Summary
Periodontal diseases result from the interaction of bacterial pathogens with the host’s gingival tissue. Gingival epithelial cells are constantly challenged by microbial cells, and respond by altering their transcription profiles, inducing the production of inflammatory mediators. Different transcription profiles are induced by oral bacteria and little is known about how the gingival epithelium responds after interaction with the periodontopathogenic organism Aggregatibacter actinomycetemcomitans. In the present study, we examined the transcription of genes involved in signaling transduction pathways in gingival epithelial cells exposed to viable A. actinomycetemcomitans.
Methods
immortalized gingival epithelial cells (OBA-9) were infected with A. actinomycetemcomitans JP2 for 24 hours and the transcription profile of genes encoding Human Signal Transduction Pathways was determined. Functional analysis of inflammatory mediators positively transcribed was performed by ELISA in culture supernatant and in gingival tissues.
Results
15 of 84 genes on the array were over-expressed (p<0.01) after 24 hours infection with viable A. actinomycetemcomitans. Over-expressed genes included those implicated in tissue remodeling and bone resorption, such as CSF2, genes encoding components of the LDL pathway, NF-kB–dependent genes and other cytokines. ELISA data confirmed that GM-CSF/CSF2, TNF-α and ICAM-1 were highly expressed by infected gingival cells when compared to control non infected cells, and presented higher concentrations in tissues from aggressive and chronic periodontitis patients than from healthy controls.
Conclusion
The induction in epithelial cells of factors such as the proinflammatory cytokine CSF2, which is involved in osteoclastogenesis, may help explaining the outcomes of A. actinomycetemcomitans infection.
Keywords: Aggregatibacter actinomycetemcomitans, gene expression, epithelial cell, infection
INTRODUCTION
Although the oral cavity harbors over 700 bacterial species (Aas et al., 2005), only a small subset of species is associated with the destruction of periodontal tissues. Aggregatibacter actinomycetemcomitans is strongly implicated in the etiology of aggressive periodontitis (Slots et al., 1980) but is also associated with systemic infectious (Paturel et al., 2004; Muhle et al., 1979). A. actinomycetemcomitans is part of the HACEK group, a group of gram-negative, fastidious, slow-growing oropharingeal bacilli, which contributes to infective endocarditis in humans (Paturel et al., 2004). The main niche for this species is the oral mucosa, particularly the gingival sulcus/pocket. The epithelial cells act as sensors during microbial infections (Dale, 2002) through the recognition of microbe-associated molecular patterns (MAMPs) by Toll-like receptors and other pattern recognition receptors (Kikkert et al., 2007). Following the receptor-ligand interaction, signal transduction is initiated (Laube et al., 2008) inducing and maintaining an inflammatory response in the periodontium (Silva et al., 2008). Although the host response is intended to fight off the bacterial infection associated with periodontitis, the elevated levels of inflammatory cytokines may result in an exacerbated host response, leading to production of matrix methalloproteinases, osteoclast activation and further bone resorption (Fukushima et al., 2005).
Different pathogens induce a variety of epithelial cell responses (Milward et al., 2007; Shan et al., 2007), and little is known about the gingival epithelial cell response to A. actinomycetemcomitans. In addition to adhesion to host cells, A. actinomycetemcomitans can invade non phagocythic cells such as epithelial cells (Meyer et al., 1996), and interact with intracellular receptors (Shan et al, 2007). Thus, pathogenic bacteria manipulate the host response in order to improve their survival by activating or inhibiting different signaling pathways. The increase in our knowledge on the strategies used by the pathogens during the infection may result in the development of new therapeutic strategies in the treatment of periodontal diseases, such as pharmacological products antagonizing the effect induced by bacteria and their products (Yoshida & Yoshikawa, 2008; Shan et al., 2007).
In order to contribute to the understanding of the inflammatory scenario in the periodontium seen in aggressive periodontitis, this study compared the transcription of genes encoding components of signal transduction pathways in A. actinomycetemcomitans infected and uninfected gingival epithelial cells. In addition, the induction of positively regulated genes was confirmed by determining the concentration of these factors by ELISA in the supernatant of A. actinomycetemcomitans infected cells as well as in the gingival tissues of patients with aggressive and chronic periodontitis and in healthy controls.
METHODS
Bacterial strain and culture conditions
A. actinomycetemcomitans strain JP2 was used to infect oral epithelial cells. Bacteria were grown in 5% CO2 (microaerophilic conditions) at 37 °C in brain heart infusion (BHI) (Difco, Sparks, MD, USA) broth supplemented with 40 mg of NaHCO3 per liter.
Epithelial Cell culture
Immortalized normal gingival epithelial cells line (OBA-9) were grown in serum–free keratinocyte growth medium (KSFM- Invitrogen) containing insulin, EGF and FGF (Invitrogen), supplemented with 100 μg/ml streptomycin and 100 U/ml penicillin (Sigma, St. Louis, MO) and cultured at 37 °C in 5% CO2 (Shell Lab, TC 2123, Cornelius, OR, USA).
Adhesion and invasion assays
Gingival epithelial cells (OBA-9) (~2×105 cells/well) were inoculated in 24 well tissue culture plates (Corning Inc., Corning, NY, USA) and incubated to reach a semiconfluent monolayer (~3×105 OBA-9 cells/well) in KSFM. Prior to infection, the wells were washed thrice with phosphate-buffered saline (PBS) (pH 7.5, 0.8%NaCl). Overnight bacterial culture were inoculated in BHI to reach the exponential growth phase/mid log, harvested by centrifugation, and resuspended in antibiotic-free KSFM medium. The bacterial CFU levels were confirmed by viable counting. OBA-9 cells in semiconfluent monolayers were inoculated with bacteria in antibiotic-free KSFM medium at the multiplicity of infection (MOI) 100:1 (~3×107 bacteria/well). After incubation for 2, 12 and 24 hours, the wells were washed thrice and treated with 0.25% trypsin/EDTA. The number of viable bacterial cells adhesive to cells/well was estimated by culture in BHI agar plates.
