Abstract
The Krüppel-like factor (KLF) family of transcription factors plays an important role in differentiation, function and homeostasis of many cell types. While their role in lymphocytes is still being determined, it is clear that these factors influence processes as varied as lymphocyte quiescence, trafficking, differentiation and function. This review will present an overview of how these factors operate and coordinate with each other in lymphocyte regulation.
Introduction
The founding member of the KLF family was found to regulate development in Drosophila melanogaster, mutants in this gene leading to embryonic deformity (1, 2), hence the name “Kruppel” (meaning cripple in German). All KLFs (which are related to the sp1 family of transcription factors) share a highly homologous set of three DNA-binding zinc fingers at the C-terminus that imparts specificity for CACCC boxes and related GC-rich regions (3, 4). Individual factors are distinguished by regions that dictate regulation and binding partner specificity. There are at least 17 KLFs in mammals, and they have been implicated in numerous biological processes, especially in the context of cell differentiation and quiescence. For example, KLF1 (also called EKLF) is critical for the switch to adult hemoglobin expression in developing erythrocytes, while KLF4 (GKLF) is well studied as a factor involved in reprogramming mature cells to become induced pluripotent stem cells (3, 4).
Interest in the KLF family among immunologists was sparked by a report from Leiden’s group which showed that deficiency for KLF2 (LKLF) caused upregulation of T cell activation markers and a dramatic loss of peripheral T cells (5), leading to the hypothesis that KLF2 enforced naïve T cell quiescence (5–7). However, as we discuss next, further studies on KLF2’s function have lead to reinterpretation of its function. This serves as a good example of how the varied activities of KLFs in lymphocyte biology can confound simple characterization of their role.
While the function of individual KLFs is still being deciphered, some general principles are emerging. One is that KLFs participate in multiple aspects of lymphocyte differentiation, trafficking and function, especially in the context of regulating late stages of maturation. Another is the appreciation that distinct KLFs may balance each other in control of certain differentiation steps – this is illustrated by the reciprocal effects of KLF2 and KLF13 deficiency in NKT cells differentiation, and by examples of both cooperation and antagonism in the control of B cell subset differentiation by KLF2 and KLF3. The well characterized functions of KLFs are illustrated in Figure 1, but it is likely that we have only scratched the surface of regulation by this versatile family of transcription factors.
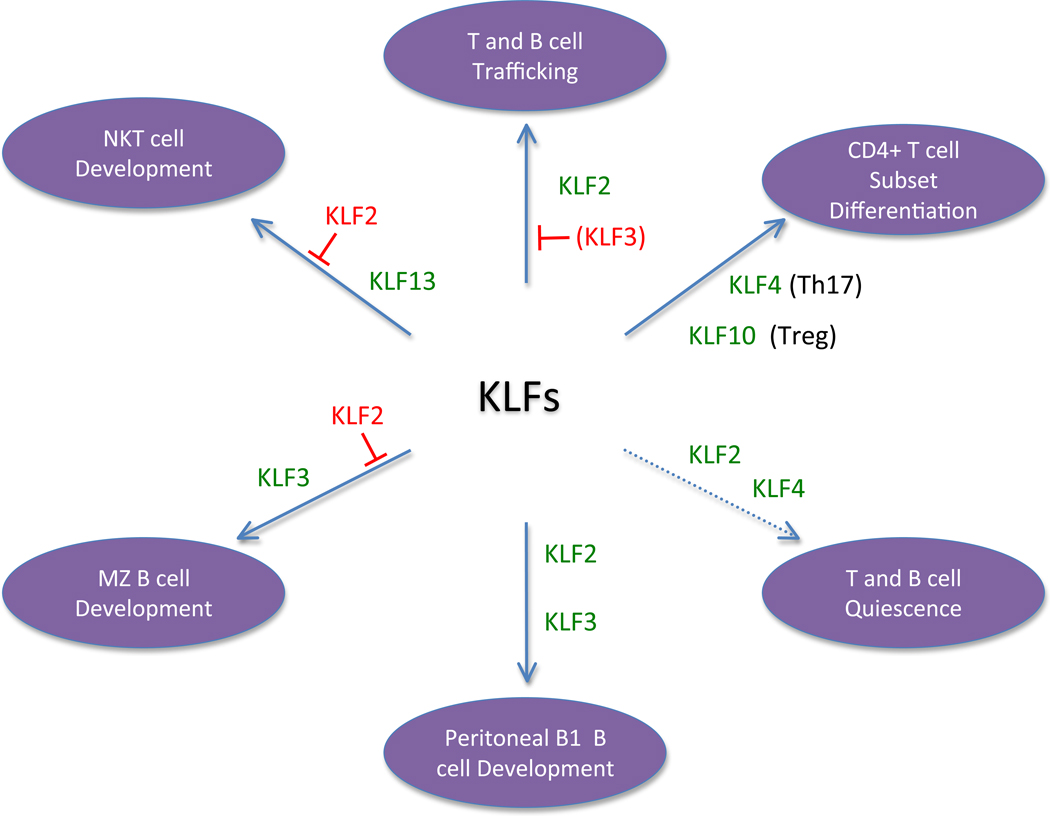
Figure 1. KLFs regulate numerous processes in lymphocytes.
