Abstract
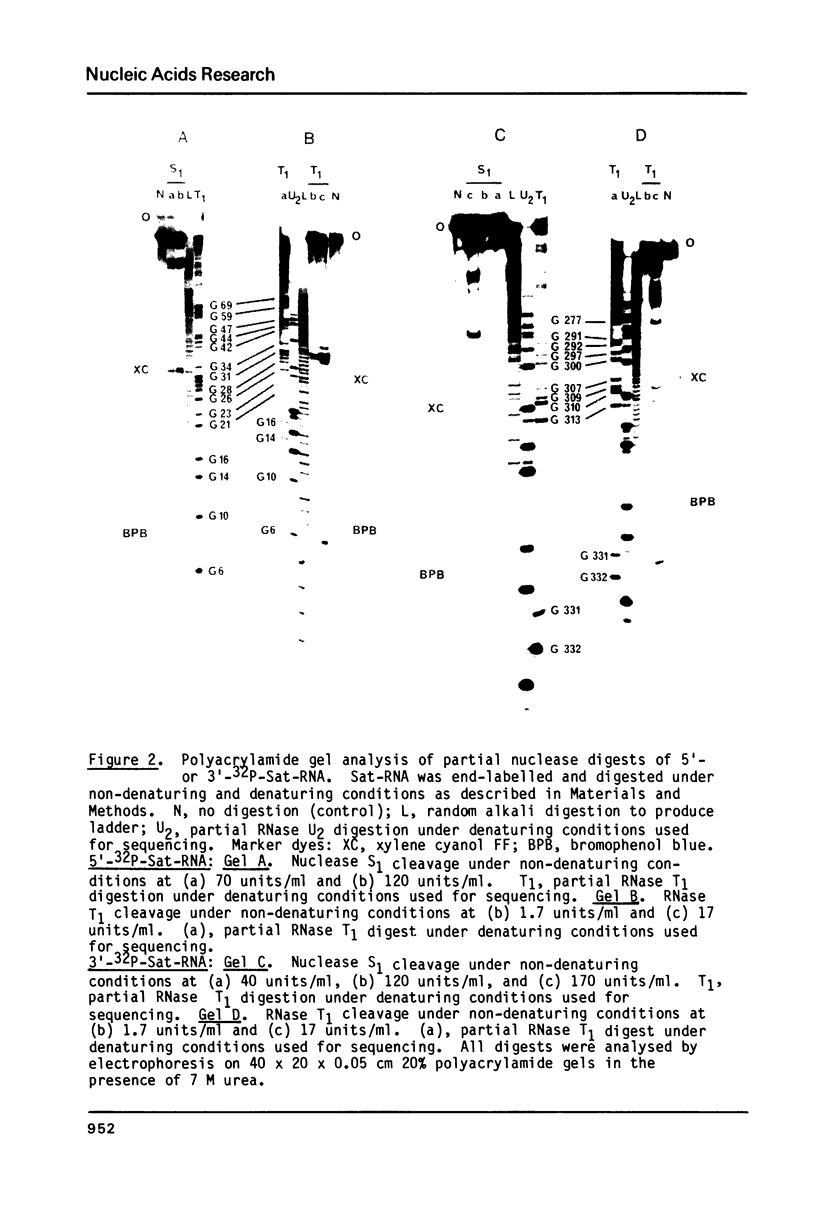
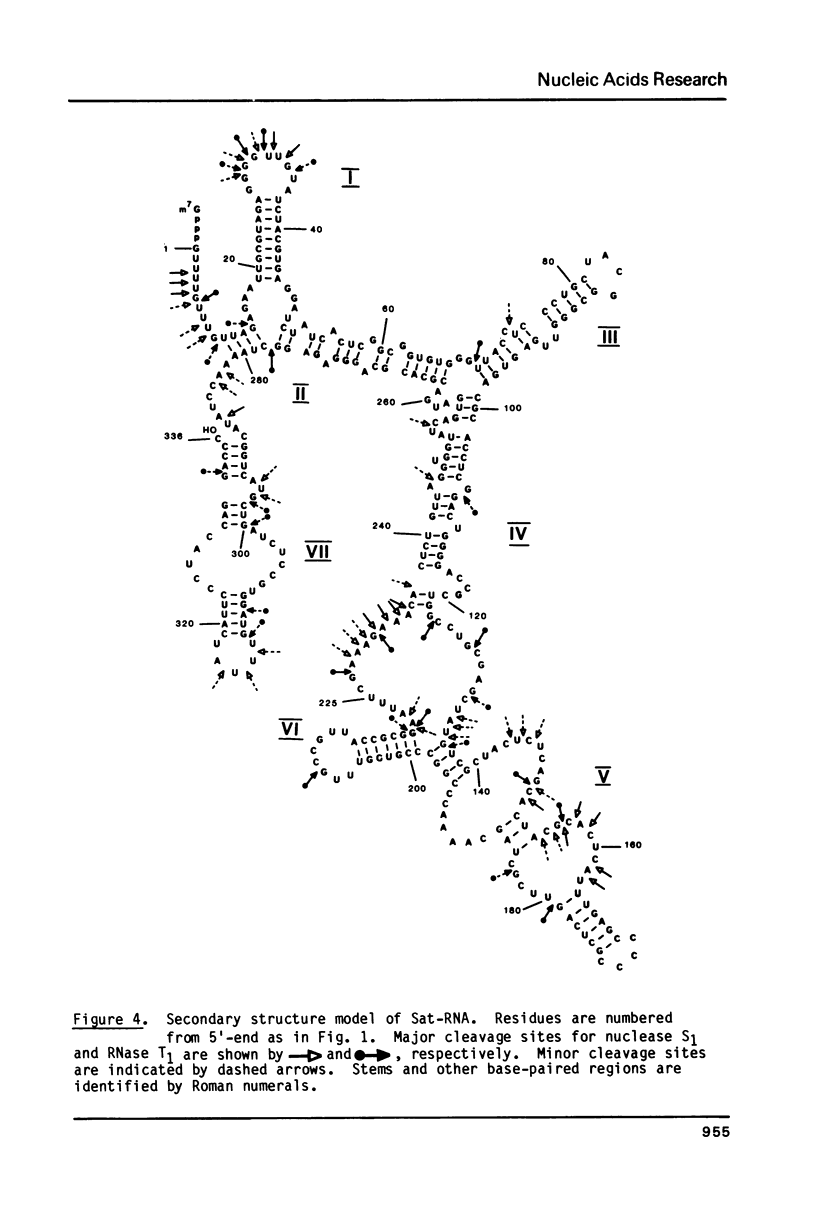
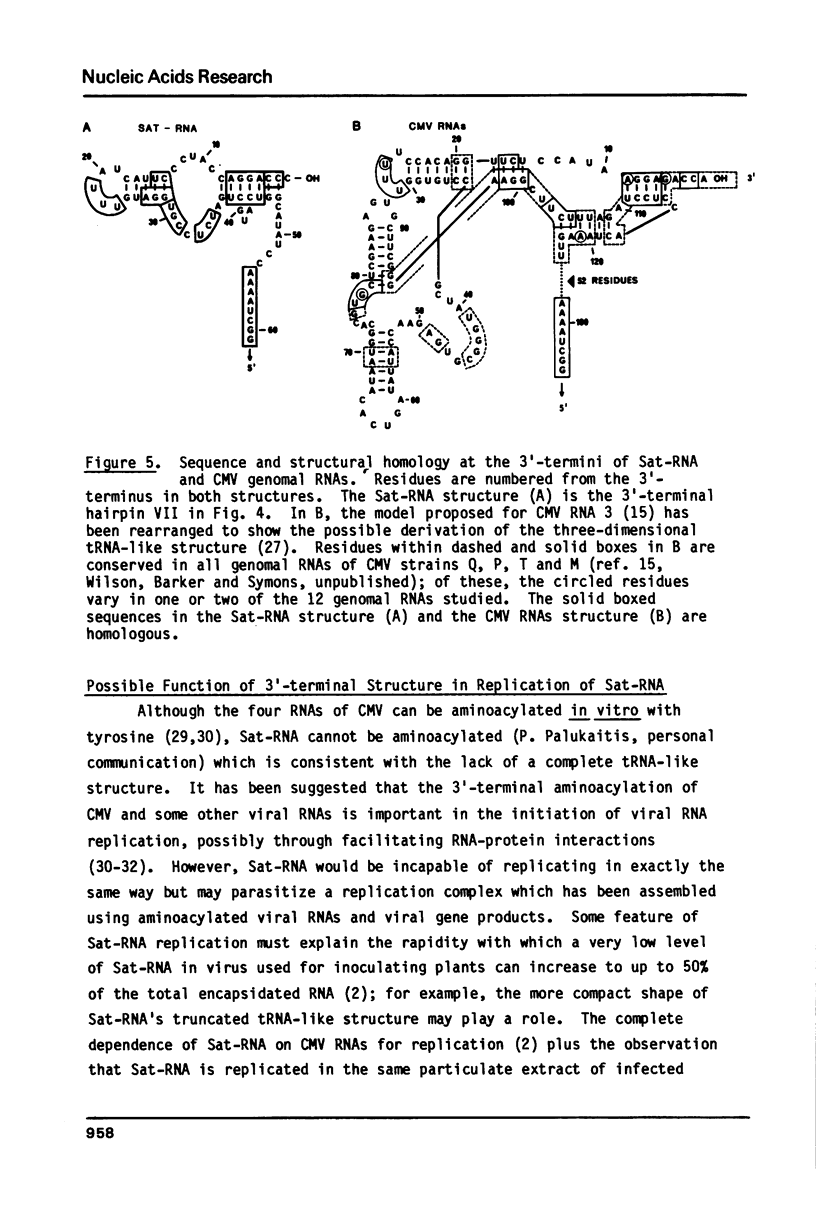
Sat-RNA is one of several replicating satellite RNAs which have been isolated from RNA encapsidated in cucumber mosaic virus (CMV) and which are totally dependent on CMV for replication. The 336 residue sequence of Sat-RNA obtained using the dideoxynucleotide chain termination and partial enzymic digestion procedures shows only a few short stretches (up to 11 residues) of sequence homology with one of the three CMV genomal RNAs so far sequenced. Sat-RNA has 88% sequence homology with another, previously sequenced, satellite RNA of CMV, CARNA 5. Analysis of partial digests of 5'- or 3' -32P-Sat-RNA with nuclease S1 or RNase T1 under non-denaturing conditions showed that only about 10% of the residues in Sat-RNA were cleaved. Further data on base-paired segments of Sat-RNA were obtained using digestion with RNase T1 followed by electrophoretic fractionation of the resulting fragments under both non-denaturing and denaturing conditions. On the basis of this data, a complete secondary structure model is proposed for Sat-RNA with 52% of its residues involved in base pairs. A prominent hairpin at the 3'-terminus of Sat-RNA shows considerable sequence and structural homology with parts of the 3'-terminal tRNA-like structure of the CMV genomal RNAs.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diaz-Ruiz J. R., Kaper J. M. Cucumber mosaic virus-associated RNA 5. III. Little nucleotide sequence homology between CARNA 5 and helper RNA. Virology. 