Abstract
Bioactive peptides have been identified in a range of foods, including plant, milk and muscle, e.g., beef, chicken, pork and fish muscle proteins. Bioactive peptides from food proteins offer major potential for incorporation into functional foods and nutraceuticals. The aim of this paper is to present an outline of the bioactive peptides identified in the muscle protein of meat to date, with a focus on muscle protein from domestic animals and fish. The majority of research on bioactives from meat sources has focused on angiotensin-1-converting enzyme (ACE) inhibitory and antioxidant peptides.
Keywords: angiotensin converting enzyme inhibitors, bioactive peptides, meat, fish
1. Introduction
Food proteins have long been recognized for their nutritional and functional properties. The nutritional properties of proteins are associated with their amino acid content in conjunction with the physiological utilization of specific amino acids upon digestion and absorption [1,2]. On the other hand, the functional properties of proteins relate to their contribution to the physiochemical and sensory properties of foods [2,3]. In recent years, a considerable amount of research has also focused on the liberation of bioactive peptides which are encrypted within food proteins, with a view to utilizing such peptides as functional food ingredients aimed at health maintenance.
Bioactive peptides have been defined as “food derived components (genuine or generated) that, in addition to their nutritional value exert a physiological effect in the body” [4]. Interestingly, within the parent protein sequence, the peptides are inactive and thus must be released to exert an effect. These bioactive peptides are usually 2–20 amino acid residues in length, although, some have been reported to be >20 amino acid residues. Bioactive peptides may be absorbed through the intestine where they subsequently enter the circulatory system intact to exert various physiological effects, or they may produce local effects in the digestive tract [5]. Food derived bioactive peptides have been shown to display a wide range of physiological functions including antihypertensive, antioxidative, opioid agonistic, immunomodulatory, antimicrobial, prebiotic, mineral binding, antithrombotic and hypocholesterolemic effects [6].
Meat and fish provide valuable sources of protein for many populations around the world; furthermore, meat and fish proteins offer huge potential as novel sources of bioactive peptides. To date, bioactive peptides displaying antihypertensive, antioxidant, antimicrobial and antiproliferative effects have been found in the hydrolysates of meat and fish proteins [7,8,9,10].
History of Bioactive Peptide Discovery
The first food-derived bioactive peptide was identified in 1950 when Mellander reported that casein phosphorylated peptides enhanced vitamin D-independent bone calcification in rachitic infants [11]. However, interest in this field has increased considerably in the last two decades, with the majority of research focusing on the identification of bioactive peptides from milk proteins [12,13]. Bioactive peptides are inactive or latent in the parent protein but are released in an active form after proteolytic digestion [14]. Methods employed in the proteolytic digestion of parent proteins include hydrolysis by digestive enzymes [15], plant and bacterial proteases [16,17], and following microbial fermentation [18,19,20].
The most widely reported bioactive peptides from milk and other food sources display antihypertensive activity, particularly those peptides which inhibit the action of angiotensin-1-converting enzyme (ACE) [21,22]. Indeed, currently a number of commercial products which contain such peptides have been released onto the market. Perhaps the two most widely known products available are both fermented milk-based products, i.e., Calpis® and Evolus®. Calpis® is a sour milk product from Japan which contains the antihypertensive peptides VPP and IPP both derived from milk caseins. Evolus® is a Finnish product that claims to reduce blood pressure. Evolus® also contains the peptides VPP and IPP which correspond to the fragments of β-casein f (84–86) and κ-casein f (74–76) [14]. Various milk protein hydrolysates which claim to contain bioactive peptides are also available as food ingredients. For example C12®, a casein derived peptide supplied by DMV International, claims to reduce blood pressure.
2. Production of Bioactive Peptides
While numerous methods have been utilized to release bioactive peptides from food proteins, enzymatic hydrolysis of whole protein is the most widely used technique. For example, a number of bioactive peptides have been isolated from meat using digestive enzymes such as pepsin, trypsin and chymotrypsin. Indeed, peptic digestion of porcine skeletal muscles has resulted in the generation of numerous ACE inhibitory peptides [23,24]. Various proteases from bacterial, animal and plant origin have also been used to generate bioactive peptides from meat sources [10,25,26].
Several researchers have described the isolation of bioactive peptides following bacterial fermentation of milk proteins where lactobacilli have been commonly used [27,28,29]. However, microbial fermentation of meat proteins has been less successful, presumably due to the poor proteolytic activity of the lactobacilli used in meat fermentations [30,31]. Indeed, to the best of our knowledge, no bioactive peptides have been produced from the microbial fermentation of muscle proteins.