The figure illustrates known roles of KLFs in various aspects of lymphocyte differentiation and function. The KLFs written in green promote the indicated activity, while KLFs indicted by red text inhibit the response. Note that KLFs expressed in the same cell may cooperate or antagonize with each other. The role of KLF2 and KLF4 in control of T cell quiescence is shown with a dotted line, since the physiological significance of this pathway is unclear. The capacity of KLF3 to oppose KLF2 in control of some lymphocyte trafficking molecules has been shown in B cells but not yet addressed in T cells, hence KLF3 is written in parenthesis. Details of individual functions are provided in the text.
Lymphocyte Quiescence
T cells
KLF2 is induced late during maturation of thymocytes, and is maintained in peripheral naïve T cells (5, 8–11). Upon T cell activation, KLF2 expression is lost, a process which is thought to initiate with KLF2 protein degradation (probably involving ubiquitination) and subsequent loss of KLF2 mRNA (5, 7, 12–15). Re-expression of KLF2 occurs late in the effector phase of the CD8 T cell response, as memory cells begin to differentiate (a process which can be directed by appropriate cytokines) (5, 7, 12–15). These expression patterns, together with data showing that KLF2 deficient thymocytes display activation markers suggested KLF2 was important for maintaining naïve T cell quiescence: In this model, the loss of KLF2 induced inappropriate activation of mature thymocytes and their subsequent cell death (5, 6). This idea was reinforced by studies indicating that KLF2 inhibits cell cycle progression – as dramatically shown by the capacity of KLF2 overexpression to halt tumor cell line proliferation and by evidence that KLF2 inhibits expression of c-myc while promoting transcription of p21WAF1/CIP1 (7, 16–19). KLF4, a close relative of KLF2, is also downregulated upon T cell activation (20), and it is interesting to note that KLF4 deficient memory CD8+ T cells show a gradual increase in representation over time, in keeping with a role for KLF4 in restraining cell cycle progression (20).
Subsequent data has led to re-evaluation of the role played by KLFs in quiescence, however. For example, the deficit of naïve KLF2−/− T cells in peripheral lymphoid sites is explained by KLF2’s role in lymphocyte trafficking rather than spontaneous cell death (8, 15), and the immune response of KLF2−/− CD8+ T cells shows normal magnitude and kinetic characteristics (13).
But what of the spontaneous activated phenotype of KLF2-deficient T cells? This is especially marked on KLF2−/− CD8+ thymocytes, which show upregulation of markers such as CXCR3, CD44 and CD122 typically observed on memory cells, and CD69 expression which is normally induced upon activation (5, 8, 21–23). It has become clear that the acquisition of memory markers on CD8+ T cells is actually due to production of IL-4 from an expanded population of KLF2−/− NKT cells (22, 23), and that this activated phenotype is not autonomous to KLF2 deficiency. Indeed, KLF2-deficient thymocytes maturing in the absence of the aberrant NKT cell population did not upregulate memory markers (22, 23), showing that the KFL2 does not itself cause a loss of the naïve, quiescent T cell phenotype.
We will return to the specific roles of KLF2 in T cell trafficking and differentiation later, but highlighting these points here is important to illustrate that what appeared to be a clear-cut role of KLF2 in quiescence regulation could be explained by a composite of different functions.
At the same time, overexpression studies clearly show that KLF2 can inhibit cell cycle progression, and hence we should not completely exclude its potential for control of quiescence. At issue is the function of KLF2 expressed at physiological levels. Studies on activated CD8+ T cells provides a useful opportunity to test how normal KLF2 re-induction influences expression of cell cycle regulatory factors. Interestingly, expression of c-myc and p21CIP1 mRNA were found to be independent of KLF2 expression in post-activated CD8+ T cells, and KLF2 deficiency did not lead to dysregulated T cell expansion in vivo (13). Such findings do not rule out redundancy between distinct KLFs for control of cell cycle progression in T cells, or that KLF2 (and/or related KLFs) enforce T cell quiescence under certain circumstances. However, in contrast with more clear-cut examples of factors regulating naïve T cell quiescence (such as recent studies on Foxp1 (24, 25)), the role of KLFs is currently unclear.
