Abstract
Polyphenols are found ubiquitously in plants and their regular consumption has been associated with a reduced risk of a number of chronic diseases, including cancer, cardiovascular disease (CVD) and neurodegenerative disorders. Rather than exerting direct antioxidant effects, the mechanisms by which polyphenols express these beneficial properties appear to involve their interaction with cellular signaling pathways and related machinery that mediate cell function under both normal and pathological conditions. We illustrate that their interactions with two such pathways, the MAP kinase (ERK, JNK, p38) and PI3 kinase/Akt signaling cascades, allow them to impact upon normal and abnormal cell function, thus influencing the cellular processes involved in the initiation and progression of cancer, CVD and neurodegeneration. For example, their ability to activate ERK in neurons leads to a promotion of neuronal survival and cognitive enhancements, both of which influence the progression of Alzheimer’s disease, whilst ERK activation by polyphenols in vascular endothelial cells influences nitric oxide production, blood pressure and ultimately CVD risk. The main focus of this review is to provide an overview of the role that polyphenols play in the prevention of cancer, cardiovascular disease and neurodegeneration. We present epidemiological data, human intervention study findings, as well as animal and in vitro studies in support of these actions and in each case we consider how their actions at the cellular level may underpin their physiological effects.
Keywords: polyphenols, cancer, cardiovascular disease, neurodegeneration, advanced glycation end products, signaling pathways
1. Introduction
Epidemiological studies suggest that high dietary intake of polyphenols is associated with decreased risk of a range of diseases including cardiovascular disease (CVD), specific forms of cancer [1] and neurodegenerative diseases [2]. In particular, a group of polyphenols known as flavonoids have been strongly linked with beneficial effects in many human, animal and in vitro studies [3]. With respect to cardiovascular health, flavonoids may alter lipid metabolism [4], inhibit low-density lipoprotein (LDL) oxidation [5], reduce atherosclerotic lesion formation [6] inhibit platelet aggregation [7], decrease vascular cell adhesion molecule expression [8], improve endothelial function [9] and reduce blood pressure [10]. However, flavonoids have also been shown to exert beneficial cognitive effects and to reverse specific age-related neurodegeneration [11] and to exert a variety of anti-carcinogenic effects, including an ability to induce apoptosis in tumor cells [12,13,14], inhibit cancer cell proliferation [15,16] and prevent angiogenesis and tumor cells invasion [17]. This review will detail the evidence for the role of polyphenols in the context of these three chronic diseases and where relevant, the probable modes by which they exert their activity in vivo.
2. Polyphenols and Cancer
Cancer refers to a group of diseases that are associated with a disturbance in the control of cell growth and metabolism [18]. Indeed, the unbalanced control of cellular proliferation is a primary characteristic of cancer cells and, as such, any molecule capable of inhibiting cancer cell proliferation may also be useful as a potential chemo-preventive agent [19,20,21,22]. There are many different types of cancer, although breast (predominately women), lung, colorectal and prostate cancer accounts for over half of all new cases. It is widely believed that a high daily intake of fruit and vegetables helps to prevent the onset of, and progression of, cancer. Over the past 20 years, case-control studies have indicated an inverse correlation between regular fruit and vegetable consumption and the development of various types of cancer [23,24]. More recently, data from large cohort investigations have gone some way to confirm these epidemiological associations [25,26,27,28,29]. However, there is a degree of controversy, in that some studies have reported no reduction in bladder, pancreatic and stomach cancer incidence due to fruit and vegetables intake [30,31,32] and a recent epidemiological study has provided evidence for no, or little, association between fruit and vegetable intake and overall cancer risk [25,33]. Despite this, it remains a possibility that specific fruits or vegetables, or specific polyphenols found within these, may exert protective effects against cancer development, particularly in the gastrointestinal tract where they will be at highest concentration. In fact, many studies have shown that various polyphenol-rich fruits and vegetables are particularly effective in protecting against colon cancer development [34,35].
At the cellular level, there is good evidence that polyphenols present in tea, red wine, cocoa, fruit juices, and olive oil influence carcinogenesis and tumor development [36]. For example, they may interact with reactive intermediates [37] and activated carcinogens and mutagens [38], may modulate the activity of key proteins involved in controlling cell cycle progression [39] and influence the expression of many cancer-associated genes [40]. Perhaps most notably, the anticancer properties of green tea flavanols have been reported in animal models [41], human cell lines [42], as well as in human intervention studies [43]. Furthermore, green tea consumption has been proposed to significantly reduce the risk of cancer of the biliary tract [44], bladder [45], breast [46] and colon [47]. Many of the anti-cancer properties associated with green tea are believed to be mediated by the flavanol, epigallocatechin gallate (EGCG), which has been shown to induce apoptosis and inhibit cancer cell growth by altering the expression of cell cycle regulatory proteins and the activity of signaling proteins involved in cell proliferation, transformation and metastasis [48]. In addition to flavonoids, phenolic alcohols, lignans and secoiridoids (all found at high concentration in olive oil) are also thought to induce anti-carcinogenic effects [49] and have been reported in large intestinal cancer cell models [50], in animals [51,52] and in humans [49]. These effects may be mediated by the ability of olive oil phenolics to inhibit the initiation, promotion and metastasis in human colon adenocarcinoma cells [53,54] and to down-regulate the expression of COX-2 and Bcl-2 proteins that have a crucial role in colorectal carcinogenesis [50] (Figure 1).
Polyphenols may exert these anticancer effects via a variety of mechanisms, including removal of carcinogenic agents [37,49], modulation of cancer cell signaling [48,55] and cell cycle progression [15,16], promotion of apoptosis [12,13,14] and modulation of enzymatic activities [56]. For example, the enhancement of glutathione peroxidase, catalase, NADPH-quinone oxidoreductase, glutathione S-transferase and/or cytochrome P450 enzyme activity by polyphenols may aid in the detoxification of carcinogenic agents [57]. Furthermore, they may modulate the activity of signaling pathways [58,59,60] (i.e., MAPK kinase and PI3 Kinase), which are involved in cancer cell proliferation [61,62,63]. The MAPK signaling pathway has long been viewed as an attractive pathway for anticancer therapies, based on its central role in regulating the growth and survival of cells from a broad spectrum of human cancers [64], and its role in the transcriptional and post-transcriptional activation of COX-2 [65] (Figure 1). In this context, certain polyphenols have been shown to exert a strong inhibitory effect on the growth of colon adenocarcinoma cells through the inhibition of p38/CREB signaling, a decrease in COX-2 expression and the stimulation of a G2/M phase cell cycle block [55]. In addition, hydroxytyrosol [66], epicatechin and dimer B2 [67] have been shown to strongly inhibit ERK1/2 phosphorylation and downstream cyclin D1 expression leading to a block in cell cycle progression (Figure 1). Alternatively, polyphenols such as hydroxytyrosol and tea flavanols such as EGCG have been shown to reduce COX-2 over-expression, which is associated with colorectal neoplasia in colorectal cancer [68,69,70,71].
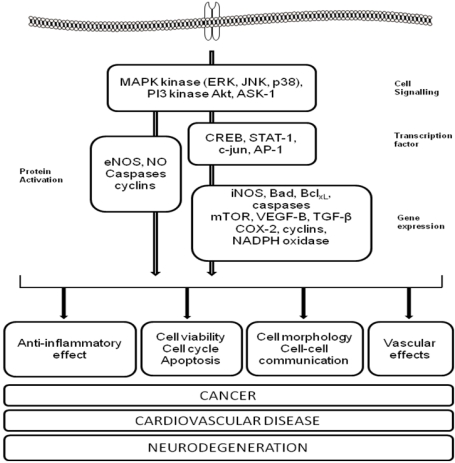
Figure 1.
The interaction of polyphenols with cellular signaling pathways involved in chronic disease. Flavonoid-induced activation and/or inhibition of MAP kinase and PI3 kinase signaling leads to the activation of transcription factors which drive gene expression. For example, activation of ERK/Akt and the downstream transcription factor CREB by flavonoids may promote changes in neuronal viability and synaptic plasticity, which ultimately influence neurodegenerative processes. Polyphenol-induced inhibition of the JNK, ASK1 and p38 pathways leads to inhibition of both apoptosis in neurons and a reduction of neuroinflammatory reactions in microglia (reduced iNOS expression and NO• release). Alternatively, their interaction with signaling may lead to direct activation of proteins such as eNOS, which controls nitric oxide release in the vasculature and thus influences CVD risk.
Tumors are also characterized by an increase in glucose uptake and a high rate of glycolysis, which can led to the non-enzymatic glycation of proteins and the generation of so called advanced glycation end products (AGEs). Indeed, the measurement of the AGEs, NЄ-carboxymethyllysine (CML) and argpyrimidine in several human tumors has been linked to their involvement in cancer progression [72]. Certain polyphenols have been proposed to counteract AGE formation both in vivo and in vitro and thus may limit their impact on the carcinogenesis process [73,74,75,76]. Furthermore, receptors for AGEs, such as RAGE, have also been recognized to play an important role in regulating cancer cell invasion and metastasis [77,78] (Figure 2) and flavanols such as EGCG may inhibit the cancer cell proliferation by blocking RAGE related signaling [79].