For the invasion assay, OBA-9 cells were co-cultured with bacterial cells for 2, 12 and 24 hours, unattached bacteria were removed by washings, and extracellular bacteria were killed by incubation with gentamicin (100μg/ml) (Sigma, St Louis, MO, USA) for 60 min. After washings, internalized bacteria were released by epithelial cell lyses with 0.5% Triton X-100 (Sigma, St Louis, MO, USA) (Meyer et al., 1996) and the number of viable bacterial cells/well was determined. The experiments were performed in triplicate in three independent assays.
Transcription analysis of genes involved in cell signaling pathway
Similar assays to the adhesion assay were performed for gene transcription analysis after 24 hours of co-cultured with A. actinomycetemcomitans, except for the trypsin treatment. The transcription of 84 genes was evaluated in cells infected with A. actinomycetemcomitans and compared to control non- infected cells. Total RNA was obtained by using Trizol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. After chloroform extraction, RNA was precipitated with isopropanol and washed with 70% ethanol. RNA quantity and integrity were determined by spectrophotometer (Nanodrop ND-1000, Wilmington, DE, USA). Contaminating genomic DNA was removed by DNase digestion. First strand synthesis was performed on 1 μg of RNA using RT2PCR Array First Strand Kit (Bioscience Corporation, Frederick, MD, USA). Gene expression was determined by real Time PCR using the Human Signal Transduction Pathway Finder (Bioscience Corporation, Frederick, MD, USA). Experimental and control samples were analyzed in triplicate.
Expression profiles of the target genes were measured relative to the mean cycle threshold (CT) values of the five different calibrator genes (b2m, hprt1, rpl13a, gapdh and actb) using ΔΔCT method. For statistical comparison of the control and experimental groups, Student’s t-test was performed using mean CT values derived from the triplicate samples. Significant difference in gene expression was considered when p<0.01 at greater than or equal to a 4 fold change in expression.
Detection of apoptosis
Interaction between bacteria and eukaryotic cells was performed as described for the adhesion assay. After 24 h of interaction, apoptosis was evaluated by using the annexin V assay as described by manufacturer (FITC AnnexinV apoptosis detection kit, BD Pharmigen, San Jose, CA, USA) (Vermes et al., 1995). Cells stained with annexin V FITC conjugated and propidium iodide were analyzed by flow cytometry (Guava Easy cyteFlow Cytometer, Millipore, Billerica, MA, USA). Ten thousand events were analyzed in FL-1 (FITC) and FL-2 (PI) channels. As a positive control, camptothecin (6 μM final concentration) (Sigma) was added to the OBA-9 control cells 4 hours before reading. Cells with the phenotype of annexin V-positive and propidium iodide- negative were defined as early apoptotic cells.
Quantification of CSF2/GM-CSF, TNF-α and ICAM-1 in the supernatant of infected GECs and in gingival tissues
The supernatants of gingival epithelial cells (OBA-9) exposed to bacteria cells for 24 hours, as well as control cells, as well as of homogeneized gingival tissues were evaluated. This study was approved previously by the Ethical Committee in human research of both Institutions (University of São Paulo, and University of Guarulhos). The protocol was explained to each subject and signed informed consents were obtained. Gingival tissue samples were obtained from surgery from subjects with different periodontal conditions selected from the population referred to the periodontal clinic of the Guarulhos University: periodontally healthy (n=12), aggressive periodontitis (n=10) and chronic periodontitis (n=11). Subjects were diagnosed based on the periodontal classification of the American Academy of Periodontology (Armitage 1999), and followed the criteria as previously described (Faveri et al., 2009).
After surgery, 100 mg of gingival tissue was added to 500 μl lyses buffer [PBS (1x)/0.05 % Tween 20/1 mM protease inhibitor - Sigma] and homogenized with Dounce Glass homogenizer. Insoluble debris was removed by centrifugation. The levels of CSF2/GM-CSF, TNF-α and ICAM -1 were determined in the soluble fraction of gingival tissue samples and in the cell supernatants by ELISA, according to the manufacturer’s protocols [CSF2/GM-CSF and TNF-α (Peprotech Inc., Rocky Hill, NJ, USA), and ICAM-1 (R&D Systems, Minneapolis, MN, USA)]. The absorbance at 450 nm was read using a microplate reader (Bio- Rad, model 680) with a wavelength correction set at 550 nm.
In order to access the levels of colonizing A.actinomycetemcomitans in each studied site, subgingival biofilm adjacent of each excised gingival site was collected and bacteria levels were determined checkerboard DNA-DNA hybridization (Faveri et al., 2009).
Student’s t-test was used in order to determinate differences in inflammatory mediators levels secreted into supernatant of control and infected gingival epithelial cells (OBA-9). Kruskal Wallis was used to compare the levels of inflammatory mediators in gingival tissues of subjects with healthy gingiva, aggressive and chronic periodontitis. Significant differences were considered when p < 0.05.