B cells
KLFs have also been proposed to control B cell quiescence. As for T cells, KLF2 and KLF4 are expressed in naïve B cells and are downregulated with B cell activation (26–28). KLF4 overexpression was shown to induce cell cycle arrest of transformed mouse pro-/pre-B cell lines (29), while forced expression of KLF4 or KLF9 caused reduced proliferation of naïve and memory human B cells following stimulation through the B cell receptor (BCR) or CD40(28). Since expression of KLF4 and KLF9 were found to be lower in memory versus naïve B cells, these data offer a potential explanation for the superior proliferation of memory B cells (28). On the other hand, Klaewsongkram et al reported that KLF4 deficiency also causes a block in B cell proliferation (26). KLF4−/− B cells (activated by BCR/CD40) exhibited decreased progression through the cell cycle: this correlated with reduced expression of cyclin D2 and the finding that KLF4 directly binds the cyclin D2 promoter(26). The apparent contradictions between these reports reinforces the emerging concept that the function of KLF4 (and perhaps other KLFs) depends on the context of other cell-cycle-regulatory factors (30). This adds to the difficulty in determining the significance of KLFs for enforcing lymphocyte quiescence.
Overall then, while several KLFs have been shown to have the potential to strongly limit lymphocyte cycle entry, their physiological role in controlling quiescence is not well defined.
Lymphocyte trafficking
T cells
In order to exit the thymus and peripheral lymphoid tissues, mature CD4+ and CD8+ T cells need to express sphingosine 1-phosphate receptor 1 (S1PR1), which detects the high levels of S1P maintained in blood and lymph (31, 32). We and others found that KLF2 is required for thymocyte expression of S1PR1 (8, 15), suggesting that the lack of peripheral T cells in KLF2-deficient mice may arise as a consequence of thymic retention (8). Indeed, the accumulation of mature KLF2-deficient T cells in the thymus is prevented by over-expression of S1PR1 (33), suggesting this factor is the key target of KLF2 for thymic emigration. Incidentally, the upregulation of CD69 on KLF2−/− thymocytes can also be attributed to impaired S1PR1 expression, since basal cell surface expression of CD69 is restrained by a protein-protein interaction with S1PR1 (34, 35). In addition, KLF2 directly promotes expression of L-selectin (CD62L), relevant for access of naïve T cells to lymph nodes (8, 15), indicating that this KLF coordinates distinct aspects of lymphocyte trafficking. While those studies focused on TCRαβ T cells, similar changes in trafficking molecule expression were observed for TCRγδ T cells (36).
KLF2 deficient thymocytes also show depressed levels of β7 integrin and elevated expression of the CXCR3 chemokine receptor (8, 21), yet subsequent studies suggest that these changes are induced by IL-4 (produced by KLF2 deficient NKT cells), not a consequence of direct gene control by KLF2 (22, 23).
KLF2 is constitutively expressed in naïve T cells but rapidly extinguished following T cell stimulation, a situation which may help retain activated T cells in lymphoid sites through transcriptional downregulation of S1PR1 (12–15). Likewise, KLF2 re-expression in post-activated CD8+ T cells is necessary for S1PR1 and CD62L re-expression (13, 15), and this may be key to allow late effector CD8+ T cells to begin recirculation again.
Such studies on the trafficking role of KLF2 have helped define signaling pathways which control its expression. A key mechanism in regulation of KLF2 expression involves PI3K signaling. Strong induction of this pathway leads to activation of AKT, which in turn induces sequestration of the transcription factor FOXO1 outside the nucleus (37). Foxo1 promotes expression of KLF2 in mature T cells (38–40), and additional FOXO factors may also contribute to this regulation (37). As has been discussed in a recent review, this pathway provides a mechanism by which the strength of PI3K signaling can directly regulate T cell trafficking mediated, at least in part, by control of KLF2 (41). Cytokines play a central role in dictating KLF2 regulation: For activated mouse CD8+ T cells, high dose IL-2 promotes efficient PI3K activation, leading to low KLF2 expression, while exposure to IL-7, IL-15 or weaker IL-2R signals permits KLF2 re-expression (13–15, 42). Also, cytokines involved in active effector differentiation, such as IL-12 and IL-4, act to acutely repress KLF2 re-expression (14, 15, 43, 44). The signaling pathways involved in these processes are not well defined, although JAK/STAT signaling appear to be critical (15, 43, 44). An emerging picture is that changes in the cytokine milieu that an activated T cell encounters has a dramatic effect on whether the T cell is equipped to traffic (via KLF2 reexpression and subsequent induction of S1PR1) or whether the cell is retained in its local tissue. Indeed, recent studies have indicated that Th2 cells utilize a specialized mechanism to initiate trafficking from lymph nodes. Th2 cells producing the protein ECM-1 which attenuates IL-2R signaling allowing for expression of KLF2 and S1PR1, allowing for successful egress from peripheral lymphoid tissues (45).
