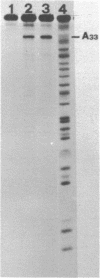
Abstract
We have examined the accessibility to diethylpyrocarbonate of spinach chloroplast 4.5S ribosomal RNA when free and when it is part of the ribosomal structure. The modifications in free 4.5S RNA were found mostly in single-stranded regions of the secondary structure model proposed in our previous paper (Kumagai, I. et al. (1982) J.B.C. 257, 12924-28): adenines at positions 17, 19, 33, 36, 54, 55, 60, 64, 68, 72, 77, 86 and 87 were identified as the reactive residues. On the other hand, in 4.5S RNA in 70S ribosomes or 50S subunits, adenine 33 was exclusively modified, and its reactivity was much higher than in free 4.5S RNA. This highly accessible A33 of spinach 4.5S RNA is located within a characteristic seven nucleotide sequence, which is found in the 4.5S rRNAs from spinach, tobacco and a fern but deleted in 4.5S RNAs from maize and wheat.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch M., Kimura M., Subramanian A. R. Purification, primary structure, and homology relationships of a chloroplast ribosomal protein. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6871–6875. doi: 10.1073/pnas.79.22.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Dyer T. A. 4.5S ribonucleic acid, a novel ribosome component in the chloroplasts of flowering plants. Biochem J. 1979 Dec 1;183(3):605–613. doi: 10.1042/bj1830605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Kössel H. The rRNA operon from Zea mays chloroplasts: nucleotide sequence of 23S rDNA and its homology with E.coli 23S rDNA. Nucleic Acids Res. 1981 Jun 25;9(12):2853–2869. doi: 10.1093/nar/9.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Hartley M. R. The synthesis and origin of chloroplast low-molecular-weight ribosomal ribonucleic acid in spinach. Eur J Biochem. 1979 May 15;96(2):311–320. doi: 10.1111/j.1432-1033.1979.tb13042.x. [DOI] [PubMed] [Google Scholar]
- Kumagai I., Pieler T., Subramanian A. R., Erdmann V. A. Nucleotide sequence and secondary structure analysis of spinach chloroplast 4.5 S RNA. J Biol Chem. 1982 Nov 10;257(21):12924–12928. [PubMed] [Google Scholar]
- Lo A. C., Nazar R. N. Accessibility of the 5 S RNA in yeast ribosomes. J Mol Biol. 1982 Jul 5;158(3):559–565. doi: 10.1016/0022-2836(82)90215-7. [DOI] [PubMed] [Google Scholar]
- Lo A. C., Nazar R. N. Topography of 5.8 S rRNA in rat liver ribosomes. Identification of diethyl pyrocarbonate-reactive sites. J Biol Chem. 1982 Apr 10;257(7):3516–3524. [PubMed] [Google Scholar]
- Machatt M. A., Ebel J. P., Branlant C. The 3'-terminal region of bacterial 23S ribosomal RNA: structure and homology with the 3'-terminal region of eukaryotic 28S rRNA and with chloroplast 4.5s rRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1533–1549. doi: 10.1093/nar/9.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Herr W. Chemical probing of the tRNA--ribosome complex. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2273–2277. doi: 10.1073/pnas.78.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Kusuda M., Sugiura M. The nucleotide sequence of chloroplast 4.5S rRNA from a fern, Dryopteris acuminata. Nucleic Acids Res. 1982 Apr 10;10(7):2257–2260. doi: 10.1093/nar/10.7.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. The nucleotide sequence of 4.5S ribosomal RNA from tobacco chloroplasts. Nucleic Acids Res. 1980 Sep 25;8(18):4125–4129. doi: 10.1093/nar/8.18.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. R., Leaver C. J., Bottomley W., Atchison B. Low-molecular-weight (4.5S) ribonucleic acid in higher-plant chloroplast ribosomes. Biochem J. 1978 Dec 1;175(3):1103–1112. doi: 10.1042/bj1751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Nazar R. N. Nucleotide sequence of wheat chloroplastid 4.5 S ribonucleic acid. Sequence homologies in 4.5 S RNA species. J Biol Chem. 1980 Dec 25;255(24):11896–11900. [PubMed] [Google Scholar]
- Wrede P., Wurst R., Vournakis J., Rich A. Conformational changes of yeast tRNAPhe and E. coli tRNA2Glu as indicated by different nuclease digestion patterns. J Biol Chem. 1979 Oct 10;254(19):9608–9616. [PubMed] [Google Scholar]