RESULTS
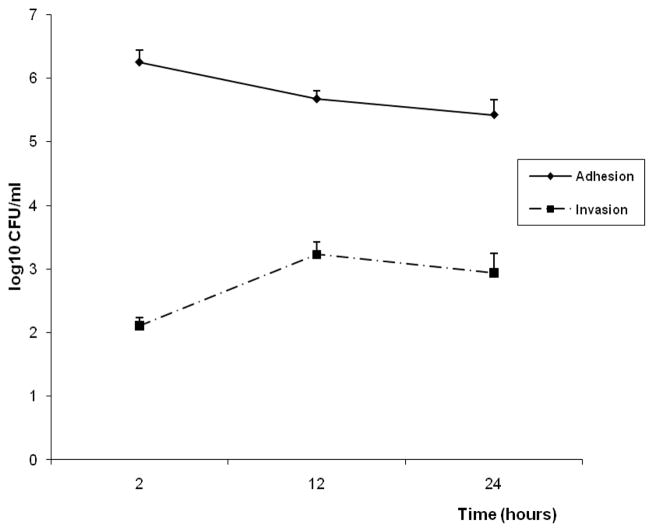
The adhesion and invasion properties of A.actinomycetemcomitans JP2 were established at different periods of interaction in order to select the appropriate time for signaling transduction analysis in OBA-9 cells. Adhesion was more efficient after 2 hours of infection and decreased after 24 hours of interaction, while invasion increased from 2 hours to 12 h, and remained stable untill 24 hours (Figure 1).
Figure 1.
Number of bacterial cell expressed as log10CFU/ml, determined in adhesion and invasion assay to gingival epithelial cells (OBA-9) after 2, 12 and 24 hours of co-cultured with A.actinomycetemcomitans JP2. Multiplicity of infection (MOI) 100:1
Gene expression assay
The transcription profile in OBA-09 cells after infection with A. actinomycetemcomitans for 24 hours indicated up-regulation of 15 genes among 84 target genes of Human Signal Transduction Pathway (Table1) with statistically significant differences (p<0.01). The gene csf2, encoding CSF2/GM-CSF, was the most up-regulated gene (170 fold change) as shown in Table 1. The genes encoding tumor necrosis factor (TNF-α) and EGR-1 were the second (28.93 change) and the third (14.08 change) most positively regulated gene in infected cells.
Table 1.
Genes in the signaling transduction pathway up-regulated in A. actinomycetemcomitans infected OBA-9 gingival epithelial cells, in comparison with non infected control cells. Student’s t-test, significant difference in gene expression was considered when p<0.01.
| Symbol | Pathway | Gene | Gen Bank | Fold Changes | p-value |
|---|---|---|---|---|---|
| BIRC3 | NF-κB | Baculoviral IAP repeat-containing 3 | NM_001165 | 5.92 | 0.000022 |
| CCL2 | LDL | Chemokine (C-C motif) ligand 2 | NM_002982 | 12.18 | 0.000003 |
| CCL20 | NF-κB | Chemokine (C-C motif) ligand 20 | NM_004591 | 6.49 | 0.0012 |
| CDKN1A | TGF-β/p53/Androgen | Cyclin-dependent kinase inhibitor 1A | NM_000389 | 4.88 | 0.0015 |
| CSF2 | LDL/calcium and protein quinase C/NF-κB (Zhang et al., 2011) | Colony stimulating factor 2 | NM_000758 | 170.04 | 0.00002 |
| EGR1 | Mitogenic/CREB/PhospholipaseC | Early growth response 1 | NM_001964 | 14.08 | 0.00066 |
| FOS | Mitogenic/Stress/PhospholipaseC/JNK/p38 (Zhang et al., 2011) | V-fos FBJ murine osteosarcoma viral oncogene homolog | NM_005252 | 4.47 | 0.004 |
| GADD45A | p53/Stress/DNA repair/cell cycle arrest (Zhang et al., 1999) | Growth arrest and DNA-damage- inducible, alpha | NM_001924 | 8.00 | 0.00058 |
| GREB1 | Estrogen | GREB-1 protein | NM_014668 | 10.13 | 0.0088 |
| ICAM1 | NF-κB/Phospholipase C | Intercellular adhesion molecule 1 (CD54), human rhiono virus receptor | NM_000201 | 7.67 | 0.00003 |
| IL1A | NF-κB | Interleukin 1, alpha | NM_000575 | 4.01 | 0.0009 |
| IRF1 | JAK-STAT/IRF (Zhang et al., 2011) | Interferon regulatory factor 1 | NM_002198 | 5.39 | 0.004 |
| JUN | Mitogenic/WNT/PI3 kinase-AKT/Calcium and protein kinase C/PhospholipaseC JNK/p38 (Zhang et al., 2011) | Jun oncogene | NM_002228 | 7.16 | 0.00023 |
| TNF | NF-κB | Tumor necrosis factor (TNF superfamily, member 2) | NM_000594 | 28.93 | 0.0097 |
| VEGFA | WNT | Vascular endothelial growth factor A | NM_003376 | 4.59 | 0.000246 |
Apoptosis assay
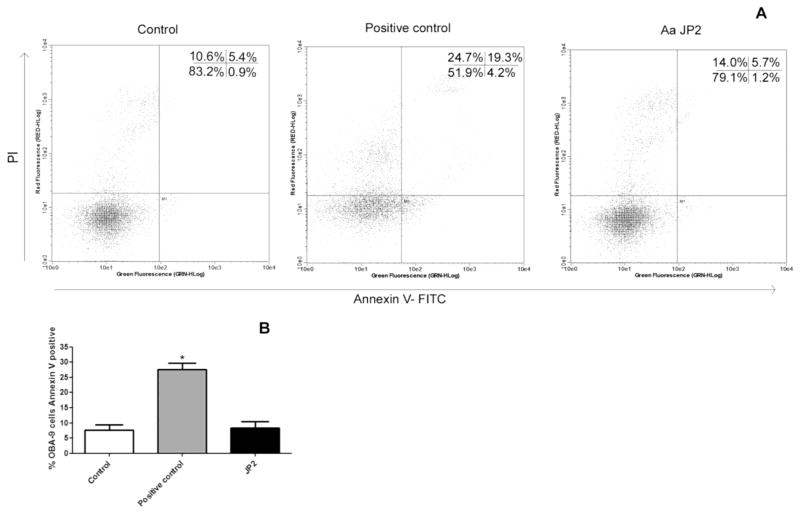
To verify the apoptosis after 24 hours of interaction, Annexin V staining was performed. As shown in Figure 2, A. actinomycetemcomitans JP2 did not lead to apoptosis when compared to negative control.
