 (82) | 47 (69) |
| Marital status: Single/widower | 12 (18) | 21 (31) |
| Current smoker | 19 (28) | 25 (37) |
| Currently exercisinga | 22 (32) | 20 (29) |
| Keeping restricted dietb | 30 (44) | 23 (34) |
| Working | 33 (49) | 42 (62) |
| Not working | 35 (51) | 26 (38) |
| Medically insured | 29 (43) | 36 (53) |
| Medically uninsured | 39 (57) | 32 (47) |
| Education: None | 2 (3) | 10 (15) |
| Education: Educated | 66 (97) | 58 (84) |
Abbreviations: AT, adherence therapy; TAU, treatment as usual.
Minimum exercise of walking 30 min per day,
Adhering to low-salt, low-fat diet.
Table 2. Baseline clinical characteristics of participants.
| Characteristics | AT Group n=68 Mean (s.d.) | TAU Group n=68 Mean (s.d.) |
|---|---|---|
| Systolic blood pressure | 165.6 (10.1) | 163.4 (9.7) |
| Diastolic blood pressure | 103.2 (7.0) | 101.3 (6.9) |
| Number of antihypertensives prescribed daily | 2.7 (0.8) | 2.4 (0.6) |
| Number of other medications prescribed daily | 0.9 (1.0) | 0.5 (0.8) |
| BMQ: General harm | 13.5 (2.4) | 12.4 (3.0) |
| BMQ: General overuse | 15.4 (2.3) | 14.9 (2.0) |
| BMQ: Sensitivity to drugs | 14.9 (2.7) | 15.0 (2.1) |
| BMQ: General benefit | 13.6 (2.4) | 14.3 (2.3) |
Table 3 summarizes the change from baseline in BP, adherence and beliefs for both groups. The results show that SBP is reduced in the AT group by 23.4 (95% CI: 20.7, 26.2) mm Hg more than in the TAU group and similarly, DBP is reduced in the AT group by 15.6 (95% CI: 13.2, 17.9) mm Hg more than in the TAU group. Because of the imbalance between treatment groups at baseline in terms of gender, economic status, education and medical insurance, an analysis was conducted adjusting for these factors and the baseline value of the outcome. The adjusted and unadjusted analyses produced very similar results.
Table 3. Analysis of outcome measures (change from baseline).
| Outcome |
AT |
TAU |
Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (s.d.) | N | Mean (s.d.) | Mean difference (95% CI) | P-value | Mean difference (95% CI) | P-value | |
| SBP | 68 | −22.1 (9.7) | 68 | 1.0 (5.5) | −23.1 (−25.9, −20.4) | <0.01 | −21.6 (−24.4, −18.8) | <0.01 |
| DBP | 68 | −14.7 (8.6) | 68 | 0.5 (3.9) | −15.2 (−17.6, -12.8) | <0.01 | −12.8 (−15.0, −10.6) | <0.01 |
| G-H | 68 | −5.8 (2.4%) | 68 | −0.1 (1.1) | −5.67 (−6.3, −5.0) | <0.01 | −5.0 (−5.6, −4.4) | <0.01 |
| G-O | 68 | −1.2 (1.9) | 68 | 0.1 (1.3) | −1.3 (−1.9, −0.8) | <0.01 | −1.4 (−2.1, −0.8) | <0.01 |
| G-S | 68 | −1.8 (1.6) | 68 | 0.02 (1.32) | −1.8 (−2.3, −1.3) | <0.01 | −1.9 (−2.4, −1.4) | <0.01 |
| G-B | 68 | 2.6 (2.4) | 68 | 0.37 (1.59) | 2.3 (1.5, 3.0) | <0.01 | 1.8 (1.2, 2.5) | <0.01 |
| Percentage adherence at 11 weeks | 68 | 97.2% (4.0) | 68 | 70.6% (10.7) | 26.7% (23.9, 29.4) | <0.01 | 26.4% (23.4, 29.4) | <0.01 |
| Medication changes over 11 weeks | 68 | 8.2 (12.1%) | 68 | 16.8 (24.7%) | OR (95% CI) 0.4 (0.2, 0.6) | 0.08 | OR (95% CI) 0.6 (0.2, 1.7) | 0.30 |
Abbreviations: AT, adherence therapy; DBP, diastolic blood pressure; G-H, general harm; G-O, general overuse; G-S, general sensitivity; G-B, general benefit; OR, odds ratio; SBP, systolic blood pressure; TAU, treatment as usual.