Identification of Bioactive Peptides
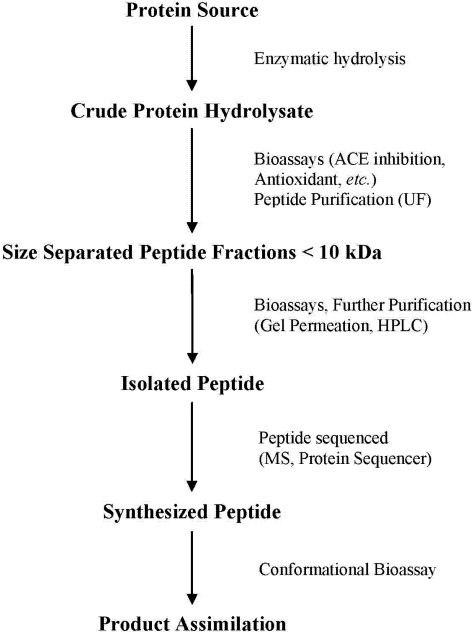
Following the hydrolysis of the protein substrate (Figure 1), the hydrolysates are assayed for various bioactivities. After the detection of bioactivity within the crude protein hydrolysates, the hydrolysates are then fractionated based on peptide size, which is typically performed using ultrafiltration [32,33,34]. The hydrolysate fraction displaying the highest bioactivity is then further purified to separate individual peptides using different techniques, most notably reverse phase high performance liquid chromatography (RP-HPLC) or gel permeation chromatography [15,22,35]. Individual peptide fractions are identified using the combined techniques of mass spectrometry and protein sequencing. Lastly, a synthetic version of the peptide is synthesized and the assay is repeated to verify bioactivity [21,36,37].
Figure 1.
Procedure for the isolation and identification of bioactive peptides from food proteins, adapted from Arihara & Ohata [30].
3. Antihypertensive Peptides
Bioactive peptides have been found in a wide range of foods, and as already mentioned, the most extensively researched of these are the antihypertensive peptides, particularly those which inhibit ACE [3,22]. ACE inhibitory peptides were first discovered in snake venom [38] and since then numerous synthetic ACE inhibitors have been produced, with Captopril being the most common. Additionally, Captopril and other synthetic ACE inhibitors are known to exert various side-effects such as coughing, taste disturbances and skin rashes [35,39]. These side effects, coupled with the fact that hypertension affects one third of the Western worlds’ population and is a known risk factor for stroke and cardiovascular disease, has contributed to the ongoing search for food derived antihypertensive peptides for exploitation as antihypertensive agents in functional foods and nutraceuticals [40,41].
3.1. Mechanism of ACE Inhibition
ACE, or kininase ІІ, is a dipeptidyl carboxy peptidase (EC 3.4.15.1) found in various tissues in the body and is integral to the moderation of blood pressure and normal heart function [40]. In the rennin-angiotensin system, ACE catalyses the conversion of the inactive form of angiotensin I (Ang І) to the potent vasoconstrictor angiotensin II (Ang ІІ). Additionally, ACE is involved in the deactivation of the hypotensive peptide, bradykinin [32]. This is as a result of ACE cleaving the C-terminal dipeptide from Ang І (His-Leu) and bradykinin. Ang ІІ is a documented potent vasoconstrictor which acts directly on vascular smooth muscle cells. Ang ІІ is also responsible for the expansion of vascular volume via sodium retention and fluid retention [42,43,44,45]. Bradykinin is responsible for uterine and ileal smooth muscle contraction, enhanced vascular permeability, activation of peripheral and C fibers and increases in mucous secretion [42,46]. Furthermore, and more notably bradykinin contributes to vasodilation by advancing the assembly of arachidonic acid metabolites, nitric oxide, and endothelium-derived hyperpolarizing factor in the vascular endothelium [42,47]. Therefore, ACE inhibitors function by maintaining the balance between the associated vasoconstrictive and salt-retentive attributes of Ang ІІ with the vasodilatory effect of bradykinin. The balance is maintained by decreasing the production of Ang ІІ and reducing the degradation of bradykinin [42]. While synthetic ACE inhibitors, such as Captopril function by directly blocking the action of ACE, ACE inhibitory peptides function by reacting with ACE, thus ACE is unavailable to cleave Ang І and prevent the production of the vasoconstrictor Ang ІІ [48]. In the past decade, it has been reported that muscle proteins from animals are a viable source of ACE inhibitory peptides in vitro and may potentially be incorporated into nutraceutical products to exert antihypertensive effects in vivo [10,25,49].