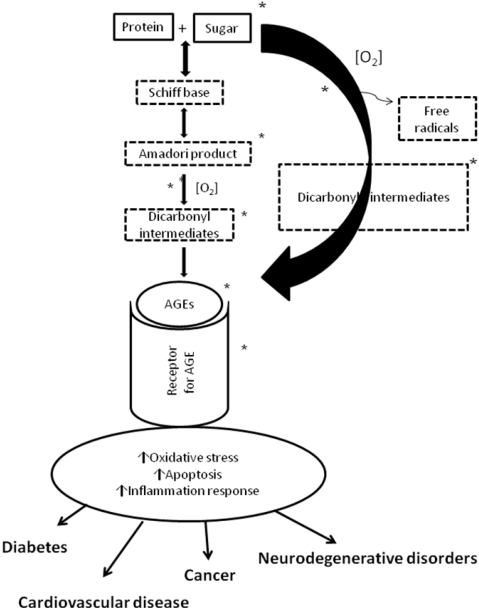
Figure 2.
Formation of Advanced Glycation Endproducts (AGEs) and the sites where flavonoids may inhibit their formation (*). These include monosaccharide autoxidation, glycation, glycoxidation, as well as AGE receptor binding, which results in the activation and release of inflammation mediators.
3. Polyphenols and Cardiovascular Disease
Cardiovascular disease (CVD), in particular coronary heart disease and stroke, is a major cause of mortality in developed nations [80]. CVD is a chronic, multi-factorial disease in which a range of genetic and environmental factors plays a role in its initiation, progression and development. For example, smoking, high saturated fat diets and physical inactivity are well known environmental factors that are known to increase the risk of CVD [81,82,83,84]. This array and variety of factors makes it difficult to explore the impact that an individual factor, for example a specific dietary nutrient, has on the progression of CVD. Despite this, numerous epidemiological and human intervention studies have suggested that regular consumption of polyphenol-rich foods, such as fruits, vegetables, cocoa, tea and wine, may exert cardio-protective effects in humans [85,86,87,88,89,90,91,92,93,94]. Prospective studies have indicated a correlation between the intake of flavonols, flavones and flavanols and a reduced risk of coronary artery disease [95] and anthocyanin and flavanone intake and reduced CVD related mortality [90]. Furthermore, meta-analyses have indicated that the consumption of three cups of tea per day reduces CVD risk by 11% [96] and regular, moderate red wine consumption is associated with a 32% reduced risk of CVD [97]. However, there remains significant debate over which polyphenols are active, or most active, in the context of CVD. Indeed, a recent systematic review has concluded that soy and cocoa flavonoids have the most beneficial effect on reducing cardiovascular risk [98], whilst other polyphenols are ineffective [87,99,100,101]. The reasons for these inconsistencies may relate to a number of factors, including the use of different dietary intake questionnaires and food composition tables, differences in the levels and types of polyphenols studied and differences in the populations investigated, such as well-nourished populations and populations with high polyphenol intake showing no effect [102].
Various human, animal and cell studies have suggested that polyphenols may exert beneficial effects on the vascular system via an induction of antioxidant defenses [103,104,105], by lowering blood pressure [98,106,107,108,109,110,111], by improving endothelial function [108,112,113,114,115,116,117,118,119,120,121], by inhibiting platelet aggregation [107,122,123,124] and low density lipoprotein oxidation [105,125] and by reducing inflammatory responses [126,127]. A daily intake of flavanol containing cocoa was found to be the causal factor in determining the relatively low incidence of hypertension and CVD incidence in the Kuna Amerinds of the San Blas Island in Panama [128]. In support of these findings, three recent meta-analyses have confirmed the blood pressure lowering capacity of flavanol-rich cocoa [98,106,110]. Whilst a correlation between high black tea consumption and decreased blood pressure has been reported [129,130], the effects of tea polyphenols have proved less consistent, with reports indicating they both reduce blood pressure [131] or have no effect in animal models [132]. Furthermore, unlike those studies with cocoa, human intervention studies investigating the short-term effect of tea consumption on blood pressure have failed to show positive effects [133,134,135,136] and there are inconsistent data with regards to the effect of red wine or grapes on blood pressure [88,89,111,137,138,139,140]. However, in general there is a growing body of evidence to support the short-term and long-term benefits of cocoa, purple grape juice, tea and red wine consumption with regards to endothelial function and CVD risk [104,108,112,113,114,115,133,135,141,142,143,144,145,115,133.
One suggested mechanism for the action of polyphenols on vascular function involves their ability to modulate the levels of and activity of nitric oxide synthase (eNOS) and therefore nitric oxide (NO) bioavailability to the endothelium [112,146,147,148,149,150] (Figure 1). In support of this, aortic ring experiments using physiological concentrations of polyphenols have shown that polyphenols induce endothelium-dependent relaxation [148,151,152,153,154,155,156]. This regulation of vascular nitric oxide is thought to involve the ability of polyphenols to interact with kinase signaling pathways such as the PI3-kinase/Akt pathway and intracellular Ca+2 on eNOS phosphorylation and subsequent NO production [157,158] (Figure 1). As well as activating eNOS, many polyphenols have also been shown to increase eNOS expression, to induce prostacyclin production, to inhibit endothelin-1 and endothelial NADPH oxidase [149,159,160,161,162] and to inhibit angiogenesis and the migration and proliferation of vascular cells and matrix metalloproteinase (MMP) activation [158]. They have also been proposed to inhibit platelet aggregation [163,164] with cocoa, purple grape juice, red wine, black tea, coffee and berry interventions all effective in acutely and chronically inhibiting platelet activation and aggregation [107,122,123,164,165,166,167,168,169]. Lastly, flavanols and flavonols may act to prevent AGE-related vascular injury [170,171] via their regulation of MAPK signaling through RAGE [172] and the down-regulation of transcription factors such as NF-kB leading to the suppression of NADPH oxidase [173] (Figure 1).
4. Polyphenols and Neurodegeneration
Neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases represent an increasing problem in our aging societies, primarily as there is an increased prevalence of both Alzheimer’s disease [174,175] and Parkinson’s disease [175,176,177] with age. These and other neurodegenerative disorders appear to be triggered by multi-factorial events including neuroinflammation, glutamatergic excitotoxicity, increases in oxidative stress, iron and/or depletion of endogenous antioxidants [178,179,180]. In terms of dietary modulation of these diseases, epidemiological studies have suggested that moderate wine consumption may reduce the incidence of certain age-related neurological disorders including Alzheimer’s disease [181,182,183]. Furthermore, regular dietary intake of flavonoid-rich foods and/or beverages has been associated with 50% reduction in the risk of dementia [184], a preservation of cognitive performance with ageing [185,186], a delay in the onset of Alzheimer’s disease [187] and a reduction in the risk of developing Parkinson’s disease [2].
Many studies have reported the bioavailability of polyphenols in the systemic circulation [188,189,190,191]. Whilst less is known regarding their degree of brain bioavailability, flavanones such as hesperetin, naringenin and their in vivo metabolites, have been shown to traverse the BBB in relevant in vitro and in situ models [192]. Moreover, several anthocyanins have also been identified in the cortex and cerebellum of rat [193] and pig [194,195] following feeding with blueberries. Together, these results suggest that polyphenols are able to transverse the BBB, albeit to varying degrees depending on their structure. Thus, such compounds are likely to be candidates for direct neuroprotective and neuromodulatory actions.
Flavonoids may act to protect the brain in a number of ways, including by protection of vulnerable neurons, the enhancement of existing neuronal function or by stimulating neuronal regeneration [196]. For example, polyphenols have been shown to protect neurons against oxidative stress [197] and Aβ-induced-induced neuronal injury [198] and polyphenol-rich Ginkgo biloba extracts have been shown to be neuroprotective [199] by protecting hippocampal neurons from nitric oxide- and beta-amyloid-induced neurotoxicity [200]. Furthermore, anthocyanins and isoflavones [201,202] may be capable of reducing the neurodegeneration associated with the accumulation AGEs during normal [203] and abnormal brain ageing [204]. In the context of Parkinson’s disease, the citrus flavanone tangeretin has been observed to maintain nigro-striatal integrity and functionality following lesioning with 6-hydroxydopamine, suggesting that it may serve as a potential neuroprotective agent against the underlying pathology associated with Parkinson’s disease [205]. In addition to the neuroprotection elicited by flavonoids, phenolic compounds such as caffeic acid and tyrosol has also been shown to protect against 5-S-cysteinyl-dopamine [206] and peroxynitrite neurotoxicity [207] in vitro.