Figure 2.
Detection of apoptosis after 24 hours of interaction between OBA-9 cells and A. actinomycetemcomitans JP2 at multiplicity of infection (MOI) 100:1, using Annexin V-FITC/PI staining and flow cytometry. A: Dual parameter representing by dot plots, and the percentage of living, dead, late apoptotic and early apoptotic cells. B: Percentage of Annexin V positive cells, considering 100% of viability in negative control viable cells. Positive control was OBA-9 cells with camptothecin in a final concentration of 6 μM.* Statistically significant when compared to negative control (Control), when p <0.05 (ANOVA-Tukey).
Enzyme-linked immunosorbent assay
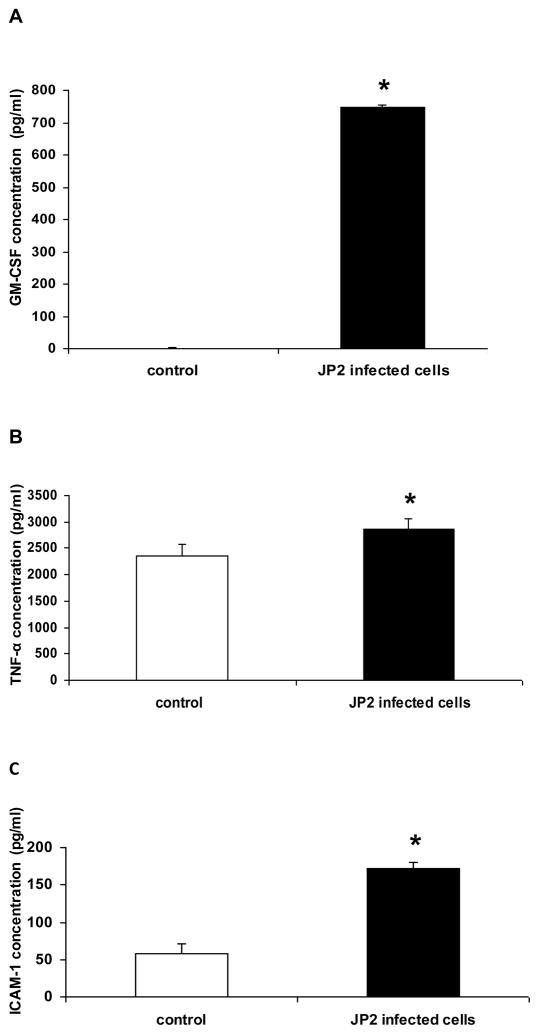
The concentrations of GM-CSF/CSF2, TNF-α and ICAM-1 were evaluated by ELISA, in order to confirm whether factors encoded by genes positively transcribed in the array were expressed at increased levels in culture supernatant by the infected epithelial cells. The concentration of GM-CSF/CSF2 reached high levels in infected cells, and was not detected in the supernatant of control cells (Figure 3A). High levels of TNF-α were observed in the JP2 infected cells, although has been observed TNF-α levels in control cells (Figure 3B). Infected gingival epithelial cells expressed also higher levels of the adhesion molecule ICAM-1 than controls (Figure 3C).
Figure 3.
Inflammatory mediator levels measured by ELISA in the supernatant of OBA-09 cells culture infected for 24 h with 3×107 CFU/ml A. actinomyctemcomitans JP2. Control – non infected cells. In A: GM-CSF, in B: TNF-α and in C: ICAM-1 (Student’s t-test; * p<0,05).
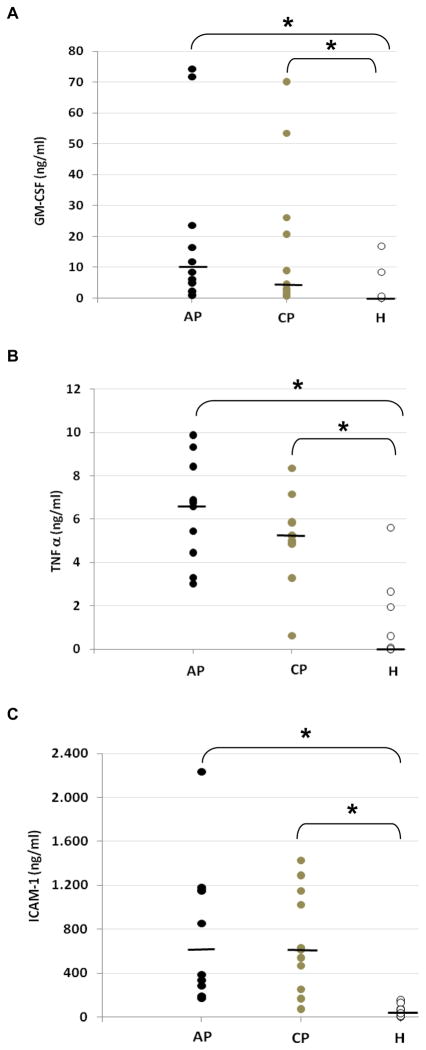
Significant differences in the levels for GM-CSF/CSF2, TNF-α and ICAM-1 were also shown in tissues from periodontitis when compared to periodontally health, but not between chronic and aggressive periodontitis (Figure 4).
Figure 4.