B cells
Since S1PR1 is also required for recirculation of B cells and, like T cells, B cells downregulate KLF2 after activation, it would be reasonable to predict that KLF2 induces S1PR1 expression in the B cell pool. However, three recent studies describing B cell specific KLF2 deficient mice reached the surprising conclusion that S1PR1 expression and function on follicular B cells was minimally altered (46, 47) or actually enhanced (48) by KLF2 loss. Whether distinct KLFs regulate S1PR1 in B cells is unclear, but the role of KLF2 is clearly different in T and follicular B cells. At the same time, the representation of KLF2-deficient B cells in the blood was significantly reduced, arguing for a partial effect on follicular B cell recirculation. KLF2 deficient follicular B cells also exhibited reduced CD62L and α4β7 integrin expression (46, 47), mirroring the phenotypic changes observed in KFL2−/− mature thymocytes (8). In one study, it was proposed that KLF2 loss also leads to reciprocal patterns of dysregulated CXCR5 expression by follicular and marginal zone B cells (leading to mislocalization of FO cells in the marginal zone), and that KLF2 is required for S1PR1 expression by MZ but not FO B cells (48). These findings were not confirmed in other reports, however(46, 47) and hence further studies will be needed to test the intriguing idea that KLF2 exerts distinct effects on trafficking molecule expression in different B cell subsets.
Interestingly, manipulation of another KLF family member, KLF3 mirrors some phenotypic changes observed in KLF2 deficient B cells. For example, a subset of KLF3 transgenic follicular B cells expressed reduced CD62L levels (while expression and function of CXCR5 and S1PR1 was not affected) (49). Likewise, KLF3 deficient MZ B cells demonstrate elevated expression of CD62L and β7-integrin (50). Together these data suggest KLF3 and KLF2 may exert opposing functions on these aspects of B cell trafficking (Fig 1).
Lymphocyte Development
Studies on KLF factors have suggested a minimal role in early lymphocyte development – exceptions being a proposed role for KLF5 in germline transcription from the Dβ1 promoter in thymocytes (51) and data showing that KLF4 deficiency leads to slightly decreased Pre-B cell numbers (26). However, recent work has revealed significant roles for KLFs in differentiation of mature T and B cell subsets.
NKT and TCRγδ T cells
It has become clear that distinct KLFs promote and oppose differentiation of NKT cells during thymic differentiation. Loss of KLF2 leads to an increase in thymic CD1d-restricted iNKT cells (23). In addition, KLF2-deficient mice have an exaggerated representation of an unusual subset of TCRγδ T cells that utilize Vδ6.3/2 and bear the CD4 coreceptor (36). Like iNKT cells these non-conventional TCRγδ T cells express the transcription factor PLZF, and hence have been termed γδ-NKT. The altered representation of these populations is not simply a consequence of impaired S1PR1 expression by KLF2−/− T cells, because the frequency of these subsets is not enhanced in S1PR1−/− animals (23, 36). However, other genetic defects (including loss of Itk, CBP and forced expression of Class II MHC molecules on mouse thymocytes) leads to a similar enhancement of PLZF+ iNKT and/or γδ-NKT cells (23, 52).
A surprising consequence of the enhanced production of thymic iNKT cells is the appearance of memory-phenotype T cells (especially CD8+ T cells) in the KLF2 deficient thymus(22, 23). Through a series of studies, this effect was found to result from PLZF+ NKT cell production of IL-4, which acted directly on mature thymocytes, leading to upregulation of the transcription factor Eomes and acquisition of memory markers (22, 23, 52). Hence, this striking change in thymocyte differentiation is not a direct effect of KLF2 deficiency, but reflects a non-autonomous effect. Such findings are useful to underscore the difficulty in dissecting direct and indirect effects of KLF manipulation.