There is also a growing interest in the potential of polyphenols to improve memory, learning and general cognitive ability [208,209,210,211]. Human investigations have suggested that fruits and vegetables may have an impact on memory [212,213,214] and depression [215] and there is a large body of animal behavioral evidence to suggest that berries, in particular blueberries and strawberries, are effective at reversing age-related deficits in spatial working memory [216,217,218,219,220,221], in improving object recognition memory [222] and in modulating inhibitory fear conditioning [220,221]. The beneficial effects of flavonoid-rich foods and beverages on psychomotor activity in older animals have also been reported [217,223]. In addition to berries, tea [35,224], pomegranate [225], Ginkgo biloba [226,227,228,229,230,231,232,233,234,235] and pure flavonols such as quercetin, rutin [236] and fisetin [237] have also been shown to be beneficial in reversing neuronal and behavioral aging. Furthermore, Ginkgo biloba has been shown to promote inhibitory avoidance conditioning in rats with high-dose intake leading to short-term, but not long-term, passive avoidance learning in senescent mice [238,239].
The effects of polyphenols on cognition and against neurodegenerative processes appear to be mediated via their interactions with neuronal and glial signaling pathways that affect gene expression and interfere with the cell death mechanisms [233,234]. For example, flavonoids may exert direct modulation of protein and lipid kinase signaling pathways [209,232,234], via the inhibition of MAPK signaling cascades, such as p38 or ERK1/2 [226,240] (Figure 1). The effects of flavonoids on these kinases may influence downstream transcription factors [240], including nuclear factor-Kappa B (NF-κB) [202,241], which responds to p38 signaling and is involved in iNOS induction [242]. This suggests that there may be interplay between signaling pathways, transcription factors and cytokine production in determining the neuroinflammatory response in the CNS (Figure 1). In addition, the actions of flavonoids on neuronal signaling may mediate their ability to protect against neurotoxicity induced by AGEs [243].
5. Summary
Polyphenols are found ubiquitously in plants and are therefore consumed in relatively high quantities in the human diet. Over the last 20 years, a significant amount of data has emerged with regards to the potential health effects of several classes of polyphenolic compounds, in particular flavonoids. Along with this, reasonable understandings of the bioavailability of polyphenols and the mechanisms by which they exert such benefits in vivo have been determined. These mechanisms are now believed to involve interactions with a number of cellular signaling pathways, which are important in the normal functioning of cells. Such interactions appear to modulate these pathways in a way that acts to control various pathogenic processes relevant to chronic disease progression. In this respect, polyphenols, in particular flavonoids structurally resemble inhibitors of cell signaling cascades, such as the PD98059, a MAPK inhibitor and the LY294002, a phosphatidylinositol-3 kinase (PI3) inhibitor. Indeed, the latter inhibitor was modeled on the structure of quercetin [244]. LY294002 and quercetin fit into the ATP binding pocket of the enzyme and it appears that the number and substitution of hydroxyl groups on the B ring and the degree of un-saturation of the C2-C3 bond are important determinants of this particular bioactivity. In this regard, quercetin and some of its in vivo metabolites have been suggested to inhibit Akt/protein kinase B (PKB) signaling pathways [245], a mechanism of action consistent with quercetin and its metabolites acting at and inhibiting PI3-kinase activity. Although we have gained a better understanding of how polyphenols interact with cells, there is still a long way to go before the precise cellular targets and mechanisms of action can be established. While various lines of evidence via biomarker assessments and the use of pharmacological tools in vivo (i.e., specific enzyme inhibitors, receptor agonists or antagonist) have indicated several potential mechanisms of action, a comprehensive proof and conclusive understanding has yet to be established. This relates mainly to significant limitations with regard to current data from in vitro investigations that aimed at elucidating the mechanisms of action by which polyphenols exert their bioactivities in vivo. It is notable that in most cases, in vitro data with regards to polyphenol bioactivity have been derived via the direct use of plant/food extracts or isolated native compounds, a practice that does not take into account the processes of absorption and metabolism that polyphenols undergo in humans. As such, one should express caution when interpreting the wealth of in vitro data linking numerous polyphenols to actions in the body and effects against various disease processes, especially if no data has been collected regarding the action of physiological metabolites of polyphenols in the same cell systems. For example, if there is no evidence for the absorption of a particular polyphenol in humans, can one really gain meaningful insight into its biological effects by exposing it to cultured cells of the cardiovascular system and/or brain? There are specific exceptions, for example the gastrointestinal tract, where polyphenols may come into direct contact with the cells without having undergone absorption and metabolism. Therefore, it is perhaps relevant to investigate the effects of polyphenols and polyphenol extracts on colon cancer cells, although as the gut microbiota also extensively metabolizes them one must take account of these effects prior to concluding on a mechanism of action in vivo. These and other limitations significantly hamper the translation of in vitro data on the biological effects of flavanols and procyanidins into meaningful insight and mechanistic understanding of the in vivo effects in humans.
Whilst the case for the biological functions of polyphenols in humans is accumulating, there remains insufficient evidence to claim clear and undisputed positive health effects relating to their consumption, particularly with regards to long-term dietary ingestion and human health. Epidemiological studies have failed to show conclusive results, in some cases due to the lack of appropriate nutrient databases and/or the use of an inappropriately controlled study population. Much of the strongest data, particularly with regards to CVD, is based on short-term human studies, in many cases lacking appropriate controls and a defined polyphenol content of the foods assessed. In addition to better-defined human intervention studies aimed at assessing physiological endpoints linked to disease, further research is also required regarding the bioavailability of polyphenols, particularly with regards to the effects of food matrices on absorption and the influence on age, gender and genotype on both absorption and metabolism These studies are required in order to help determine the physiological metabolic forms responsible for activity in vivo, as well as to help define adequate biomarkers of polyphenol intake. Therefore, at present, while the vast literature regarding the potential of polyphenols to improve in human health is encouraging, more long-term, randomized, controlled, dietary intervention trials with appropriate controls are warranted in order to assess the full and unequivocal role that polyphenols play in preventing chronic human disease. The outcomes of these studies may ultimately be used to make specific dietary recommendations regarding the efficacy of polyphenols in preventing chronic disease risk and to fully validate polyphenols as the new agents against various chronic human diseases.
Acknowledgements
The authors are funded by the Biotechnology and Biological Sciences Research Council (BB/F008953/1; BB/E023185/1; BB/G005702/1), the FSA (FLAVURS) and the European Union (FP7 FLAVIOLA). There is no conflict of interest to disclose, having read the Journals guidelines. All authors contributed to the preparation of the manuscript and agreed the final version.
References
- 1.Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., Tsubono Y., Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Checkoway H., Powers K., Smith-Weller T., Franklin G.M., Longstreth W.T., Jr., Swanson P.D. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 2002;155:732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 3.Schroeter H., Spencer J.P., Rice-Evans C., Williams R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001;358:547–557. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zern T.L., Wood R.J., Greene C., West K.L., Liu Y., Aggarwal D., Shachter N.S., Fernandez M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005;135:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 5.Jeong Y.J., Choi Y.J., Kwon H.M., Kang S.W., Park H.S., Lee M., Kang Y.H. Differential inhibition of oxidized LDL-induced apoptosis in human endothelial cells treated with different flavonoids. Br. J. Nutr. 2005;93:581–591. doi: 10.1079/bjn20041397. [DOI] [PubMed] [Google Scholar]