1977 Jul 1;80(1):204–213. doi: 10.1016/0042-6822(77)90393-2. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Wells R. D. Action of single-strand specific nucleases on model DNA heteroduplexes of defined size and sequence. Biochemistry. 1977 May 31;16(11):2374–2379. doi: 10.1021/bi00630a010. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Symons R. H. The use of hybridization analysis with complementary DNA to determine the RNA sequence homology between strains of plant viruses: its application to several strains of cucumoviruses. Virology. 1978 Jul 15;88(2):361–370. doi: 10.1016/0042-6822(78)90292-1. [DOI] [PubMed] [Google Scholar]
- Gould A. R., Palukaitis P., Symons R. H., Mossop D. W. Characterization of a satellite RNA associated with cucumber mosaic virus. Virology. 1978 Feb;84(2):443–455. doi: 10.1016/0042-6822(78)90261-1. [DOI] [PubMed] [Google Scholar]
- Gould A. R., Symons R. H. Cucumber mosaic virus RNA 3. Determination of the nucleotide sequence provides the amino acid sequences of protein 3A and viral coat protein. Eur J Biochem. 1982 Aug;126(2):217–226. [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Diaz-Ruiz J. R., Tolin S. A. Peanut stunt virus-associated RNA 5: second tripartite genome virus with an associated satellite-like replicating RNA. Virology. 1978 Jul 1;88(1):166–170. doi: 10.1016/0042-6822(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Lot H. A low molecular weight replicating RNA associated with a divided genome plant virus: defective or satellite RNA? Biochem Biophys Res Commun. 1976 Oct 18;72(4):1237–1243. [PubMed] [Google Scholar]