Unadjusted analysis based on a two-sample t-test and adjusted analysis based on an analysis of covariance model.
The BMQ scores show that beliefs about medications in the AT group also move away from a view that medications are intrinsically harmful (G-H −5.7) and towards one that recognises the benefits of the medications (G-B 2.7) with little or no change in attitudes over the period of the study in the TAU group.
The patients in this study were taking an average of 2.6 antihypertensive medications and a further 0.7 other medications daily. There was no significant difference in the numbers of patients who changed their hypertensive medications during the 11-week period of the study (AT: 12%, TAU: 25%).
Patients in the AT group took substantially more of their medications (97%) compared with those in the TAU group (71%). There was no significant difference in the average adherence rate between individuals on monotherapy compared with those prescribed two or more antihypertensive medications in either the AT or TAU groups.
Our proposed mechanism of action is that AT will positively influence attitudes and beliefs about medications, which will improve adherence, and which will, in turn, reduce BP. The BMQ scores indicating negative beliefs about medication were all negatively correlated with adherence (GH: −0.73, P<0.001, G-O: −0.31, P<0.001 and G-S: −0.46, P<0.001) and the BMQ General Benefit score was positively correlated with adherence (0.44, P<0.001), that is a reduction in negative beliefs and an increase in positive beliefs about medication-improved adherence. The adherence scores were negatively correlated with BP (SBP: −0.71, P<0.001, DBP: −0.63, P<0.001) that is, an improvement in adherence reduced BP. Finally, we also identified that the BMQ scores indicating negative beliefs were positively correlated with SBP (G-H: 0.75, P<0.001; G-O: 0.20, P=0.028; G-S: 0.53, P<0.001) and the BMQ General Benefit score was negatively correlated with SBP (−0.45, P<0.001) that is, negative beliefs about medication are associated with higher SBP and positive beliefs about medication are associated with lower SBP.
We estimated the cost of the delivery of the intervention was seven-AT sessions × 20 min that is, 2 h 20 min of a mid-grade nurse (mid-Band six in the United Kingdom, including all employer's costs) plus the hire of a suitable room in an outpatient clinic for the same duration. From this we calculated that the price of a course of AT would be approximately US$ 130 per person.
Discussion
Current clinical guidelines17, 38 on strategies for enhancing adherence are based largely on observational data that has yet to be tested in a robust RCT. AT incorporates all of the key elements of the NICE and WHO guidelines,17, 38 and this study represents a rigorous test of them.
Size of effect
We were surprised by the size of effect of the AT on adherence (37% increase) and BP (SBP −23.1 mm Hg. (95% CI: −25.85, −20.36), DBP −15.2 mm Hg (95% CI: −17.55, −12.80)). Although the sample size of 136 patients was modest the confidence intervals for the effect are narrow due to the small variation in BP in this population. A previous Cochrane systematic review of RCTs of adherence therapies in hypertension19 had demonstrated up to a 41% increase in adherence, and reductions of up to 19.5 mm Hg in SBP, and 12.7 mm Hg in DBP. The duration of our follow-up was short (4 weeks) so the impact of the AT was maximised and may not have suffered from decay in effect that might be expected with a longer follow-up period.
Changes in BP compared with previous pharmacological studies
Law's review39 of 354 RCTs of BP-lowering drugs showed that the average BP of trial participants was 154/97 mm Hg (90% range: SBP 139–170, DBP 97–106), making our patient's baseline average BP (164/102 mm Hg) towards the top of the range that is usually recruited into hypertension trials. They determined that patients placed on two drugs had an average reduction of 14.6 mm Hg for SBP and 8.6 mm Hg for DBP. Our patients were prescribed 2–3 antihypertensive drugs on average, which may in part account for the larger reductions in BP observed (SBP 23.1 mm Hg, DBP 15.2 mm Hg). It may also be that patients recruited to pharmaceutical trials tend to be selected for adherent behaviours; consequently there may be less potential room for improvement (the ceiling effect). The patients in our study were specifically selected for their non-adherence to their medication at baseline, and as compliance levels improved so dramatically in the AT group, we may be observing close to the maximum change capable of being achieved by these antihypertensive drugs.