- 6.Fuhrman B., Volkova N., Coleman R., Aviram M. Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity. J. Nutr. 2005;135:722–728. doi: 10.1093/jn/135.4.722. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard G.P., Wolffram S., de Vos R., Bovy A., Gibbins J.M., Lovegrove J.A. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: A pilot study. Br. J. Nutr. 2006;96:482–488. [PubMed] [Google Scholar]
- 8.Ludwig A., Lorenz M., Grimbo N., Steinle F., Meiners S., Bartsch C., Stangl K., Baumann G., Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- 9.Hallund J., Bugel S., Tholstrup T., Ferrari M., Talbot D., Hall W.L., Reimann M., Williams C.M., Wiinberg N. Soya isoflavone-enriched cereal bars affect markers of endothelial function in postmenopausal women. Br. J. Nutr. 2006;95:1120–1126. doi: 10.1079/bjn20061734. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson J., Croft K. Dietary flavonoids: Effects on endothelial function and blood pressure. J. Sci. Food Agric. 2006;86:2492–2498. [Google Scholar]
- 11.Joseph J.A., Shukitt-Hale B., Denisova N.A., Bielinski D., Martin A., McEwen J.J., Bickford P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantena S.K., Baliga M.S., Katiyar S.K. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis. 2006;27:1682–1691. doi: 10.1093/carcin/bgl030. [DOI] [PubMed] [Google Scholar]
- 13.Fabiani R., De Bartolomeo A., Rosignoli P., Servili M., Montedoro G.F., Morozzi G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002;11:351–358. doi: 10.1097/00008469-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fini L., Hotchkiss E., Fogliano V., Graziani G., Romano M., De Vol E.B., Qin H., Selgrad M., Boland C.R., Ricciardiello L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis. 2008;29:139–146. doi: 10.1093/carcin/bgm255. [DOI] [PubMed] [Google Scholar]
- 15.Corona G., Deiana M., Incani A., Vauzour D., Dessi M.A., Spencer J.P. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009;53:897–903. doi: 10.1002/mnfr.200800269. [DOI] [PubMed] [Google Scholar]
- 16.Wang W., Heideman L., Chung C.S., Pelling J.C., Koehler K.J., Birt D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 17.Piao M., Mori D., Satoh T., Sugita Y., Tokunaga O. Inhibition of endothelial cell proliferation, in vitro angiogenesis, and the down-regulation of cell adhesion-related genes by genistein. Combined with a cDNA microarray analysis. Endothelium. 2006;13:249–266. doi: 10.1080/10623320600903940. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 19.D’Archivio M., Santangelo C., Scazzocchio B., Vari R., Filesi C., Masella R., Giovannini C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008;9:213–228. doi: 10.3390/ijms9030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W., Kong E., Meydani M. Dietary polyphenols, inflammation, and cancer. Nutr. Cancer. 2009;61:807–810. doi: 10.1080/01635580903285098. [DOI] [PubMed] [Google Scholar]
- 21.Kampa M., Nifli A.P., Notas G., Castanas E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007;159:79–113. doi: 10.1007/112_2006_0702. [DOI] [PubMed] [Google Scholar]
- 22.Stoner G.D., Mukhtar H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. Suppl. 1995;22:169–180. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S., Parpinel M., La Vecchia C., Favero A., Talamini R., Negri E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology. 1998;9:338–341. [PubMed] [Google Scholar]
- 24.La Vecchia C., Chatenoud L., Franceschi S., Soler M., Parazzini F., Negri E. Vegetables and fruit and human cancer: Update of an Italian study. Int. J. Cancer. 1999;82:151–152. doi: 10.1002/(sici)1097-0215(19990702)82:1<151::aid-ijc25>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Benetou V., Orfanos P., Lagiou P., Trichopoulos D., Boffetta P., Trichopoulou A. Vegetables and fruits in relation to cancer risk: Evidence from the Greek EPIC cohort study. Cancer Epidemiol. Biomarkers Prev. 2008;17:387–392. doi: 10.1158/1055-9965.EPI-07-2665. [DOI] [PubMed] [Google Scholar]
- 26.Feskanich D., Ziegler R.G., Michaud D.S., Giovannucci E.L., Speizer F.E., Willett W.C., Colditz G.A. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J. Natl. Cancer Inst. 2000;92:1812–1823. doi: 10.1093/jnci/92.22.1812. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S.M., Hunter D.J., Rosner B.A., Giovannucci E.L., Colditz G.A., Speizer F.E., Willett W.C. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin’s lymphoma among women. Cancer Epidemiol. Biomarkers Prev. 2000;9:477–485. [PubMed] [Google Scholar]
- 28.Gonzalez C.A., Pera G., Agudo A., Bueno-de-Mesquita H.B., Ceroti M., Boeing H., Schulz M., Del Giudice G., Plebani M., Carneiro F., et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int. J. Cancer. 2006;118:2559–2566. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 29.Favero A., Parpinel M., Franceschi S. Diet and risk of breast cancer: Major findings from an Italian case-control study. Biomed. Pharmacother. 1998;52:109–115. doi: 10.1016/S0753-3322(98)80088-7. [DOI] [PubMed] [Google Scholar]
- 30.Larsson S.C., Andersson S.O., Johansson J.E., Wolk A. Fruit and vegetable consumption and risk of bladder cancer: A prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 2008;17:2519–2522. doi: 10.1158/1055-9965.EPI-08-0407. [DOI] [PubMed] [Google Scholar]
- 31.Larsson S.C., Hakansson N., Naslund I., Bergkvist L., Wolk A. Fruit and vegetable consumption in relation to pancreatic cancer risk: A prospective study. Cancer Epidemiol. Biomarkers Prev. 2006;15:301–305. doi: 10.1158/1055-9965.EPI-05-0696. [DOI] [PubMed] [Google Scholar]
- 32.Botterweck A.A., van den Brandt P.A., Goldbohm R.A. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in The Netherlands. Am. J. Epidemiol. 1998;148:842–853. doi: 10.1093/oxfordjournals.aje.a009709. [DOI] [PubMed] [Google Scholar]
- 33.Boffetta P., Couto E., Wichmann J., Ferrari P., Trichopoulos D., Bueno-de-Mesquita H.B., van Duijnhoven F.J., Buchner F.L., Key T., Boeing H., et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) J. Natl. Cancer Inst. 2010;102:529–537. doi: 10.1093/jnci/djq072. [DOI] [PubMed] [Google Scholar]
- 34.Martinez M.E. Primary prevention of colorectal cancer: Lifestyle, nutrition, exercis. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 35.Li Q., Zhao H.F., Zhang Z.F., Liu Z.G., Pei X.R., Wang J.B., Cai M.Y., Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Middleton E., Jr., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 37.Duthie S.J., Dobson V.L. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur. J. Nutr. 1999;38:28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- 38.Calomme M., Pieters L., Vlietinck A., Vanden Berghe D. Inhibition of bacterial mutagenesis by Citrus flavonoids. Planta Med. 1996;62:222–226. doi: 10.1055/s-2006-957864. [DOI] [PubMed] [Google Scholar]
- 39.Plaumann B., Fritsche M., Rimpler H., Brandner G., Hess R.D. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–1614. [PubMed] [Google Scholar]
- 40.van Erk M.J., Roepman P., van der Lende T.R., Stierum R.H., Aarts J.M., van Bladeren P.J., van Ommen B. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. Eur. J. Nutr. 2005;44:143–156. doi: 10.1007/s00394-004-0503-1. [DOI] [PubMed] [Google Scholar]
- 41.Khan W.A., Wang Z.Y., Athar M., Bickers D.R., Mukhtar H. Inhibition of the skin tumorigenicity of (±)-7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988;42:7–12. doi: 10.1016/0304-3835(88)90232-7. [DOI] [PubMed] [Google Scholar]
- 42.Takada M., Nakamura Y., Koizumi T., Toyama H., Kamigaki T., Suzuki Y., Takeyama Y., Kuroda Y. Suppression of human pancreatic carcinoma cell growth and invasion by epigallocatechin-3-gallate. Pancreas. 2002;25:45–48. doi: 10.1097/00006676-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Inoue M., Tajima K., Mizutani M., Iwata H., Iwase T., Miura S., Hirose K., Hamajima N., Tominaga S. Regular consumption of green tea and the risk of breast cancer recurrence: Follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167:175–182. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- 44.Takada M., Ku Y., Habara K., Ajiki T., Suzuki Y., Kuroda Y. Inhibitory effect of epigallocatechin-3-gallate on growth and invasion in human biliary tract carcinoma cells. World J. Surg. 2002;26:683–686. doi: 10.1007/s00268-001-0290-2. [DOI] [PubMed] [Google Scholar]
- 45.Rieger-Christ K.M., Hanley R., Lodowsky C., Bernier T., Vemulapalli P., Roth M., Kim J., Yee A.S., Le S.M., Marie P.J., Libertino J.A., Summerhayes I.C. The green tea compound, (−)-epigallocatechin-3-gallate downregulates N-cadherin and suppresses migration of bladder carcinoma cells. J. Cell. Biochem. 2007;102:377–388. doi: 10.1002/jcb.21299. [DOI] [PubMed] [Google Scholar]
- 46.Leong H., Mathur P.S., Greene G.L. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res. Treat. 2008;107:359–369. doi: 10.1007/s10549-007-9568-x. [DOI] [PubMed] [Google Scholar]
- 47.Larsen C.A., Dashwood R.H. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: Minor role of hydrogen peroxide. Biochem. Biophys. Res. Commun. 2009;389:527–530. doi: 10.1016/j.bbrc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan N., Afaq F., Saleem M., Ahmad N., Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 49.Owen R.W., Giacosa A., Hull W.E., Haubner R., Spiegelhalder B., Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer. 2000;36:1235–1247. doi: 10.1016/s0959-8049(00)00103-9. [DOI] [PubMed] [Google Scholar]
- 50.Llor X., Pons E., Roca A., Alvarez M., Mane J., Fernandez-Banares F., Gassull M.A. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 2003;22:71–79. doi: 10.1054/clnu.2002.0627. [DOI] [PubMed] [Google Scholar]