Inflammatory mediator levels measured by ELISA in the gingival tissue samples obtained from subjects with different periodontal conditions: healthy (H) (n=12), aggressive periodontitis (AP) (n=10) and chronic periodontitis (CP) (n=11). In A: GM-CSF, in B: TNF-α and in C: ICAM-1. (Kruskal-Wallis; * p<0,05). Bars: median of individual groups
A.actinomycetemcomitans was detected by DNA-DNA hybridation in levels equivalent or higher than 105cells/sample in 5 of 10 subgingival samples of aggressive periodontitis patients adjacent to the excised gingival tissue, whereas these levels were detected in three of 11 chronic periodontitis sites and in none of the healthy subjects sites. However, there was no correlation between subgingival levels of A.actinomycetemcomitans and of the studied cytokines or ICAM-1 in the adjacent gingival tissues (Spearman Rank Correlation, p>0.05).
DISCUSSION
Data presented here provide evidence on the transcript changes of gingival epithelial cells promoted by infection with A. actinomycetemcomitans, identifying a variety of genes and pathways involved in signaling recruitment and activation of immune system cells, along with genes important for cellular and tissue protection from bacteria and other cellular insults. The bacterial challenge consisted not only of bacterial products, but adherent and internalized bacteria were found, indicating that membrane, as well as intracellular receptors (Nods) were possibly activated during A. actinomycetemcomitans infection, as previously suggested (Stathopoulou et al., 2010).
Although oral epithelial cells may respond to a bacterial stimulus after a short period of contact, the cascade of transcription increases within time and other factors inducing stress, such as media changes may result in differential transcription (Milward et al, 2007). Thus, a longer exposure (24 h infection) was chosen for the analysis, which may better represent the conditions occurring in vivo during chronic diseases, with epithelial cell multiplication and feedback mechanisms.
Fifteen genes belonging to signaling transduction pathways were up-regulated in gingival epithelial cells infected with A. actinomycetemcomitans. Our data suggest that interaction with viable adhesive and invasive A. actinomycetemcomitans activates several pathways, since the up-regulated genes were involved in mitogen-activated protein kinase (MAPK) signaling (egr1, fos, and jun), nuclear factor-κB (NF-κB) activation, increase of cytoplasmic calcium concentration and activation of protein kinase C (csf2/gm-csf, fos, jun), activation of phospholipase C (egr1, fos, icam-1, jun), LDL (ccl2 and csf2) and p53 (cdkn1a, gadd45a) pathways in OBA-9 cells. On the other hand, except for jun, genes such as bcl2, ccnd1, fn1 and mmp7, and myc, involved in the phosphoinositol-3 kinase (PI3 kinase) pathway were not induced by A. actinomycetemcomitans infection. Recent study have shown activation of FAK - PI3 kinase or Rho-GTPase signaling cascade by A. actinomycetemcomitans Omp29 resulting F-actin rearrangement, which, in turn, can promote in OBA-9 bacterial internalization (Kajiya et al., 2011), although, CdtB is involved with phosphatase activity leading to depletion of PI-3,4,5-P3 (Shenker et al., 2007).
The granulocyte-macrophage colony-stimulating factor (csf2 or gm-csf) was the most up-regulated gene (170.04 times) in gingival epithelial cells infected by A. actinomycetemcomitans. ELISA data confirmed the high expression of GM-CSF in infected gingival epithelial cells and in gingival tissues of aggressive periodontitis patients. Oral epithelial cells exposed to other bacteria, such as Fusobacterium nucleatum, had also shown a significant increase in the expression of CSF2 (17.96 times) (Zhang and Rudney, 2011), although not so drastic as seen for A.actinomycetemcomitans infected cells.
CSF2/GM-CSF plays an important role in survival, proliferation and differentiation of neutrophils and macrophages (Hamilton, 2008). However, this factor has also been identified as a key target of NF-kB, and mediates osteoclastic bone destruction (Park et al., 2007). It is expressed at higher levels at sites of inflammation, such as in arthritis (Nomura et al., 2008), and in human periodontal ligament cells, after orthodontic tooth movement (MacDonald et al., 1986).
Expression of GM-CSF is linked to the production of pro-inflammatory cytokines tumor-necrosis factor (TNF) and interleukin-1 (Hamilton, 2008). Both TNF-α and IL-1 were up-regulated in A. actinomycetemcomitans infected epithelial cells (28.93 fold change and 4.01 fold change respectively) and previous data revealed that both are significantly elevated in periodontal disease (Fukushima et al., 2005). IL-1 is the most potent mediator of bone resorption in vertebrates (Jimi et al., 1996) and promotes osteoclast formation by regulating RANKL/OPG expression (Fukushima et al., 2005).
TNF-α contributes to the pathogenicity of rheumatoid arthritis, periodontitis and other infections (Feldmann et al., 2001; Feldmann et al., 2003) by inducing pro-inflammatory chemoattractant cytokines cascades (O’Hara et al., 2009). TNF-α also activates signaling cascades required for NF-kB activation (Yamamoto &Takeda, 2008). Our data indicated that genes encoding factors induced by NF-kB activation such as CCL20, IL1A, ICAM1 and BIRC3 were also up-regulated in A. actinomycetemcomitans infected cells. Other evidences showing NF-kB activation in periodontal tissues came from studies showing that p50 and p65 transcription factors, which are components of the NF-κB complex, exhibited increased activity beneath periodontal lesions (Ambili et al., 2005). Furthermore, elevated NF-κB activity has been demonstrated in the pathogenesis of chronic inflammatory diseases associated with periodontitis and atherosclerosis (Nichols et al., 2001).
NF-kB activation influences bone resorption and osteoclast differentiation (Park et al., 2007) although it is involved in both protective and destructive responses (Spehlman & Eckmann, 2009).
Previous data have shown that interaction of epithelial cells with live A. actinomycetemcomitans for 6 hours resulted in the expression of IL-8 and intercellular adhesion molecule 1 (ICAM-1) (Huang et al, 1998). Infection with P. gingivalis results also in immediate up-regulation of IL-8 mRNA but down-regulation occurs simultaneously and is enhanced by the continuous presence of large number of bacteria (Huang et al., 2004). Thus, the lack of an up- regulation of IL-8 promoted by A. actinomycetemcomitans infection in the present study may be due to the longer exposure to live bacterial cells (24h).