Causal mechanism
The hypertensive patients all began the study with relatively neutral beliefs about medication, but poor adherence behaviours. Those in the AT group shifted their opinions so that overall they disagreed with statements regarding the intrinsically harmful nature of medicines, and overall agreed with statements regarding the intrinsically beneficial nature of medicines. This shift in beliefs led to a change in adherence behaviour (37% improvement in the number of pills taken over 1 month), which subsequently reduced the BP to close to the usual target ranges (Mean SBP/DBP 143/88 mm Hg). No such shift in medication beliefs or adherence behaviours was observed in the TAU group, and their endpoint BP had barely changed from baseline (Mean SBP/DBP 164/102 mm Hg). That we have been able to demonstrate how our AT has impacted on BP is important and particularly pleasing because few, if any, previous adherence studies have been able to establish this. To an extent this observation also counters one of the major weaknesses of our trial; a lack of a control intervention that addresses the non-specific effects of the therapy.
Adjustments to prescriptions
In our study there was no significant difference in the rates of prescribing adjustments in either group, although the TAU group did change their medication more often. Our study had a short period of follow-up (1 month) which may have reduced the likelihood of us detecting differences in the rates of medication adjustment between the two groups. Ogedegbe40 reported that the effect of motivational interviewing intervention led to steady maintenance of adherence in hypertensive African Americans, over 12 months follow-up compared with a significant reduction in adherence noted in the usual care group. This effect was also associated with greater frequency of adjustments in medication in the usual care group. Thus with a longer follow-up period we may have observed similar changes.
One factor that is known to reduce adherence is to take more than one drug. Currently, it is recommended that two anti-hypertensive medications should be prescribed for most people with hypertension in order to maximize BP control.41 However, our results showed no association between the number of prescribed drugs and the rate of adherence in either group.
Cultural issues
To our knowledge this is the first RCT that has tested the effect of AT on reduction in BP and medication adherence among mainly Muslim hypertensive patients. Griffiths42 has suggested that a belief in the predetermination (‘takdir' or destiny) of the Islamic life-course can present a barrier to the uptake of interventions that aim to improve health behaviours. The success of our intervention refutes this, although we acknowledge that Islamic beliefs and behaviours vary across the world and the fact that FA is from Jordan meant that she undoubtedly presented the AT in a culturally acceptable manner.
Global impact of hypertension
Hypertension was estimated to be present in 26% of the adult population in 2000, which represents 972 million patients. The number of adults with hypertension in 2025 is predicted to increase by about 60% to a total of 1·56 billion.2 It has been estimated that the cost of hypertension was US$ 370 billion globally in 2001, and indirect costs could be as high as US$ 3600 billion annually. This represents about 10% of the world's overall healthcare expenditure.43
Although a formal evaluation of cost effectiveness could not be conducted with the data collected from this study, we calculated that the price of a course of AT would be approximately US$ 130 per person. Observational studies have predicted that a long-term reduction of 10 mm Hg DBP would be predicted to lead to at least a 56% reduction in stroke and a 37% reduction in chronic heart disease.5 Our study showed a short-term reduction of 15.2 mm Hg DBP, which even if the size of this effect were attenuated somewhat over time, and considering the substantial costs outlined above and the sizeable preventive effect for serious sequelae of reductions in DBP, it would seem reasonable to suggest that this intervention is likely to be cost effective.
Strengths and weaknesses of the study
Our process of randomisation was only partially successful in balancing the baseline characteristics of the two groups as there were differences in terms of gender balance, employment status, education levels and medical insurance status. Although the use of a single therapist limits the generalisability of this intervention, we believe that this was mitigated as FA was trained according to the standard manual to deliver AT and the fidelity of her delivery was confirmed. This model of AT has also been used successfully to increase adherence in other long term conditions—for example, schizophrenia.44
Patients were aware of the fact that they were being monitored because this was explained to them in the patient information sheet. This may have encouraged them to be more adherent with treatment than usual. The lack of change in BP in the TAU group suggests that this did not occur. However, we recognise that because of the relationship the patients in the AT group developed with FA during therapy, they might have not wanted to let her down, and been particularly adherent during the assessment period. This effect may have been enhanced by the short follow-up period (4 weeks).
We used pill counting after 1 month to estimate the level of adherence, but we recognize that the absence of pills does not necessarily mean that the patient has correctly taken their medications. Pill counting cannot identify other types of non-adherence, such as inappropriate timing or dosage.