- 51.Bartoli R., Fernandez-Banares F., Navarro E., Castella E., Mane J., Alvarez M., Pastor C., Cabre E., Gassull M.A. Effect of olive oil on early and late events of colon carcinogenesis in rats: Modulation of arachidonic acid metabolism and local prostaglandin E(2) synthesis. Gut. 2000;46:191–199. doi: 10.1136/gut.46.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solanas M., Hurtado A., Costa I., Moral R., Menendez J.A., Colomer R., Escrich E. Effects of a high olive oil diet on the clinical behavior and histopathological features of rat DMBA-induced mammary tumors compared with a high corn oil diet. Int. J. Oncol. 2002;21:745–753. [PubMed] [Google Scholar]
- 53.Gill C.I., Boyd A., McDermott E., McCann M., Servili M., Selvaggini R., Taticchi A., Esposto S., Montedoro G., McGlynn H., Rowland I. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer. 2005;117:1–7. doi: 10.1002/ijc.21083. [DOI] [PubMed] [Google Scholar]
- 54.Hashim Y.Z., Rowland I.R., McGlynn H., Servili M., Selvaggini R., Taticchi A., Esposto S., Montedoro G., Kaisalo L., Wahala K., Gill C.I. Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. Int. J. Cancer. 2008;122:495–500. doi: 10.1002/ijc.23148. [DOI] [PubMed] [Google Scholar]
- 55.Corona G., Deiana M., Incani A., Vauzour D., Dessi M.A., Spencer J.P. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem. Biophys. Res. Commun. 2007;362:606–611. doi: 10.1016/j.bbrc.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 56.Adams L.S., Chen S. Phytochemicals for breast cancer prevention by targeting aromatase. Front. Biosci. 2009;14:3846–3863. doi: 10.2741/3493. [DOI] [PubMed] [Google Scholar]
- 57.Khan S.G., Katiyar S.K., Agarwal R., Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992;52:4050–4052. [PubMed] [Google Scholar]
- 58.Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 59.Hopfner M., Schuppan D., Scherubl H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J. Gastroenterol. 2008;14:1–14. doi: 10.3748/wjg.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramos S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 61.Fang J.Y., Richardson B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–327. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- 62.Wang W., Wang X., Peng L., Deng Q., Liang Y., Qing H., Jiang B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010;101:112–119. doi: 10.1111/j.1349-7006.2009.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corona G., Spencer J.P., Dessi M.A. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health. 2009;25:285–293. doi: 10.1177/0748233709102951. [DOI] [PubMed] [Google Scholar]
- 64.Sebolt-Leopold J.S., Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 65.Tsatsanis C., Androulidaki A., Venihaki M., Margioris A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Guichard C., Pedruzzi E., Fay M., Marie J.C., Braut-Boucher F., Daniel F., Grodet A., Gougerot-Pocidalo M.A., Chastre E., Kotelevets L., Lizard G., Vandewalle A., Driss F., Ogier-Denis E. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase PP2A and promotes the endoplasmic reticulum stress response in human colon carcinoma cells. Carcinogenesis. 2006;27:1812–1827. doi: 10.1093/carcin/bgl009. [DOI] [PubMed] [Google Scholar]
- 67.Lee S.Y., Munerol B., Pollard S., Youdim K.A., Pannala A.S., Kuhnle G.G., Debnam E.S., Rice-Evans C., Spencer J.P. The reaction of flavanols with nitrous acid protects against N-nitrosamine formation and leads to the formation of nitroso derivatives which inhibit cancer cell growth. Free Radic. Biol. Med. 2006;40:323–334. doi: 10.1016/j.freeradbiomed.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 68.Adhami V.M., Malik A., Zaman N., Sarfaraz S., Siddiqui I.A., Syed D.N., Afaq F., Pasha F.S., Saleem M., Mukhtar H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin. Cancer Res. 2007;13:1611–1619. doi: 10.1158/1078-0432.CCR-06-2269. [DOI] [PubMed] [Google Scholar]
- 69.Banerjee S., Manna S., Mukherjee S., Pal D., Panda C.K., Das S. Black tea polyphenols restrict benzopyrene-induced mouse lung cancer progression through inhibition of Cox-2 and induction of caspase-3 expression. Asian Pac. J. Cancer Prev. 2006;7:661–666. [PubMed] [Google Scholar]
- 70.Kumar N., Shibata D., Helm J., Coppola D., Malafa M. Green tea polyphenols in the prevention of colon cancer. Front. Biosci. 2007;12:2309–2315. doi: 10.2741/2233. [DOI] [PubMed] [Google Scholar]
- 71.Chell S., Kadi A., Williams A.C., Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim. Biophys. Acta. 2006;1766:104–119. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 72.van Heist J., Niessen H., Hoekman K., Schalkwij C. Advanced glycation end products in human cancer tissues: Detection of Nepsilon-(carboxymethyl)lysine and argpryrimidine. Ann. N. Y. Acad. Sci. 2005;1043:725–733. doi: 10.1196/annals.1333.084. [DOI] [PubMed] [Google Scholar]
- 73.Bengmark S. Advanced Glycation and Lipoxidation End Products–Amplifiers of Inflammation: The Role of Food. JPEN J. Parenter. Enteral Nutr. 2007;31:430–440. doi: 10.1177/0148607107031005430. [DOI] [PubMed] [Google Scholar]
- 74.Kiho T., Usui S., Hirano K., Aizawa K., Inakuma T. Tomato paste fraction inhibiting the formation of advance glycation end-products. Biosci. Biotechnol. Biochem. 2004;1:200–205. doi: 10.1271/bbb.68.200. [DOI] [PubMed] [Google Scholar]
- 75.Lo C.-Y., Li S., Tan D., Pan M.-H., Sang S., HO C.-T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006;50:1118–1128. doi: 10.1002/mnfr.200600094. [DOI] [PubMed] [Google Scholar]
- 76.Sang S., Shao X., Bai N., Lo C.-Y., Yang C., HO C.-T. Tea polyphenol (−)-Epigallocatechin-3-Gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007;20:1862–1870. doi: 10.1021/tx700190s. [DOI] [PubMed] [Google Scholar]
- 77.Kuniyasu H., Oue N., Wakikawa A., Shigeishi N., Matsutani N., Kuraoka K., Ito R., Yokozaki H., Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2001;196:163–170. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- 78.Sparvero L., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A., Zeh H., Lotze M. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J. Transl. Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takada M., Ku Y., Toyama H., Suzuki Y., Kuroda Y. Suppressive effects of tea polyphenol and conformational changes with receptor for advanced glycation en products (RAGE) expression in human hepatoma cells. Hepatogastroenterology. 2002;49:928–931. [PubMed] [Google Scholar]
- 80.WHO: Cardiovascular diseases (CVDs). Fact sheet No317. [(accessed on 10 September 2009)]. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html.
- 81.Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 82.Jia C.P., Chen M.J., Huang S.Z., Zeng Y.T. A study of inductive effect of hemin on expression of the beta-globin genes in K562 cells. Yi Chuan. 2002;24:399–402. [PubMed] [Google Scholar]
- 83.Tanasescu M., Leitzmann M.F., Rimm E.B., Willett W.C., Stampfer M.J., Hu F.B. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 84.Twisk J., Gillian-Daniel D.L., Tebon A., Wang L., Barrett P.H., Attie A.D. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 2000;105:521–532. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arts I.C., Jacobs D.R., Jr., Harnack L.J., Gross M., Folsom A.R. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 86.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 87.Hertog M.G., Feskens E.J., Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- 88.Hertog M.G., Kromhout D., Aravanis C., Blackburn H., Buzina R., Fidanza F., Giampaoli S., Jansen A., Menotti A., Nedeljkovic S., et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 89.Knekt P., Jarvinen R., Reunanen A., Maatela J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.P., Nettleton J.A., Jacobs D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 91.Nakachi K., Matsuyama S., Miyake S., Suganuma M., Imai K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors. 2000;13:49–54. doi: 10.1002/biof.5520130109. [DOI] [PubMed] [Google Scholar]
- 92.Rein D., Paglieroni T.G., Pearson D.A., Wun T., Schmitz H.H., Gosselin R., Keen C.L. Cocoa and wine polyphenols modulate platelet activation and function. J. Nutr. 2000;130:2120S–2126S. doi: 10.1093/jn/130.8.2120S. [DOI] [PubMed] [Google Scholar]
- 93.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 94.Yochum L., Kushi L.H., Meyer K., Folsom A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 95.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 96.Peters U., Poole C., Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am. J. Epidemiol. 2001;154:495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 97.Di Castelnuovo A., Rotondo S., Iacoviello L., Donati M.B., De Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation. 2002;105:2836–2844. doi: 10.1161/01.cir.0000018653.19696.01. [DOI] [PubMed] [Google Scholar]
- 98.Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., Ryder J.J., Hall W.L., Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 99.Lin J., Rexrode K.M., Hu F., Albert C.M., Chae C.U., Rimm E.B., Stampfer M.J., Manson J.E. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am. J. Epidemiol. 2007;165:1305–1313. doi: 10.1093/aje/kwm016. [DOI] [PubMed] [Google Scholar]