Our data indicated that transcription of IL-1α, CCL20, GM-CSF, BIRC3 and TNF-α were induced in epithelial cell infected with A. actinomycetemcomitans. The same factors were also induced after interaction with P. gingivalis and F. nucleatum (Milward et al., 2007). However, infection with A. actinomycetemcomitans induced the expression of the intercellular adhesion molecule (ICAM-1), which was not reported for P. gingivalis and F. nucleatum (Milward et al., 2007). The elevated levels of ICAM-1 after interaction of epithelial cells with A. actinomycetemcomitans were confirmed in the cell supernatants, as also recently shown (Shimada et al., 2008). The production of ICAM-1 is also induced by TNF-α (Tancharoen et al., 2008) and its levels were associated with the severity of cardiovascular diseases (Lawson & Wolf, 2009). This adhesion molecule is involved in the trans-endothelial migration of leukocytes to sites of inflammation, in interactions between antigen presenting cells (APC) and T cells, and it may play a direct role in osteoclast formation and function (Bloemen et al., 2009).
Genes known to encode the transcription factors Erg 1, FOS and Jun were also positively transcribed in infected cells. Early growth response -1 (Egr-1), was the third most up-regulated gene (14.08 fold change) in infected cells. It should be noted that its expression was also induced in H. pylori infected gastric and colonic cell lines (Abdel-Latif et al., 2004) and it is involved in the activation of NF-kB in certain carcinomas (Wang et al., 2009).
Egr1 plays a role in the induction of the epithelial cell suppressor TGFβ1 and p53, which promote apoptosis (Baron et al.; 2006). In addition, the cell cycle inhibitor p21 (CIP1 or CDKN1A), which is regulated by Egr1 through direct induction of TGFβ1 (Polyak et al., 1994), was also up-regulated in A. actinomycetemcomitans infected cells.
Factors related to growth inhibition such as gadd45α (growth arrest and DNA damage-inducible) and p21 (encoded by cdkn1a gene) were positively regulated (8.0 fold change and 4.88 fold change, respectively) in A. actinomycetemcomitans infected cells. Gadd45α seems to be produced during stress stimuli and could lead to apoptosis. However, it is also involved in cellular survival, cell cycle arrest and DNA repair (Liebermann and Hoffmann, 2008). In hematopoietic cells exposed to UV radiation, Gadd45α cooperate to promote cell survival by two distinct signaling pathways involving activation of a novel Gadd45α mediated p38-NF-κB-mediated survival pathway (Gupta et al., 2006). A.actinomycetemcomitans CDTB is known for its DNase activity and cell cycle arrest (Shenker et al., 2007), which is compatible with the up-regulation of gadd45α involved in DNA repair. The lack of an apoptotic phenotype, as shown in the annexin assay, suggest that interaction with A.actinomycetemcomitans whole cells may result in DNA damage, which may be repaired, leading to cell survival.
Furthermore, infected cells exhibited higher levels (5.92 times) of transcripts of the antiapoptotic factor BIRC 3 (baculoviral IAP repeat-containing 3), which inhibits apoptosis by binding to tumor necrosis factor receptor-associated factors TRAF1 and TRAF2, probably by interfering with activation of ICE-like proteases (Garrison et al., 2009).
The fast turnover of epithelial cells in the non keratinized epithelium of the gingival crevice is a key factor in controlling the number of bacterial adherent cells. Therefore, the inhibition of cell cycle progression and decreased cell proliferation promoted by A. actinomycetemcomitans would probably maintain the colonizing internalized or adherent bacteria for longer periods.
Transcription of CCL2, encoding the inflammatory chemokine monocyte chemoattractant protein-1 (CCL2/MCP-1) was up-regulated (12 times) in A. actinomycetemcomitans infected gingival epithelial cells. High levels of MCP-1 were shown in the crevicular fluid of periodontitis patients but not in healthy controls (Praddep et al., 2009). MCP-1 is typically expressed by monocytes/macrophages, but can also be produced by other cells, including epithelial cells, induced by the proinflammatory mediators TNF- α, IL-1, as well as by endotoxin and internalized bacteria (Jia et al., 2008). In addition to chemotactic activity for leukocytes, MCP-1 also plays a role in modulation of cell proliferation, apoptosis, angiogenesis and bone remodeling (Yadav et al., 2010). Recent study revealed that P. gingivalis was able to induce MCP-1 expression in human coronary artery endothelial cells induced by the transcription factor Erg1 in concert with other transcription factors and cytokines (Maekawa et al., 2010). On the other hand, Streptococcus gordonii, a commensal organism frequently isolated from healthy subjects (Aas et al., 2005), had no significant effect on the cytokine response, except for IL-8, and induced a minimal chemokine response in gingival epithelial cells, much lower than P. gingivalis, A. actinomycetemcomitans and F. nucleatum. Thus, commensals trigger a minimal inflammatory response (Stathopoulou et al., 2010), while A. actinomycetemcomitans behaved as true pathogen, eliciting a strong inflammatory response.
The ELISA data in gingival tissues confirmed the in vitro data. However, subgingival A. actinomycetemcomitans levels were not correlated with levels of pro-inflammatory cytokines or ICAM-1 levels in gingival tissues. These data may be interpreted with caution, due to the multiple bacteria colonizing the subgingival sites.
Local inflammatory immune reactions of the host in response to periodontal pathogens seem to be decisive to protect the host against infection (Garlet et al., 2003), but increased gene expression of inflammatory mediators persistent at an inflammatory site may result in pathological alterations on host tissues.