- Kim S. H. Three-dimensional structure of transfer RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:181–216. doi: 10.1016/s0079-6603(08)60070-7. [DOI] [PubMed] [Google Scholar]
- Kohl R. J., Hall T. C. Aminoacylation of RNA from several viruses: amino acid specificity and differential activity of plant, yeast and bacterial synthetases. J Gen Virol. 1974 Nov;25(2):257–261. doi: 10.1099/0022-1317-25-2-257. [DOI] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Brederode F. T., Veeneman G., van Boom J. H., Bol J. F. Nucleotide sequences at the 5'-termini of the alfalfa mosaic virus RNAs and the intercistronic junction in RNA 3. Nucleic Acids Res. 1980 Dec 11;8(23):5635–5647. doi: 10.1093/nar/8.23.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Symons R. H. Cucumber mosaic virus contains a functionally divided genome. Virology. 1973 Jun;53(2):487–492. doi: 10.1016/0042-6822(73)90232-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber J. R., Loeb L. A. S1 nuclease does not cleave DNA at single-base mis-matches. Biochim Biophys Acta. 1981 Dec 28;656(2):256–264. doi: 10.1016/0005-2787(81)90094-0. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Extensive sequence homology at the 3'-termini of the four RNAs of cucumber mosaic virus. Nucleic Acids Res. 1979 Oct 25;7(4):825–837. doi: 10.1093/nar/7.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth H. E., Kaper J. M., Tousignant M. E. CARNA 5, the Small Cucumber Mosaic Virus--Dependent Replicating RNA, Regulates Disease Expression. Science. 1979 May 25;204(4395):845–847. doi: 10.1126/science.204.4395.845. [DOI] [PubMed] [Google Scholar]