- 100.Rimm E.B., Katan M.B., Ascherio A., Stampfer M.J., Willett W.C. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann. Intern. Med. 1996;125:384–389. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 101.Sesso H.D., Gaziano J.M., Liu S., Buring J.E. Flavonoid intake and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2003;77:1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- 102.Vita J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005;81:292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 103.Rein D., Lotito S., Holt R.R., Keen C.L., Schmitz H.H., Fraga C.G. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000;130:2109–2114. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 104.Stein J.H., Keevil J.G., Wiebe D.A., Aeschlimann S., Folts J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 105.Wan Y., Vinson J.A., Etherton T.D., Proch J., Lazarus S.A., Kris-Etherton P.M. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 106.Desch S., Schmidt J., Kobler D., Sonnabend M., Eitel I., Sareban M., Rahimi K., Schuler G., Thiele H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 107.Erlund I., Koli R., Alfthan G., Marniemi J., Puukka P., Mustonen P., Mattila P., Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008;87:323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 108.Grassi D., Necozione S., Lippi C., Croce G., Valeri L., Pasqualetti P., Desideri G., Blumberg J.B., Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 109.Taubert D., Roesen R., Lehmann C., Jung N., Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 110.Taubert D., Roesen R., Schomig E. Effect of cocoa and tea intake on blood pressure: A meta-analysis. Arch. Intern. Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 111.Park Y.K., Kim J.S., Kang M.H. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. Biofactors. 2004;22:145–147. doi: 10.1002/biof.5520220128. [DOI] [PubMed] [Google Scholar]
- 112.Heiss C., Dejam A., Kleinbongard P., Schewe T., Sies H., Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 113.Heiss C., Finis D., Kleinbongard P., Hoffmann A., Rassaf T., Kelm M., Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J. Cardiovasc. Pharmacol. 2007;49:74–80. doi: 10.1097/FJC.0b013e31802d0001. [DOI] [PubMed] [Google Scholar]
- 114.Heiss C., Kleinbongard P., Dejam A., Perre S., Schroeter H., Sies H., Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 115.Engler M.B., Engler M.M., Chen C.Y., Malloy M.J., Browne A., Chiu E.Y., Kwak H.K., Milbury P., Paul S.M., Blumberg J., Mietus-Snyder M.L. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 116.Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., Sies H., Kwik-Uribe C., Schmitz H.H., Kelm M. Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang-Polagruto J.F., Villablanca A.C., Polagruto J.A., Lee L., Holt R.R., Schrader H.R., Ensunsa J.L., Steinberg F.M., Schmitz H.H., Keen C.L. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J. Cardiovasc. Pharmacol. 2006;47(Suppl. 2):S177–S186; discussion S206-S209. doi: 10.1097/00005344-200606001-00013. [DOI] [PubMed] [Google Scholar]
- 118.Grassi D., Mulder T.P., Draijer R., Desideri G., Molhuizen H.O., Ferri C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J. Hypertens. 2009;27:774–781. doi: 10.1097/HJH.0b013e328326066c. [DOI] [PubMed] [Google Scholar]
- 119.Widlansky M.E., Hamburg N.M., Anter E., Holbrook M., Kahn D.F., Elliott J.G., Keaney J.F., Jr., Vita J.A. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007;26:95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cuevas A.M., Guasch V., Castillo O., Irribarra V., Mizon C., San Martin A., Strobel P., Perez D., Germain A.M., Leighton F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids. 2000;35:143–148. doi: 10.1007/BF02664763. [DOI] [PubMed] [Google Scholar]
- 121.Papamichael C., Karatzis E., Karatzi K., Aznaouridis K., Papaioannou T., Protogerou A., Stamatelopoulos K., Zampelas A., Lekakis J., Mavrikakis M. Red wine’s antioxidants counteract acute endothelial dysfunction caused by cigarette smoking in healthy nonsmokers. Am. Heart J. 2004;147:E5. doi: 10.1016/S0002. [DOI] [PubMed] [Google Scholar]
- 122.Pearson D.A., Paglieroni T.G., Rein D., Wun T., Schramm D.D., Wang J.F., Holt R.R., Gosselin R., Schmitz H.H., Keen C.L. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb. Res. 2002;106:191–197. doi: 10.1016/S0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- 123.Rein D., Paglieroni T.G., Wun T., Pearson D.A., Schmitz H.H., Gosselin R., Keen C.L. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 124.Keevil J.G., Osman H.E., Reed J.D., Folts J.D. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J. Nutr. 2000;130:53–56. doi: 10.1093/jn/130.1.53. [DOI] [PubMed] [Google Scholar]
- 125.Mathur S., Devaraj S., Grundy S.M., Jialal I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002;132:3663–3667. doi: 10.1093/jn/132.12.3663. [DOI] [PubMed] [Google Scholar]
- 126.Mao T.K., van de Water J., Keen C.L., Schmitz H.H., Gershwin M.E. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev. Immunol. 2002;9:135–141. doi: 10.1080/1044667031000137601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schramm D.D., Karim M., Schrader H.R., Holt R.R., Kirkpatrick N.J., Polagruto J.A., Ensunsa J.L., Schmitz H.H., Keen C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003;73:857–869. doi: 10.1016/s0024-3205(03)00373-4. [DOI] [PubMed] [Google Scholar]
- 128.Hollenberg N.K., Martinez G., McCullough M., Meinking T., Passan D., Preston M., Rivera A., Taplin D., Vicaria-Clement M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension. 1997;29:171–176. doi: 10.1161/01.hyp.29.1.171. [DOI] [PubMed] [Google Scholar]
- 129.Stensvold I., Tverdal A., Solvoll K., Foss O.P. Tea consumption. relationship to cholesterol, blood pressure, and coronary and total mortality. Prev. Med. 1992;21:546–553. doi: 10.1016/0091-7435(92)90062-M. [DOI] [PubMed] [Google Scholar]
- 130.Yang Y.C., Lu F.H., Wu J.S., Wu C.H., Chang C.J. The protective effect of habitual tea consumption on hypertension. Arch. Intern. Med. 2004;164:1534–1540. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]
- 131.Negishi H., Xu J.W., Ikeda K., Njelekela M., Nara Y., Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004;134:38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- 132.Uchida S., Ozaki M., Akashi T., Yamashita K., Niwa M., Taniyama K. Effects of (−)-epigallocatechin-3-O-gallate (green tea tannin) on the life span of stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. Suppl. 1995;22:S302–S303. doi: 10.1111/j.1440-1681.1995.tb02928.x. [DOI] [PubMed] [Google Scholar]
- 133.Duffy S.J., Keaney J.F., Jr., Holbrook M., Gokce N., Swerdloff P.L., Frei B., Vita J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 134.Hodgson J.M., Puddey I.B., Burke V., Beilin L.J., Jordan N. Effects on blood pressure of drinking green and black tea. J. Hypertens. 1999;17:457–463. doi: 10.1097/00004872-199917040-00002. [DOI] [PubMed] [Google Scholar]
- 135.Hodgson J.M., Puddey I.B., Burke V., Watts G.F., Beilin L.J. Regular ingestion of black tea improves brachial artery vasodilator function. Clin. Sci. (Lond.) 2002;102:195–201. doi: 10.1042/CS20010120. [DOI] [PubMed] [Google Scholar]
- 136.Bingham S.A., Vorster H., Jerling J.C., Magee E., Mulligan A., Runswick S.A., Cummings J.H. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br. J. Nutr. 1997;78:41–55. doi: 10.1079/bjn19970117. [DOI] [PubMed] [Google Scholar]
- 137.Knekt P., Isotupa S., Rissanen H., Heliovaara M., Jarvinen R., Hakkinen S., Aromaa A., Reunanen A. Quercetin intake and the incidence of cerebrovascular disease. Eur. J. Clin. Nutr. 2000;54:415–417. doi: 10.1038/sj.ejcn.1600974. [DOI] [PubMed] [Google Scholar]
- 138.Andrade A.C., Cesena F.H., Consolim-Colombo F.M., Coimbra S.R., Benjo A.M., Krieger E.M., Luz P.L. Short-term red wine consumption promotes differential effects on plasma levels of high-density lipoprotein cholesterol, sympathetic activity, and endothelial function in hypercholesterolemic, hypertensive, and healthy subjects. Clinics (Sao Paulo) 2009;64:435–442. doi: 10.1590/S1807-59322009000500011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spaak J., Merlocco A.C., Soleas G.J., Tomlinson G., Morris B.L., Picton P., Notarius C.F., Chan C.T., Floras J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diamete. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H605–H612. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- 140.Hansen A.S., Marckmann P., Dragsted L.O., Finne Nielsen I.L., Nielsen S.E., Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005;59:449–455. doi: 10.1038/sj.ejcn.1602107. [DOI] [PubMed] [Google Scholar]
- 141.Hodgson J.M., Burke V., Puddey I.B. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J. Hypertens. 2005;23:47–54. doi: 10.1097/00004872-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 142.Agewall S., Wright S., Doughty R.N., Whalley G.A., Duxbury M., Sharpe N. Does a glass of red wine improve endothelial function? Eur. Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- 143.Hashimoto M., Kim S., Eto M., Iijima K., Ako J., Yoshizumi M., Akishita M., Kondo K., Itakura H., Hosoda K., Toba K., Ouchi Y. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am. J. Cardiol. 2001;88:1457–1460. doi: 10.1016/s0002-9149(01)02137-3. [DOI] [PubMed] [Google Scholar]