Interestingly, the expansion of PLZF+ NKT cells (and attendant IL-4-mediated bystander effect) is evident in some normal mouse strains (e.g. Balb/c) but not others (e.g. C57BL/6) (23). Recent studies show that a different KLF, KLF13 is also involved in this phenomena (53). KLF13 first came to prominence as a transcription factor induced late in T cell activation, which was found to be critical for induction of the chemokine RANTES (CCL5) and regulation of T cell apoptosis (54, 55). Newer studies show that KLF13 also mediates control of PLZF+ NKT thymocyte development, as revealed by the reduced numbers of such cells (and the absence of bystander memory-phenotype CD8+ thymocytes) in KLF13 deficient Balb/c mice (53). While KLF2 and KLF13 deficiency have reciprocal effects on PLZF+ thymocyte differentiation, it is not clear whether and how these factors intersect. KLF13 loss leads to reduced PLZF expression levels in thymic iNKT, and this could influence the efficiency of their development (53). However, KLF2 loss does not appear to change PLZF expression levels (23), and it is possible that the capacity of KLF2 to restrain proliferation acts to contain the size of the PLZF+ thymocyte pool. Regardless, these findings serve as another example of the way in different KLF family members intervene in lymphocyte differentiation programs.
B cells subsets
Recent studies suggest that KLF2 and KLF3 have opposing roles in the development of mature B cell subsets. The three major mature B cell subsets – follicular (FO), marginal zone (MZ), and B1 B cells -- show distinct phenotypic, functional and tissue localization characteristics. While the signals that determine B cell subset differentiation are incompletely defined, some transcription factors have been implicated in dictating FO, MZ or B1 generation. For example, Notch 2 and Aiolos are required for differentiation of MZ B cells (56). KLF2 shows an intriguing pattern of gene expression in B cell lineage cells, being uniformly expressed at the large pre-B cell stage(46, 57) but then showing differential expression in mature B cells, following the order MZ<FO<B1 (46, 47). This heterogeneity in expression correlates with a profound impact on B cell subset generation in KLF2-deficient animals(46–48). Using B cell specific conditional knockout approaches, loss of KLF2 was shown to have a relatively modest effect on the FO pool, but lead to an increase in the size of the MZ subset, while the peritoneal B1 population was severely depleted (46–48). Changes in the splenic transitional and marginal zone precursor populations of B cells suggest these effects reflect a role of KLF2 in B cell subset differentiation, although an impact on homeostasis has not been ruled out. Furthermore, it is currently unclear whether KLF2 deficiency causes a loss of B1 cells or alterations in their trafficking(46–48).
KLF3 may play a reciprocal role to KLF2 in controlling the differentiation/homeostasis of certain B cell subsets. KLF3 overexpression leads to substantial enhancement of MZ B cell numbers (49), while the opposite phenotype has been observed in KLF3 knockout mice (50). The capacity of KLF3 overexpression to promote MZ B cell generation was sufficiently strong as to overcome CD19 deficiency and blockade of the BAFF-family factor TACI (both of which are important for MZ cell development/survival) but could not substitute for the loss of Notch2, suggesting KLF3 is not downstream of Notch2 (49). The finding that KLF2 loss leads to increased KLF3 expression is consistent with the idea that the two factors play antagonistic roles in B cell subset differentiation. Interestingly, gene expression studies indicate that KLF2-deficient (and also KLF3 transgenic) FO B cells bear some transcriptional characteristics of MZ B cells, suggesting that the regulation of KLF2 and KLF3 is important for dictating FO B cell “identity” (46, 47, 49). Whether these differentiation effects relate to alterations in BCR signaling (as has been proposed previously (58, 59)) and/or altered trafficking remain to be seen. On the other hand, development or maintenance of B1 B cells evidently involves quite distinct regulation, since deficiency for either KLF2 or KLF3 caused a reduction in the size of the peritoneal B1 pool, while KLF3 overexpression leads to enhanced B1 B cell frequencies (46–50). Regulation between KLFs has been proposed to operate in quite complex networks (4, 50, 60), and the activity of KLF2 and KLF3 in control of B cell subset differentiation appears a good example of how factor may have cooperative or opposite roles even within the same lymphocyte lineage (Fig 1).
Activated lymphocyte differentiation
In addition to alterations in lymphocyte development, it has become clear that KLFs may also influence expansion and differentiation of activated T and B cells.
Treg
Foxp3-expressing regulatory CD4+ T cells (T regs) are vital for protecting against autoagressive responses, and TGF-β is crucial for differentiation of a least some Treg populations (61). KLF10, is rapidly induced by TGF-β (hence the alternative name of KLF10 as TGF-β induced early gene 1, or TIEG1) and is important for maturation and function of Tregs. KLF10 deficiency lead to reduced differentiation of Foxp3+ CD4+ T cells, and impaired capacity of these cells to suppress airway inflammation in vivo (62). Complementary studies showed that KLF10 overexpression induces CD4+, CD25(−) cells to express TGF-β1 and FOXP3 (63). KLF10 activity hinges on differential ubiquitination, with monoubiquination (involving the E3 ligase Itch) being necessary for KLF10 to induce Foxp3 transcription, while polyubiquitination of KLF10 (as occurs downstream of IL-6 signals) leads to KLF10 exclusion from the nucleus (62, 64).