3.1.1. ACE Inhibitor Type Peptides
An important factor when discussing ACE inhibitory peptides isolated from food proteins is the discrepancy between ACE inhibitory activity of peptides in vitro and their antihypertensive effect in vivo [3,30]. ACE inhibitory food peptides can be divided into three categories, depending on their inhibitory activity following preincubation with ACE [33]. The first group of ACE inhibitory peptides is known as “true inhibitor type” peptides. The IC50 value of these peptides is not affected by preincubation with ACE. The second category of peptides, known as “substrate type” peptides are hydrolyzed by ACE resulting in weak inhibitory activity. Finally, the third category is “pro-drug type” inhibitors. The peptides in this category are converted to “true inhibitor type” peptides by ACE or proteases of the digestive tract. In vivo studies have demonstrated that only peptides belonging to the groups of true inhibitor type or pro-drug type reduce the systolic blood pressure of spontaneously hypertensive rats (SHR) [30].
To date, the majority of ACE inhibitory peptides found in meat can be classified as true inhibitor type peptides [10,50,51]. These peptides may act in one of two ways, first the peptide may bind to the active site of the ACE enzyme or second it may bind to an inhibitor site located on the ACE enzyme thereby modifying the protein confirmation and preventing the substrate (Ang І) from binding to the enzyme active site [52].
3.1.2. Absorption of ACE Inhibitory Peptides
When determining or extrapolating the in vivo efficacy of potential ACE inhibitory peptides in humans using the SHR model, differences in bioavailability of nutrients between both species is an important consideration. Bioavailability is the term used to “express the proportion of the total amount of a nutrient that can be absorbed and utilized” [53]. The bioavailability of a nutrient is in part controlled by the physicochemical properties of the nutrient; these include molecular weight and size, lipophilicity, p Ka, charge and solubility. Factors which affect the variability of nutrient absorption and which are species-dependent include the physiological factors of pH, gastrointestinal (GI) motility, transit time, intestinal permeability as well as the diverse groups of enzymes and transporters [54,55,56]. It has been proposed that the larger relative absorptive surface area of the human GI tract results in faster absorption of nutrients and a larger uptake than that of the rat [57]. Finally, when extrapolating the results from in vivo studies of SHR to human subjects, it is important to incorporate dosage of nutrient, form of nutrient, period of administration, metabolic state of subjects and control diets used [58].
For an ACE inhibitory peptide to function in vivo, it must first enter the circulatory system intact. Peptides may be hydrolyzed by enzymes of the digestive tract as well as peptidases associated with the brush border membrane, cytoplasm or serum [59,60]. To date, studies documenting the intestinal absorption of ACE inhibitory peptides in vitro have employed the Caco-2 cell monolayer [61], as it is generally acknowledged that this cell line is a suitable model to predict the permeability of intestinal epithelial cells to pharmaceuticals [62,63,64]. Interestingly, the occurrence of peptides which demonstrate ACE inhibitory activity in vitro but poor antihypertensive activity in vivo may be explained by the possible alteration of peptides prior to reaching the target ACE enzyme in the circulatory system [62,65]. The opposite has also proven true, in that peptides determined to have poor ACE inhibitory activity in vitro have demonstrated a significant systolic blood pressure (SBP) lowering effect in vivo, possibly due to intestinal modification [23,66].
The bioavailability of the β-casein ACE inhibitory peptide LHLPLP was determined by evaluating it’s resistance to brush border peptidases, serum peptidases as well as identifying the mechanism of transepithelial transport [67]. It was concluded that LHLPLP was partly hydrolyzed to HLPLP by brush border peptidases and HLPLP was rapidly absorbed by the Caco-2 cell monolayer in a concentration-dependent manner. Furthermore, the peptide remained “practically intact” after one hour incubation with human plasma and remained unbound to plasma proteins. This suggests that the peptide LHLPLP is hydrolyzed to HLPLP, the minimum active form, which is then readily absorbed across the intestinal epithelium by paracellular diffusion and is stable to serum proteases. This offers the possibility that HLPLP may be a viable alternative in the treatment or prevention of hypertension in humans.