- 144.Karatzi K., Papamichael C., Aznaouridis K., Karatzis E., Lekakis J., Matsouka C., Boskou G., Chiou A., Sitara M., Feliou G., Kontoyiannis D., Zampelas A., Mavrikakis M. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron. Artery Dis. 2004;15:485–490. doi: 10.1097/00019501-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 145.Whelan A.P., Sutherland W.H., McCormick M.P., Yeoman D.J., de Jong S.A., Williams M.J. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Intern. Med. J. 2004;34:224–228. doi: 10.1111/j.1444-0903.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 146.Appeldoorn M.M., Venema D.P., Peters T.H., Koenen M.E., Arts I.C., Vincken J.P., Gruppen H., Keijer J., Hollman P.C. Some phenolic compounds increase the nitric oxide level in endothelial cells in vitro. J. Agric. Food Chem. 2009;57:7693–7699. doi: 10.1021/jf901381x. [DOI] [PubMed] [Google Scholar]
- 147.Schmitt C.A., Dirsch V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 148.Fitzpatrick D.F., Hirschfield S.L., Ricci T., Jantzen P., Coffey R.G. Endothelium-dependent vasorelaxation caused by various plant extracts. J. Cardiovasc. Pharmacol. 1995;26:90–95. doi: 10.1097/00005344-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 149.Wallerath T., Deckert G., Ternes T., Anderson H., Li H., Witte K., Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.CIR.0000029925.18593.5C. [DOI] [PubMed] [Google Scholar]
- 150.Leikert J.F., Rathel T.R., Wohlfart P., Cheynier V., Vollmar A.M., Dirsch V.M. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation. 2002;106:1614–1617. doi: 10.1161/01.cir.0000034445.31543.43. [DOI] [PubMed] [Google Scholar]
- 151.Karim M., McCormick K., Kappagoda C.T. Effects of cocoa extracts on endothelium-dependent relaxation. J. Nutr. 2000;130:2105S–2108S. doi: 10.1093/jn/130.8.2105S. [DOI] [PubMed] [Google Scholar]
- 152.Chin-Dusting J.P., Fisher L.J., Lewis T.V., Piekarska A., Nestel P.J., Husband A. The vascular activity of some isoflavone metabolites: Implications for a cardioprotective role. Br. J. Pharmacol. 2001;133:595–605. doi: 10.1038/sj.bjp.0704088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fitzpatrick D.F., Bing B., Rohdewald P. Endothelium-dependent vascular effects of Pycnogenol. J. Cardiovasc. Pharmacol. 1998;32:509–515. doi: 10.1097/00005344-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 154.Fitzpatrick D.F., Hirschfield S.L., Coffey R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 155.Karamsetty M.R., Klinger J.R., Hill N.S. Phytoestrogens restore nitric oxide-mediated relaxation in isolated pulmonary arteries from chronically hypoxic rats. J. Pharmacol. Exp. Ther. 2001;297:968–974. [PubMed] [Google Scholar]
- 156.Woodman O.L., Chan E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004;31:786–790. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 157.Lorenz M., Wessler S., Follmann E., Michaelis W., Dusterhoft T., Baumann G., Stangl K., Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 158.Stoclet J.C., Chataigneau T., Ndiaye M., Oak M.H., El Bedoui J., Chataigneau M., Schini-Kerth V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 159.Corder R., Douthwaite J.A., Lees D.M., Khan N.Q., Viseu Dos Santos A.C., Wood E.G., Carrier M.J. Endothelin-1 synthesis reduced by red wine. Nature. 2001;414:863–864. doi: 10.1038/414863a. [DOI] [PubMed] [Google Scholar]
- 160.Khan N.Q., Lees D.M., Douthwaite J.A., Carrier M.J., Corder R. Comparison of red wine extract and polyphenol constituents on endothelin-1 synthesis by cultured endothelial cells. Clin. Sci. (Lond.) 2002;103(Suppl. 48):72–75. doi: 10.1042/CS103S072S. [DOI] [PubMed] [Google Scholar]
- 161.Steffen Y., Gruber C., Schewe T., Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 162.Steffen Y., Schewe T., Sies H. Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 163.Pignatelli P., Pulcinelli F.M., Celestini A., Lenti L., Ghiselli A., Gazzaniga P.P., Violi F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am. J. Clin. Nutr. 2000;72:1150–1155. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- 164.Freedman J.E., Parker C. III, Li L., Perlman J.A., Frei B., Ivanov V., Deak L.R., Iafrati M.D., Folts J.D. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 165.Natella F., Nardini M., Belelli F., Pignatelli P., Di Santo S., Ghiselli A., Violi F., Scaccini C. Effect of coffee drinking on platelets: Inhibition of aggregation and phenols incorporation. Br. J. Nutr. 2008;100:1276–1282. doi: 10.1017/S0007114508981459. [DOI] [PubMed] [Google Scholar]
- 166.Steptoe A., Gibson E.L., Vuononvirta R., Hamer M., Wardle J., Rycroft J.A., Martin J.F., Erusalimsky J.D. The effects of chronic tea intake on platelet activation and inflammation: A double-blind placebo controlled trial. Atherosclerosis. 2007;193:277–282. doi: 10.1016/j.atherosclerosis.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 167.Gresele P., Pignatelli P., Guglielmini G., Carnevale R., Mezzasoma A.M., Ghiselli A., Momi S., Violi F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J. Nutr. 2008;138:1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 168.Holt R.R., Actis-Goretta L., Momma T.Y., Keen C.L. Dietary flavanols and platelet reactivity. J. Cardiovasc. Pharmacol. 2006;47(Suppl. 2):S187–S196; discussion S206-S209. doi: 10.1097/00005344-200606001-00014. [DOI] [PubMed] [Google Scholar]
- 169.Murphy K.J., Chronopoulos A.K., Singh I., Francis M.A., Moriarty H., Pike M.J., Turner A.H., Mann N.J., Sinclair A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 170.Peppa M., Raptis S. Advanced glycation end products and cardiovascular disease. Curr. Diabetes Rev. 2008;4:92–100. doi: 10.2174/157339908784220732. [DOI] [PubMed] [Google Scholar]
- 171.Schramm D., German J. Potential effects of flavonoids on the etiology of vascular disease. J. Nutr. Biochem. 1998;9:560–566. [Google Scholar]
- 172.Huang S.-M., Wu C.-H., Yen G.-C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006;50:1129–1139. doi: 10.1002/mnfr.200600075. [DOI] [PubMed] [Google Scholar]
- 173.Kim J., Lee E., Kim D., Yu B., Chung H. Kaempferol modulates pro-inflammatory NF-kB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age (Dordr.) 2010;32:197–208. doi: 10.1007/s11357-009-9124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hy L.X., Keller D.M. Prevalence of AD among whites: A summary by levels of severity. Neurology. 2000;55:198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- 175.Nussbaum R.L., Ellis C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 176.de Lau L.M., Breteler M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 177.Tanner C.M., Goldman S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barzilai A., Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol. Med. 2003;9:126–132. doi: 10.1016/s1471-4914(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 179.Jellinger K.A. Cell death mechanisms in neurodegeneration. J. Cell. Mol. Med. 2001;5:1–17. doi: 10.1111/j.1582-4934.2001.tb00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spires T.L., Hannan A.J. Nature, nurture and neurology: Gene-environment interactions in neurodegenerative disease. FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005;272:2347–2361. doi: 10.1111/j.1742-4658.2005.04677.x. [DOI] [PubMed] [Google Scholar]
- 181.Lindsay J., Laurin D., Verreault R., Hebert R., Helliwell B., Hill G.B., McDowell I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 182.Orgogozo J.M., Dartigues J.F., Lafont S., Letenneur L., Commenges D., Salamon R., Renaud S., Breteler M.B. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. (Paris) 1997;153:185–192. [PubMed] [Google Scholar]
- 183.Truelsen T., Thudium D., Gronbaek M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]
- 184.Commenges D., Scotet V., Renaud S., Jacqmin-Gadda H., Barberger-Gateau P., Dartigues J.F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000;16:357–363. doi: 10.1023/A:1007614613771. [DOI] [PubMed] [Google Scholar]
- 185.Letenneur L., Proust-Lima C., Le G.A., Dartigues J.F., Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 186.Morris M.C., Evans D.A., Tangney C.C., Bienias J.L., Wilson R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dai Q., Borenstein A.R., Wu Y., Jackson J.C., Larson E.B. Fruit and vegetable juices and Alzheimer’s disease: The Kame Project. Am. J. Med. 2006;119:751–759. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 189.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 190.Manach C., Williamson G., Morand C., Scalbert A., Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230–242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 191.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243–255. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 192.Youdim K.A., Qaiser M.Z., Begley D.J., Rice-Evans C.A., Abbott N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 193.Passamonti S., Vrhovsek U., Vanzo A., Mattivi F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005;53:7029–7034. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- 194.Kalt W., Blumberg J.B., McDonald J.E., Vinqvist-Tymchuk M.R., Fillmore S.A., Graf B.A., O’Leary J.M., Milbury P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 195.Milbury P.E., Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J. Agric. Food Chem. 2010;58:3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- 196.Youdim K.A., Joseph J.A. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radic. Biol. Med. 2001;30:583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 197.Inanami O., Watanabe Y., Syuto B., Nakano M., Tsuji M., Kuwabara M. Oral administration of (-)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Radic. Res. 1998;29:359–365. doi: 10.1080/10715769800300401. [DOI] [PubMed] [Google Scholar]