Th17
Another family member, KLF4, has recently been implicated in differentiation of Th17 CD4+ T cells, which are significant for their role in control of certain extracellular bacteria as well as autoimmune diseases (65, 66). KLF4 was found to be necessary for Th17 cell differentiation in vitro, and in an adoptive transfer EAE model, and ChIP assays revealed that KLF4 occupies the promoter of the IL-17 gene(67, 68). How KLF4 integrates with other transcription factors that dictate Th17 differentiation (including RORγt, RORα and STAT3 (65, 66)) is not yet clear, and neither is it known whether KLF4 plays a similar role in control of CD8+ Tc17 cells.
Recent studies have reported that HIF-1α is important for the differentiation of Th17 and Treg cells(69). Interestingly, studies in myeloid cells indicate KLF2 can act as a negative regulator for HIF-1α expression(70) and it will be important to determine whether KLF2 has any similar activity in lymphocytes.
B cell activation
Studies on KLF2 deficient B cells indicated they were compromised in the proliferation, survival, and maintenance of responding follicular B cells (46–48). Following in vitro anti-BCR stimulation, KLF2 deficient follicular B cells exhibited impaired proliferation and a substantial survival defect, a situation that could be corrected by simultaneous stimulation of BCR and CD40(46). As CD40 engagement is potent at activation of NF-kB signals(71), this pathway may be promising for further investigations into the role of KLF2 in B cell priming. Following in vivo priming for T dependent responses, Wilkelmann et al reported a reduction in antigen specific immunoglobulin, a result that may be partially explained by fewer KLF2−/− plasma cells/antibody secreting cells in the bone marrow (47). Whether this relates to compromised survival of activated B cells, or relates to the proposed relevance of KLF2 in migration of plasma cells to the bone marrow (72) is unclear.
Conclusions
It is becoming clear that KLFs play multiple and varied roles in lymphocyte development and function, and that individual KLF family members may reinforce or antagonize each others’ functions. However, substantial questions remain, based partly on the complexity of this family. While mRNA expression of individual KLFs within lymphocyte subsets can be readily determined, post-transcriptional regulation of KLFs has been observed in several systems, making it harder to predict functional KLF protein expression. The fact that many KLFs share a similar DNA binding site motif, together with the shortage of studies (such as ChIP) to define which genes are actually occupied by a given factor, means that there is a dearth of information on identification and characterization of key transcriptional targets. Redundancy and competition between KLFs for gene regulation adds yet another layer of complexity to understanding their roles. Still, as exemplified by the burst of information over the last few years, we are approaching a renaissance in understanding the functions and significance of KLFs in lymphocyte biology.
Acknowledgments
We acknowledge Jamequist lab members, past and present, for lively discussions and thank Merlin Crossley and Richard Pearson for communicating data ahead of publication.
Footnotes
This work was supported by the NIH (AI R3738903 to SCJ).
References
- 1.Wieschaus E, Nusslein-Volhard C, Kluding H. Kruppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Developmental biology. 1984;104:172–186. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 2.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 3.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiological reviews. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. The international journal of biochemistry & cell biology. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science (New York, N.Y. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 6.Di Santo JP. Lung Krupple-like factor: a quintessential player in T cell quiescence. Nature immunology. 2001;2:667–668. doi: 10.1038/90598. [DOI] [PubMed] [Google Scholar]
- 7.Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc--dependent pathway. Nature immunology. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 8.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 9.Endrizzi BT, Jameson SC. Differential role for IL-7 in inducing lung Kruppel-like factor (Kruppel-like factor 2) expression by naive versus activated T cells. International immunology. 2003;15:1341–1348. doi: 10.1093/intimm/dxg133. [DOI] [PubMed] [Google Scholar]
- 10.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. The Journal of experimental medicine. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinreich MA, Jameson SC, Hogquist KA. Postselection thymocyte maturation and emigration are independent of IL-7 and ERK5. Journal of immunology. 2011;186:1343–1347. doi: 10.4049/jimmunol.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 13.Takada K, Wang X, Hart GT, Odumade OA, Weinreich MA, Hogquist KA, Jameson SC. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J Immunol. 2011;186:775–783. doi: 10.4049/jimmunol.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schober SL, Kuo CT, Schluns KS, Lefrancois L, Leiden JM, Jameson SC. Expression of the transcription factor lung Kruppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J Immunol. 1999;163:3662–3667. [PubMed] [Google Scholar]