In conclusion, our results showed that interaction between viable A. actinomycetemcomitans and epithelial cells results in transcription and expression of factors involved in immune cells recruitment and differentiation, including osteoclastogenesis, and these factors were shown in high levels in gingival tissues from periodontitis patients.
Acknowledgments
We thank D. F. Kinane for providing OBA-9 cells and Johnah Galicia for help us technical suggestions.
This study was supported by FAPESP 03/08598-0 and 05/58903-0 and Public Health Service grant RO1DE14605 from the NIDCR.
We thank Rosana Prisco for help in the statistical analysis.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif MMM, Windle HJ, Fitzgerald KA, Ang YS, NíEidhin D, Li-Weber M, Sabra K, Kelleher D. Helicobacter pylori activates the early growth response 1 protein in gastric epithelial cells. Infect Immun. 2004;72:3549–3560. doi: 10.1128/IAI.72.6.3549-3560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambili R, Santhi WS, Janam P, Nandakumar K, Pilai MR. Expression of activated transcription factor nuclear factor-kappaB in periodontally diseased tissues. J Periodontol. 2005;76:1148–1153. doi: 10.1902/jop.2005.76.7.1148. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr-1 is a direct regulator of multiple tumor suppressors including TGFβ-1, PTEN, p53 and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen V, Vries TJ, Shoenmaker T, Everts V. Intercellular adhesion molecule-1 clusters during osteoclastogenesis. Biochem Biophys Res Commun. 2009;385:640–645. doi: 10.1016/j.bbrc.2009.05.145. [DOI] [PubMed] [Google Scholar]
- Dale BA. Periodontal epithelium: a newly recognized role in health and disease. Periodontol 2000. 2002;30:70–8. doi: 10.1034/j.1600-0757.2002.03007.x. [DOI] [PubMed] [Google Scholar]
- Faveri M, Figueiredo LC, Duarte PM, Mestnik MJ, Mayer MPA, Feres M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol. 2009;36:739–749. doi: 10.1111/j.1600-051X.2009.01449.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Foxwell BM, Maini RN. The role of TNF-α and IL-1 in rheumatoid arthritis. Curr Dir Autoimmun. 2001;3:188–199. doi: 10.1159/000060522. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Lasker Clinical Medical Research Award: TNF defined as a therapeutic target for a rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Jimi E, Okamoto F, Motokawa W, Okabe K. IL-1-induced receptor activator of NF-kB ligand in human periodontal ligament cells involves ERK-dependent PGE2 production. Bone. 2005;36:267–275. doi: 10.1016/j.bone.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Martins W, Jr, Ferreira BR, Mailanezi CM, Silva JS. Patterns of chemokines and chemokines receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003;38:210–217. doi: 10.1034/j.1600-0765.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- Garrison JB, Samuel T, Reed JC. TRAF2-binding BIR1 domain of c-IAP2/MALT1 fusion protein is essential for activation of NF-kB. Oncogene. 2009;28:1584–1593. doi: 10.1038/onc.2009.17. [DOI] [PubMed] [Google Scholar]
- Gupta M, Gupta SK, Hoffman B, Liebermann DA. Gadd45a and gadd45b Protect Hematopoietic Cells From UV Induced Apoptosis Via Distinct Signaling Pathways including p38 activation and JNK inhibition. J Biol Chem. 2006;281:17552–17558. doi: 10.1074/jbc.M600950200. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Huang GTJ, Kinder Haake S, Kim JW, Park NH. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral Microbiol Immunol. 1998;13:301–309. doi: 10.1111/j.1399-302x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Huang TJG, Zhang HB, Dang HN, Haake SK. Differential regulation of cytokines genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microb Pathog. 2004;37:303–312. doi: 10.1016/j.micpath.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Jia T, Serbina NV, Brandl K, et al. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Ikebe T, Takahashi N, Hirata M, Suda T, Koga T. Interleulin-1α activates an NF-kB-like factor in osteoclast-like cells. J Biol Chem. 1996;271:4605–4608. doi: 10.1074/jbc.271.9.4605. [DOI] [PubMed] [Google Scholar]
- Kajiya M, Komatsuzawa H, Papantonakis A, Seki M, Makihira S, Ouhara K, Kusumoto Y, Murakami S, Taubman MA, Kawai T. Aggregatibacter actinomicetemcomitansOmp29 is associated with bacterial entry to gingival epithelial cells by F-actin rearrangement. PLos One. 2011;6:e18287. doi: 10.1371/journal.pone.0018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkert R, Laine ML, Aarden LA, Van Winkelhoff AJ. Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol Immunol. 2007;22:145–151. doi: 10.1111/j.1399-302X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Laube DM, Dongari-Bagtzoglou A, Kashleva H, Eskadale J, Gallagher G, Diamond G. Differential regulation of innate immune response genes in gingival epithelial cells stimulated with Aggregatibacter actinomycetemcomitans. J Periodontol Res. 2008;43:116–123. doi: 10.1111/j.1600-0765.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B. Gadd45 in stress signaling. J Mol Signal. 2008;12:3–15. doi: 10.1186/1750-2187-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BR, Mundy GR, Clark S, et al. Effects of human recombinant CSCM and highly purified CSF-1 on the formation of multi-nucleated cells with osteoclasts characteristics in long term bone marrow cultures. J Bone Min Res. 1986;1:977–985. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- Maekawa Y, Takahashi N, Honda T, Yonezawa D, Miyashita H, Okui T, Tabeta K, Yamasaki K. Porphyromonas gingivalis antigens and interleukin-6 stimulate the production of monocyte chemoattractant protein-1 via the upregulation of early growth response-1 transcription in human coronary artery endothelial cells. J Vasc Res. 2010;47:346–354. doi: 10.1159/000265568. [DOI] [PubMed] [Google Scholar]