- 198.Luo Y., Smith J.V., Paramasivam V., Burdick A., Curry K.J., Buford J.P., Khan I., Netzer W.J., Xu H., Butko P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bastianetto S., Zheng W.H., Quirion R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: Involvement of its flavonoid constituents and protein kinase C. J. Neurochem. 2000;74:2268–2277. doi: 10.1046/j.1471-4159.2000.0742268.x. [DOI] [PubMed] [Google Scholar]
- 200.Tchantchou F., Xu Y., Wu Y., Christen Y., Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 201.Hsieh H.-M., Wua W.-M., Hu M.-L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem. Toxicol. 2009;47:625–632. doi: 10.1016/j.fct.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 202.Shan Q., Lu J., Zheng Y., Li J., Zhou Z., Hu B., Zhang Z., Fan S., Mao Z., Wang Y.-J., Ma D. Purple Sweet Potato Color Ameliorates Cognition Deficits and Attenuates Oxidative Damage and Inflammation in AgingMouse Brain Induced by D-Galactose. J. Biomed. Biotechnol. 2009;2009:564737. doi: 10.1155/2009/564737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Munch G., Thome J., Foley P., Schinzel R., Riederer P. Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res. Brain Res. Rev. 1997;23:134–143. doi: 10.1016/s0165-0173(96)00016-1. [DOI] [PubMed] [Google Scholar]
- 204.Ramasamy R., Vannucci S., Yan S., Herold K., Yan S., Schmidt A. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16–28. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 205.Datla K.P., Christidou M., Widmer W.W., Rooprai H.K., Dexter D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport. 2001;12:3871–3875. doi: 10.1097/00001756-200112040-00053. [DOI] [PubMed] [Google Scholar]
- 206.Vauzour D., Corona G., Spencer J.P. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010;501:106–111. doi: 10.1016/j.abb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 207.Vauzour D., Vafeiadou K., Corona G., Pollard S.E., Tzounis X., Spencer J.P. Champagne wine polyphenols protect primary cortical neurons against peroxynitrite-induced injury. J. Agric. Food Chem. 2007;55:2854–2860. doi: 10.1021/jf063304z. [DOI] [PubMed] [Google Scholar]
- 208.Spencer J.P. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008;67:238–252. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- 209.Spencer J.P. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008;99(E-Suppl. 1):ES60–ES77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- 210.Rendeiro C., Spencer J.P., Vauzour D., Butler L.T., Ellis J.A., Williams C.M. The impact of flavonoids on spatial memory in rodents: From behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009;4:251–270. doi: 10.1007/s12263-009-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Krikorian R., Nash T.A., Shidler M.D., Shukitt-Hale B., Joseph J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010;103:730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- 213.Macready A.L., Kennedy O.B., Ellis J.A., Williams C.M., Spencer J.P., Butler L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009;4:227–242. doi: 10.1007/s12263-009-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.How P.S., Cox R., Ellis J.A., Spencer J.P.E. The impact of plant-derived flavonoids on mood, memory and motor skills in UK adults. Proc. Nutr. Soc. 2007;66:87A. [Google Scholar]
- 215.Krikorian R., Shidler M.D., Nash T.A., Kalt W., Vinqvist-Tymchuk M.R., Shukitt-Hale B., Joseph J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Joseph J.A., Shukitt-Hale B., Denisova N.A., Prior R.L., Cao G., Martin A., Taglialatela G., Bickford P.C. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J. Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Joseph J.A., Shukitt-Hale B., Denisova N.A., Bielinski D., Martin A., McEwen J.J., Bickford P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Casadesus G., Shukitt-Hale B., Stellwagen H.M., Zhu X., Lee H.G., Smith M.A., Joseph J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 219.Williams C.M., El Mohsen M.A., Vauzour D., Rendeiro C., Butler L.T., Ellis J.A., Whiteman M., Spencer J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 220.Ramirez M.R., Izquierdo I., do Carmo Bassols Raseira M., Zuanazzi J.A., Barros D., Henriques A.T. Effect of lyophilised Vaccinium berries on memory, anxiety and locomotion in adult rats. Pharmacol. Res. 2005;52:457–462. doi: 10.1016/j.phrs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 221.Barros D., Amaral O.B., Izquierdo I., Geracitano L., do Carmo Bassols Raseira M., Henriques A.T., Ramirez M.R. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol. Biochem. Behav. 2006;84:229–234. doi: 10.1016/j.pbb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 222.Goyarzu P., Malin D.H., Lau F.C., Taglialatela G., Moon W.D., Jennings R., Moy E., Moy D., Lippold S., Shukitt-Hale B., Joseph J.A. Blueberry supplemented diet: Effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr. Neurosci. 2004;7:75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- 223.Shukitt-Hale B., Carey A., Simon L., Mark D.A., Joseph J.A. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 224.Chan Y.C., Hosoda K., Tsai C.J., Yamamoto S., Wang M.F. Favorable effects of tea on reducing the cognitive deficits and brain morphological changes in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. 2006;52:266–273. doi: 10.3177/jnsv.52.266. [DOI] [PubMed] [Google Scholar]
- 225.Hartman R.E., Shah A., Fagan A.M., Schwetye K.E., Parsadanian M., Schulman R.N., Finn M.B., Holtzman D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 226.Bhat N.R., Zhang P., Lee J.C., Hogan E.L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clostre F. Gingko Biloba extract (EGb 761). State of knowledge in the dawn of the year 2000. Ann. Pharm. Fr. 1999;57:1S8–1S88. [PubMed] [Google Scholar]
- 228.Cohen-Salmon C., Venault P., Martin B., Raffalli-Sebille M.J., Barkats M., Clostre F., Pardon M.C., Christen Y., Chapouthier G. Effects of Ginkgo biloba extract (EGb 761) on learning and possible actions on aging. J. Physiol. Paris. 1997;91:291–300. doi: 10.1016/S0928-4257(97)82409-6. [DOI] [PubMed] [Google Scholar]
- 229.Diamond B.J., Shiflett S.C., Feiwel N., Matheis R.J., Noskin O., Richards J.A., Schoenberger N.E. Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 2000;81:668–678. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 230.Itil T.M., Eralp E., Ahmed I., Kunitz A., Itil K.Z. The pharmacological effects of Gingko Biloba, a plant extract, on the brain of dementia patients in comparinson with tacrine. Psychopharmacology. 1998;34:391–396. [PubMed] [Google Scholar]
- 231.Shif O., Gillette K., Damkaoutis C.M., Carrano C., Robbins S.J., Hoffman J.R. Effects of Ginkgo biloba administered after spatial learning on water maze and radial arm maze performance in young adult rats. Pharmacol. Biochem. Behav. 2006;84:17–25. doi: 10.1016/j.pbb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 232.Spencer J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–273. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Vauzour D., Vafeiadou K., Rice-Evans C., Williams R.J., Spencer J.P. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J. Neurochem. 2007;103:1355–1367. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- 234.Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 235.Winter J.C. The effects of an extract of Ginkgo biloba, EGb 761, on cognitive behavior and longevity in the rat. Physiol. Behav. 1998;63:425–433. doi: 10.1016/S0031-9384(97)00464-2. [DOI] [PubMed] [Google Scholar]
- 236.Pu F., Mishima K., Irie K., Motohashi K., Tanaka Y., Orito K., Egawa T., Kitamura Y., Egashira N., Iwasaki K., Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 2007;104:329–334. doi: 10.1254/jphs.fp0070247. [DOI] [PubMed] [Google Scholar]
- 237.Maher P., Akaishi T., Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stoll S., Scheuer K., Pohl O., Muller W.E. Ginkgo biloba extract (EGb 761) independently improves changes in passive avoidance learning and brain membrane fluidity in the aging mouse. Pharmacopsychiatry. 1996;29:144–149. doi: 10.1055/s-2007-979561. [DOI] [PubMed] [Google Scholar]
- 239.Topic B., Hasenohrl R.U., Hacker R., Huston J.P. Enhanced conditioned inhibitory avoidance by a combined extract of Zingiber officinale and Ginkgo biloba. Phytother. Res. 2002;16:312–315. doi: 10.1002/ptr.870. [DOI] [PubMed] [Google Scholar]
- 240.Vafeiadou K., Vauzour D., Lee H.Y., Rodriguez-Mateos A., Williams R.J., Spencer J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009;484:100–109. doi: 10.1016/j.abb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 241.Wang X., Chen S., Ma G., Ye M., Lu G. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport. 2005;16:267–270. doi: 10.1097/00001756-200502280-00013. [DOI] [PubMed] [Google Scholar]
- 242.Bhat N.R., Feinstein D.L., Shen Q., Bhat A.N. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J. Biol. Chem. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 243.Lee S.-J., Lee K.-W. Protective Effect of (−)-Epigallocatechin Gallate against Advanced Glycation Endproducts-Induced Injury in Neuronal Cells. Biol. Pharm. Bull. 2007;30:1369–1373. doi: 10.1248/bpb.30.1369. [DOI] [PubMed] [Google Scholar]
- 244.Vlahos C.J., Matter W.F., Hui K.Y., Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 245.Spencer J.P., Rice-Evans C., Williams R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003;278:34783–34793. doi: 10.1074/jbc.M305063200. [DOI] [PubMed] [Google Scholar]