- 15.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Srinivasan SV, Lingrel JB. WWP1-dependent ubiquitination and degradation of the lung Kruppel-like factor, KLF2. Biochemical and biophysical research communications. 2004;316:139–148. doi: 10.1016/j.bbrc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Lingrel JB. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene. 2004;23:8088–8096. doi: 10.1038/sj.onc.1207996. [DOI] [PubMed] [Google Scholar]
- 18.Conkright MD, Wani MA, Lingrel JB. Lung Kruppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. The Journal of biological chemistry. 2001;276:29299–29306. doi: 10.1074/jbc.M103670200. [DOI] [PubMed] [Google Scholar]
- 19.Haaland RE, Yu W, Rice AP. Identification of LKLF-regulated genes in quiescent CD4+ T lymphocytes. Molecular immunology. 2005;42:627–641. doi: 10.1016/j.molimm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Yamada T, Park CS, Mamonkin M, Lacorazza HD. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Kruppel-like factors KLF4 and KLF2. Nature immunology. 2009;10:618–626. doi: 10.1038/ni.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nature immunology. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 22.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nature immunology. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nature immunology. 12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, Ippolito GC, Tian L, Wiehagen K, Oh S, Sambandam A, Willen J, Bunte RM, Maika SD, Harriss JV, Caton AJ, Bhandoola A, Tucker PW, Hu H. Foxp1 is an essential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood. 115:510–518. doi: 10.1182/blood-2009-07-232694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaewsongkram J, Yang Y, Golech S, Katz J, Kaestner KH, Weng NP. Kruppel-like factor 4 regulates B cell number and activation-induced B cell proliferation. J Immunol. 2007;179:4679–4684. doi: 10.4049/jimmunol.179.7.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuf I, Kharas MG, Chen J, Peralta RQ, Maruniak A, Sareen P, Yang VW, Kaestner KH, Fruman DA. KLF4 is a FOXO target gene that suppresses B cell proliferation. International immunology. 2008;20:671–681. doi: 10.1093/intimm/dxn024. [DOI] [PubMed] [Google Scholar]
- 28.Good KL, Tangye SG. Decreased expression of Kruppel-like factors in memory B cells induces the rapid response typical of secondary antibody responses. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13420–13425. doi: 10.1073/pnas.0703872104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharas MG, Yusuf I, Scarfone VM, Yang VW, Segre JA, Huettner CS, Fruman DA. KLF4 suppresses transformation of pre-B cells by ABL oncogenes. Blood. 2007;109:747–755. doi: 10.1182/blood-2006-03-011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 31.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annual review of immunology. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature reviews. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science (New York, N.Y. 2010;328:1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 35.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. The Journal of biological chemistry. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odumade OA, Weinreich MA, Jameson SC, Hogquist KA. Kruppel-like factor 2 regulates trafficking and homeostasis of gammadelta T cells. J Immunol. 2010;184:6060–6066. doi: 10.4049/jimmunol.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nature immunology. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nature immunology. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 40.Gubbels Bupp MR, Edwards B, Guo C, Wei D, Chen G, Wong B, Masteller E, Peng SL. T cells require Foxo1 to populate the peripheral lymphoid organs. European journal of immunology. 2009;39:2991–2999. doi: 10.1002/eji.200939427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nature reviews. Immunology. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nature immunology. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. Journal of immunology. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Zhang Y, Liu Z, Wu X, Zheng Y, Tao Z, Mao K, Wang J, Lin G, Tian L, Ji Y, Qin M, Sun S, Zhu X, Sun B. ECM1 controls T(H)2 cell egress from lymph nodes through re-expression of S1P(1) Nature immunology. 2011;12:178–185. doi: 10.1038/ni.1983. [DOI] [PubMed] [Google Scholar]