The well documented ACE inhibitory milk peptides IPP and VPP have also been subject to in vitro permeability studies [59]. Both peptides were found to be readily transported across the intestinal epithelium of the various models used. It has been reported that IPP avoids gastrointestinal digestion and can enter the circulatory system of humans intact [68]. Studies involving the oral administration of foods rich in both IPP and VPP have demonstrated that such foods exhibit blood pressure lowering effects on hypertensive patients [69,70,71]. This clearly demonstrates that certain ACE inhibitory peptides are sufficiently well absorbed by the body to be effective at reducing blood pressure of hypertensive subjects. The challenge for researchers now is to identify bioactive peptides in vitro which can enter the circulatory system intact and remain active at their target site.
3.1.3. Structure Correlation of ACE Inhibitory Peptides
As stated previously, the mode of action of the majority of ACE inhibitory peptides is thought to be as competitive substrates for ACE. The structure activity relationships of ACE inhibitory peptides has not yet been confirmed due to the assorted amino acid sequence properties of ACE inhibitory peptides identified to date [21,30]. The tripeptide sequence at the C-terminal end of ACE inhibitory peptides has been suggested as the controlling factor of ACE inhibitory peptides, which regularly contains hydrophobic amino acid residues. It has been reported that at the penultimate positions, aliphatic (V, I, and A), basic (R) and aromatic (Y and F) residues are preferred, while aromatic (W, Y, and F), proline (P) and aliphatic (I, A, L and M) residues are favored at the ultimate position of the C-terminal end of the peptide [33,72,73,74,75,76]. This is because of the interaction of these residues with the three hydrophobic sub-sites located on the active site of ACE [49,77]. The positive charge associated with arginine at the C-terminus has also been associated with the ACE inhibitory activity of some peptides, as has the presence of lysine (K) which possesses a positive charge on the є-amino group at the C-terminus [27]. Another common feature of ACE inhibitory peptides is the hydrophobic nature of the N-terminal end of the peptide [52,78]. In the case of peptides greater than three amino acids in length, it has been suggested that the presence of hydroxyproline (Hyp) is critical for the binding of peptides to the active sites of ACE [79]. Furthermore, the overall hydrophobicity of the peptide is important. Hydrophilic peptides possess weak or no ACE inhibitory activity, since hydrophilic peptides are incompatible with the active sites of ACE. Indeed, inhibition of ACE is achieved by hydrophobic peptides which display high affinity to the active sub-sites of ACE [8,21,80].
3.2. Antihypertensive Peptides from Animal Sources
The generation of force (i.e., contraction) by skeletal muscle is the responsibility of the muscle proteins, actin and myosin. Both of these proteins are associated with two distinct types of muscle filaments. Myosin proteins are associated with the thick filaments of skeletal muscle while actin is associated with the thin filaments of skeletal muscle. Also present in the thin filaments are the proteins troponin and tropomyosin [3,81]. In striated muscle, the myosin thick filaments extend along the thin filaments of actin and are known as sacromeres. Sacromeres are further longitudinally repeated to form myofibrils. Myofibrils are then arranged in parallel to form myofibers, these myofibers then form muscle fibers. The muscle proteins arranged in parallel bands result in skeletal and cardiac muscle being striped or striated in appearance [3,81]. Contraction of striated muscle is due to the interaction of the thick and thin filaments. Nerve impulses release Ca2+ from the sacroplasmic reticulum, which pass through the myofibrils resulting in the sliding of myosin and actin filaments over one another, causing the sacromeres to shorten resulting in muscle contraction [82]. Interestingly, ACE inhibitory peptides have been identified in the hydrolysates of actin, myosin and troponin [50,83,84].
3.2.1. Antihypertensive Peptides from Myosin Sources
Arihara and colleagues [23] identified two ACE inhibitory pentapeptides from the thermolysin digestion of porcine myosin. These were named myopentapeptides A and B and their sequences determined. The amino acid sequence of myopentapeptide A was found to be MNPPK, which corresponded to positions 79–83 on the myosin heavy chain, while myopentapeptide B, with the amino acid sequence ITTNP, corresponded to positions 306–310 on the myosin heavy chain. The antihypertensive activities of myopentapeptide A and myopentapeptide B were investigated in SHR, in addition to other thermolysin hydrolysates of porcine skeletal muscle proteins. Administration of myopentapeptide A and myopentapeptide B at a concentration of 1 mg per kilogram of animal weight resulted in a maximum decrease in SBP of 23.4 ± 3.0 mmHg and 21.0 ± 3.1 mmHg after 6 h, respectively. At 24 h, the SBP of both test groups was still significantly lower than that of the control group, indicating that both myopentapeptide A and myopentapeptide B are potent antihypertensive peptides in vivo [66].