- Meyer DH, Lippmann JE, Fives-Taylor Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect Immun. 1996;64:2988–2997. doi: 10.1128/iai.64.8.2988-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward MR, Chapple ILC, Wright HJ, Millard JL, Matthews JB, Cooper PR. Differential activation of NF-kB and gene expression in oral epithelial cells by periodontal pathogens. Clin Exp Immunol. 2007;148:307–324. doi: 10.1111/j.1365-2249.2007.03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle I, Rau J, Ruskin J. Vertebral osteomyelitis due to Actinobacillus actinomycetemcomitans. JAMA. 1979;241:1824–1825. [PubMed] [Google Scholar]
- Nichols TC, Fischer TH, Deljarqvris EN, Baldwin AS., Jr Role of nuclear factor-kappa B (NF-kappa B) inflammation, periodontits and atherogenesis. Ann Periodontol. 2001;6:20–29. doi: 10.1902/annals.2001.6.1.20. [DOI] [PubMed] [Google Scholar]
- Nomura K, Kuroda S, Yoshikawa H, Tomita T. Inflammatory osteoclastogenisis can be induced by GM-CSF and activated under TNF immunity. Bioch Biophysical Res Comm. 2008;367:881–887. doi: 10.1016/j.bbrc.2008.01.023. [DOI] [PubMed] [Google Scholar]
- O’Hara AM, Bhattacharyya A, Bai J, Mifflin RC, Ernst PB, Mitra S, Crowe SE. Tumor necrosis factor (TNF)-α induced IL-8 expression in gastric epithelial cells: Role of reactive oxygen species and AD endonuclease-1/redox factor (Ref)-1. Cytokine. 2009;46:359–369. doi: 10.1016/j.cyto.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. Actinobacillus actinomycetemcomitans endocarditis. Clin Microbiol Infect. 2004;10:98–118. doi: 10.1111/j.1469-0691.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Thomas G, Gu K, Shah V, Pei L, Zarbo RJ, McCauley L, Shi S, Chen S, Wang CY. NF-kB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, et al. p27kip1, a cyclin-cdk inhibitor, links transforming growth factor-beta and contact and inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Pradepp AR, Daisy H, Hadge P, Grarg G, Thorat M. Correlation of gingival crevicular fluid interleukin-18 and monocyte chemoattractant protein-1 levels in periodontal health and disease. J Periodontol. 2009;80:1454–1461. doi: 10.1902/jop.2009.090117. [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Sheen J. Intercepting host MAPK signaling cascades by bacterial type III efectors. Cell Host Microbe. 2007;1:167–174. doi: 10.1016/j.chom.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Dlakic M, Walker LP, Besack D, Jaffe E, LaBelle E, Boesze-Battaglia K. A novel mode of activation for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol 3,4,5-triphosphate phosphatase activity. J Immunol. 2007;178:5099–5108. doi: 10.4049/jimmunol.178.8.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Sugano N, Nishihara R, Suzuki K, Tanaka H, Ito K. Differential effects of five Aggregatibacter actinomycetemcomitans strains on gingival epithelial cells. Oral Microbiol Immunol. 2008;23:455–458. doi: 10.1111/j.1399-302X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- Silva N, Dutzan N, Hernandez M, Dezerega A, Rivera O, Aquillon JC, Aravena O, Lastres P, Pozo P, Vernal R, Garmonal J. Characterization of progressive lesions in chronic periodontits patients: levels of chemokines, cytokines, matrix metalloproteinase-13, periodontal pathogens and inflammatory cells. J Clin Periodontol. 2008;35:206–214. doi: 10.1111/j.1600-051X.2007.01190.x. [DOI] [PubMed] [Google Scholar]
- Slots J, Reynolds HS, Genco R. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross sectional microbiological investigation. Infect Immun. 1980;29:1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehlmann ME, Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction. Curr Opin Gastroenterol. 2009;25:92–99. doi: 10.1097/MOG.0b013e328324f857. [DOI] [PubMed] [Google Scholar]
- Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. Epithelial cell proinflammatory cytokine response differs across dental bacterial species. J Clin Periodontol. 2010;37:24–29. doi: 10.1111/j.1600-051X.2009.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancharoen S, Matsuyama T, Abeyama K, Matsushita K, Kawahara K, Sangalungkarn V, Tokuda M, Hashiguchi T, Maruyama I, Izumi Y. The role of water channel aquaporin 3 in the mechanism of TNF-α-mediated proinflammatory events: implication in periodontal inflammation. J Cell Physiol. 2008;217:338–349. doi: 10.1002/jcp.21506. [DOI] [PubMed] [Google Scholar]
- Vermes AI, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using uorescein labeled annexinV. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Wang B, Khachigian LM, Esau L, Birrer MJ, Zhao X, Parker MI, Hendricks DT. A key role for early growth response-1 and nuclear factor-κb in mediating and maintaining gro/cxcr2 proliferative signaling in esophageal cancer. Mol Cancer Res. 2009;7:755–764. doi: 10.1158/1541-7786.MCR-08-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Saini V, Sarika A. MCP-1: Chemoattractant with a role beyond immunity: A review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Takeda K. Role of nuclear IkB proteins in the regulation of immune responses. J Infect Chemother. 2008;14:265–269. doi: 10.1007/s10156-008-0619-y. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Yoshikawa T. Basic and translational research on proteinase-activated receptors: implication of proteinase/proteinase-activated receptor in gastrointestinal inflammation. J Pharmacol Sci. 2008;108:415–421. doi: 10.1254/jphs.08r31fm. [DOI] [PubMed] [Google Scholar]
- Zhang G, Rudney JD. Streptococcus cristatus attenuates Fusobacterium nucleatum-induced cytokine expression by influencing pathways converging on nuclear factor-kB. Mol Oral Microbiol. 2011;26:150–163. doi: 10.1111/j.2041-1014.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]