Implications for future research
All of the AT was delivered by a single person (FA) in this study. This reduces our ability to state that its effect could be as easily achieved by other clinicians. Future research should be conducted with more therapists to counter the potential ‘halo' effect of a single highly motivated nurse delivering treatment. Future studies should also follow more participants over a longer period to increase our understanding of the durability of the effect in a larger and potentially more diverse population. Adherence to medication is complex and future trials may wish to determine whether drug doses were taken in the correct amount and at the correct time. Future studies should also collect data to enable a cost effectiveness analysis to be conducted. An important question when applying AT in clinical practice is whether a short-term intervention (one session per week over 7 weeks) is sufficient to maintain BP-lowering effect over time. Other aspects of AT need to be tested to determine the robustness and generalisability of this therapy. Aspects to be tested could include frequency and intensity of the therapy, the location of its delivery, the mode of delivery (for example, by telephone), the numbers to whom it is delivered (for example, one-to-one or group sessions), the character of the person delivering the AT (for example, lay advisors), and whether the duration of effect could be enhanced by the use of top-up sessions.
Implications for practice
From our findings it can be concluded that a relatively short dose of AT is sufficient to reduce BP and improve adherence to medication for at least 1 month. It is possible that these results reflected changes in patient's behaviour, attitudes and beliefs that would lead to regular drug intake eventually becoming a habitual behaviour.

Acknowledgments
We thank Dr Jane Skinner, Edward Wilson, Professor Max Bachmann and Dr Jessica Walburn for their advice in the development of this paper. Funded by a doctoral studentship grant from Philadelphia University, Jordan and the University of East Anglia, UK. The funders were not involved in the design or analysis of this study.
Author Contributors: RG is the Chief Investigator of the project, FA delivered the AT, AC proposed and undertook the analysis, RG, KD, FA and AN designed the trial, KD wrote the first draft of the paper, RG, FA, AN and AC participated in editing and redrafting the paper. All authors read and commented on the final manuscript. The UEA CRTU undertook the randomisation. RG is the guarantor.
Richard Gray developed the Adherence Therapy intervention and has provided consultancy work to AstraZeneca, Bristol-Myers Squibb, Jannsen Cilag, Eli Lilly and Co. Otsuka Pharmceutical Europe Ltd, Pfizer, received honoraria from AstraZeneca, Bristol-Myers Squibb, Jannsen Cilag, Eli Lilly and Co. Otsuka Pharmceutical Europe Ltd, Pfizer, Wyeth and had research support from AstraZeneca. Katherine Deane has had research support from Orion Pharmaceuticals, and unrestricted educational grants from GlaxoSmithKline and Amersham Pharmacia Biotech.
References
- World Health Organization, International Society of Hypertension Writing Group World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. The Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez A, Rodgers A, Vander Hoorn S, Murray C. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. The Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Peto R, Collins R, Godwin J, Cutler J, Sorlie P, et al. Blood pressure, stroke, and coronary heart disease: Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. The Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- Asia Pacific Cohort Studies Collaboration Blood pressure indices and cardiovascular disease in the Asia Pacific region: A pooled analysis. Hypertension. 2003;42:69–75. doi: 10.1161/01.HYP.0000075083.04415.4B. [DOI] [PubMed] [Google Scholar]
- Williams B, Lindholm L, Sever P. Systolic pressure is all that matters. The Lancet. 2008;371:2219–2221. doi: 10.1016/S0140-6736(08)60804-1. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: The Suita Study. Hypertension. 2008;52:652–659. doi: 10.1161/HYPERTENSIONAHA.108.118273. [DOI] [PubMed] [Google Scholar]
- Kearney P, Whelton M, Reynolds K, Whelton P, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22:1.19-1. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003. p. 289.219.2560. [DOI] [PubMed]
- Elzubier AG, Husain AA, Suleiman IA, Hamid ZA. Drug compliance among hypertensive patients in Kassala, Eastern Sudan. East Mediterr Health J. 2000;6:100–105. [PubMed] [Google Scholar]
- Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. Br Med J. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sluijs E, Dulmen SV, Dijk LV, de Ridder D, Heerdink R, Bensing J. Patient Adherence to Medical Treatment: a Meta Review. Nivel, Utrecht; 2006. [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Nunes V, Neilson J, O'Flynn N, Calvert N, Kuntze S, Smithson H, et al. Clinical Guidelines and Evidence Review for Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. National Collaborating Centre for Primary Care and Royal College of General Practitioners: London; 2009. [PubMed] [Google Scholar]
- Haynes R, Ackloo E, Sahota N, McDonald H, Yao X.Interventions for enhancing medication adherence Cochrane Database of Systematic Reviews 2008(2): Art. No.: CD000011. [DOI] [PubMed]
- Schroeder K, Fahey T, Ebrahim S.Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings Cochrane Database of Systematic Reviews 2004(3): Art. No.: CD004804. [DOI] [PMC free article] [PubMed]
- Morrison A, Wertheimer AI, Berger ML. Interventions to improve antihypertensive drug adherence: A quantitative review of trials. Formulary. 2000;35:234–255. [Google Scholar]
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;4:1–24. [Google Scholar]
- Kemp R, Kirov G, Everitt B, Hayward P, David A. Randomised controlled trial of compliance therapy. 18-month follow-up. Br J Psychiatry. 1998;172:413–419. doi: 10.1192/bjp.172.5.413. [DOI] [PubMed] [Google Scholar]
- Belbeisi A, Zindah M, Walke H, Jarrar B, Mokdad A. Assessing risk factors for chronic disease—Jordan, 2004. MMWR. 2006;55:653–655. [PubMed] [Google Scholar]
- Yousef A-MM, Al-Bakri AG, Bustanji Y, Wazaify M. Self-Medication patterns in Amman, Jordan. Pharmacy World Sci. 2008;30:24–30. doi: 10.1007/s11096-007-9135-x. [DOI] [PubMed] [Google Scholar]
- Morisky D, Ang A, Krousel-Wood M, Ward H. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Capewell S, Ford E, Croft J, Critchley J, Greenlund K, Labarthe D. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in the United States of America. Bulletin WHO. 2010;88:120–130. doi: 10.2471/BLT.08.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Goldstein MK, Lavori P, Coleman R, Advani A, Hoffman BB. Improving adherence to guidelines for hypertension drug prescribing: cluster-randomized controlled trial of general versus patient-specific recommendations. Am J Managed Care. 2005;11:677–685. [PubMed] [Google Scholar]
- Schroeder K, Fahey T, Hollinghurst S, Peters TJ. Nurse-led adherence support in hypertension: a randomized controlled trial. Family Practice. 2005;22:144–151. doi: 10.1093/fampra/cmh717. [DOI] [PubMed] [Google Scholar]
- Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. Cochrane Database of Systematic Reviews. 2010. [DOI] [PubMed]
- World Health Organisation Process of translation and adaptation of instruments, 2007 . http://www.who.int/substance_abuse/research_tools/ translation/en/index.html .
- Brislin RW. Back-translation for cross-cultural research. J Cross-cultural Psychol. 1970;1:185–216. [Google Scholar]
- Brislin RW.Translation and content analysis of oral and written materialsIn: Trialdis HC, Berry JW (eds).Handbook of Cross-cultural Psychology: Methodology Allyn and Bacon Inc: Boston; 1986 [Google Scholar]
- Brislin RW, Lonner WJ, Throndike RM. Cross-cultural Research Methods. John Wiley & Sons: New York; 1973. [Google Scholar]
- Craven RF, Hirnle CJ. Fundamentals of Nursing. Lippincott Williams and Wilkins: Philadelphia, USA; 2003. [Google Scholar]
- Michigan Department of Community Health Procedure for measurement of blood pressure, 2003 . http://www.michigan.gov/documents/BPprocedure_79896_7.pdf .
- Rubin DB.Multiple Imputation for Nonresponse in Surveys9th edn.West Sussex; 1987 [Google Scholar]
- World Health Organisation . Non-communicable Diseases and Mental Health. Adherence to long-term therapies project. World Health Organization; 2003. Adherence to long-term therapies evidence for action. [Google Scholar]
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. Br Med J. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogedegbe G, Chaplin W, Schoenthaler A, Statman D, Berger D, Richardson T, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21:1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Poulter N, Brown M, Davis M, McInnes G, Potter J, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004—BHS IV. J Hum Hypertens. 2004;18:139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- Griffiths C, Motlib J, Azad A, Ramsay J, Eldridge S, Feder G, et al. Randomised controlled trial of a lay-led self-management programme for Bangladeshi patients with chronic disease. Br J Gen Pract. 2005;55:831–837. [PMC free article] [PubMed] [Google Scholar]
- Gaziano TA, Bitton A, Anand S, Weinstein MC. The global cost of nonoptimal blood pressure. J Hypertensi. 2009;27:1472–1477. doi: 10.1097/HJH.0b013e32832a9ba3. [DOI] [PubMed] [Google Scholar]
- Gray R, Leese M, Bindman J, Becker T, Bburti L, David A, et al. Adherence therapy for people with schizophrenia. Br J Psychiatry. 2006;189:508–514. doi: 10.1192/bjp.bp.105.019489. [DOI] [PubMed] [Google Scholar]