- 46.Hart GT, Wang X, Hogquist KA, Jameson SC. Kruppel-like factor 2 (KLF2) regulates B-cell reactivity, subset differentiation, and trafficking molecule expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:716–721. doi: 10.1073/pnas.1013168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkelmann R, Sandrock L, Porstner M, Roth E, Mathews M, Hobeika E, Reth M, Kahn ML, Schuh W, Jack HM. B cell homeostasis and plasma cell homing controlled by Kruppel-like factor 2. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:710–715. doi: 10.1073/pnas.1012858108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoek KL, Gordy LE, Collins PL, Parekh VV, Aune TM, Joyce S, Thomas JW, Van Kaer L, Sebzda E. Follicular B cell trafficking within the spleen actively restricts humoral immune responses. Immunity. 2010;33:254–265. doi: 10.1016/j.immuni.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turchinovich G, Vu TT, Frommer F, Kranich J, Schmid S, Alles M, Loubert JB, Goulet JP, Zimber-Strobl U, Schneider P, Bachl J, Pearson R, Crossley M, Agenes F, Kirberg J. Programming of marginal zone B-cell fate by basic Kruppel-like factor (BKLF/KLF3) Blood. 2011;117:3780–3792. doi: 10.1182/blood-2010-09-308742. [DOI] [PubMed] [Google Scholar]
- 50.Vu T, Gatto TD, Turner V, Funnell AP, Mak KS, Norton LJ, Kaplan W, Cowley MJ, Agenes F, Kirberg J, Brink R, Pearson RC, Crossley M. Impaired B Cell Development in the Absence of Kruppel-like Factor 3. Journal of immunology. 2011 doi: 10.4049/jimmunol.1101450. [DOI] [PubMed] [Google Scholar]
- 51.Yang XO, Doty RT, Hicks JS, Willerford DM. Regulation of T-cell receptor D beta 1 promoter by KLF5 through reiterated GC-rich motifs. Blood. 2003;101:4492–4499. doi: 10.1182/blood-2002-08-2579. [DOI] [PubMed] [Google Scholar]
- 52.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends in immunology. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, Clayberger C, Krensky AM. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. The Journal of experimental medicine. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou M, McPherson L, Feng D, Song A, Dong C, Lyu SC, Zhou L, Shi X, Ahn YT, Wang D, Clayberger C, Krensky AM. Kruppel-like transcription factor 13 regulates T lymphocyte survival in vivo. Journal of immunology. 2007;178:5496–5504. doi: 10.4049/jimmunol.178.9.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krensky AM, Ahn YT. Mechanisms of disease: regulation of RANTES (CCL5) in renal disease. Nat Clin Pract Nephrol. 2007;3:164–170. doi: 10.1038/ncpneph0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 57.Schuh W, Meister S, Herrmann K, Bradl H, Jack HM. Transcriptome analysis in primary B lymphoid precursors following induction of the pre-B cell receptor. Molecular immunology. 2008;45:362–375. doi: 10.1016/j.molimm.2007.06.154. [DOI] [PubMed] [Google Scholar]
- 58.Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunological reviews. 2004;197:206–218. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 59.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nature reviews. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 60.Eaton SA, Funnell AP, Sue N, Nicholas H, Pearson RC, Crossley M. A network of Kruppel-like Factors (Klfs). Klf8 is repressed by Klf3 and activated by Klf1 in vivo. The Journal of biological chemistry. 2008;283:26937–26947. doi: 10.1074/jbc.M804831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu YC. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nature immunology. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Z, Wara AK, Icli B, Sun X, Packard RR, Esen F, Stapleton CJ, Subramaniam M, Kretschmer K, Apostolou I, von Boehmer H, Hansson GK, Spelsberg TC, Libby P, Feinberg MW. Kruppel-like factor KLF10 targets transforming growth factor-beta1 to regulate CD4(+)CD25(−) T cells and T regulatory cells. The Journal of biological chemistry. 2009;284:24914–24924. doi: 10.1074/jbc.M109.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng DJ, Zeng M, Muromoto R, Matsuda T, Shimoda K, Subramaniam M, Spelsberg TC, Wei WZ, Venuprasad K. Noncanonical K27-linked polyubiquitination of TIEG1 regulates Foxp3 expression and tumor growth. Journal of immunology. 2011;186:5638–5647. doi: 10.4049/jimmunol.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nature immunology. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebson L, Gocke A, Rosenzweig J, Alder J, Civin C, Calabresi PA, Whartenby KA. Cutting edge: The transcription factor Kruppel-like factor 4 regulates the differentiation of Th17 cells independently of RORgammat. J Immunol. 2010;185:7161–7164. doi: 10.4049/jimmunol.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An J, Golech S, Klaewsongkram J, Zhang Y, Subedi K, Huston GE, Wood WH, 3rd, Wersto RP, Becker KG, Swain SL, Weng N. Kruppel-like factor 4 (KLF4) directly regulates proliferation in thymocyte development and IL-17 expression during Th17 differentiation. Faseb J. 2011 doi: 10.1096/fj.11-186924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1{alpha}-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahabeleshwar GH, Kawanami D, Sharma N, Takami Y, Zhou G, Shi H, Nayak L, Jeyaraj D, Grealy R, White M, McManus R, Ryan T, Leahy P, Lin Z, Haldar SM, Atkins GB, Wong HR, Lingrel JB, Jain MK. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34:715–728. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nature immunology. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, Cyster JG. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. The Journal of experimental medicine. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]