Another ACE inhibitory octapeptide VKKVLGNP was discovered corresponding to positions 47–54 on the myosin light chain [84]. This peptide was generated following the digestion of crude myosin light chain with pepsin. The IC50 value of this peptide was calculated to be 28.5 µM. Following the administration of this purified peptide to SHR at a concentration of 10 mg per kilogram of animal weight, the SBP decreased up to 3 h post-administration with SBP returning to the pre administration value after 9 h. It was therefore postulated that the peptide VKKVLGNP was an effective hypotensor in vivo.
Porcine skeletal muscle also was found to be a source of antihypertensive peptides when crude myosin B was hydrolyzed with pepsin. [83]. Indeed, within this hydrolysate a novel peptide M6 was identified with the amino acid sequence KRVITY. This peptide corresponded to positions 191–196 on the myosin heavy chain. The SBP of SHR was immediately reduced following oral administration of M6 peptide with a maximum decrease of 23 mmHg after 6 h, with the SBP of test animals returning to the control after 9 h. This reduction in SBP of SHR would indicate that the M6 peptide is a potent hypotensor in vivo. Furthermore, the M6 peptide retained its ACE inhibitory activity after heating the myosin B to 98 °C for 10 min prior to hydrolysis by pepsin. Retention of ACE inhibitory activity after such thermal treatment clearly indicates that bioactivity is retained even after the cooking process.
3.2.2. Antihypertensive Peptides from Troponin Sources
In a study to identify the most active ACE inhibitory peptides from porcine skeletal muscle, a bioactive peptide of 9 amino acids was released from the regulatory protein troponin C [85]. This peptide with the amino acid sequence RMLGQTPTK had an IC50 value of 34 µM and was categorized as being a non-competitive inhibitor [50]. Subsequently, the same researchers described a novel ACE inhibitory peptide from the pepsin hydrolysate of crude porcine troponin [24]. This peptide with the amino acid sequence KRQKYDI (corresponding to amino acid positions 198–204 of troponin T) had an IC50 value of 26.2 µM. Incubation of the peptide with ACE resulted in a decrease in the observed IC50 value suggesting that the peptide KRQHYDI is a substrate type inhibitor. Administration of the peptide to SHR resulted in temporary reduction of SBP. It has therefore been suggested that the peptide KRQHYDI may inhibit ACE activity in vivo in the short term.
As demonstrated in the above studies, bioactive peptides can be released from the digestion of meat proteins by various digestive enzymes. ACE inhibitory peptides were released following in vitro gastric simulated digestion of pork meat using a combination of the digestive enzymes pepsin and pancreatin [86]. The active ACE inhibitory peptides generated in this study were found to be homologous to the amino acid sequence of the muscle protein titin, representing the first documented ACE inhibitory peptides released from the myofibrillar protein titin. The amino acid sequence of the most active peptide present in this hydrolysate was identified as KAPVA, the IC50 value of this peptide was determined to be 46.56 µM.
3.2.3. Antihypertensive Peptides from Collagen
Connective tissue, which functions to convert force (i.e., contraction) into movement by attaching muscle to bone via robust strips of connective tissue called tendons, is mainly composed of fibrillar collagen. There are numerous collagens present in the extracellular matrix and they are the major structural constituent of connective tissue. All collagens contain repetition of the proline rich tripeptide Gly-X-Y; that forms the trimeric collagen triple helices [87].
Recently, it was reported that ACE inhibitory peptides were purified from the hydrolysate of bovine skin gelatine [34]. Five proteases were employed, Alcalase, α chymotrypsin, Neutrase, Pronase E, and trypsin. Hydrolysis was performed using sequential protease treatments and a three-step ultrafiltration membrane reactor. This involved collagen first being hydrolyzed by Alcalase, fractioned with a 10 kDa membrane, followed by hydrolysis with Pronase E, fractionated with a 5 kDa membrane and finally hydrolyzed with collagenase and separated using a 1 kDa molecular weight cut-off (MWCO) membrane. Two peptides responsible for ACE inhibitory activity were subsequently isolated from this low molecular weight fraction. The first peptide referred to as EIIICIII, with the amino acid sequence GPV had an IC50 value of 4.7 µM, while the second peptide known as EIIICIV, with amino acid sequence GPL had an IC50 value of 2.55 µM.
Chicken breast muscle was identified as a potential source of ACE inhibitory peptides by Saiga et al. 2003 [79]. The researchers fed two chicken breast muscle extracts to SHR, the first an untreated chicken muscle extract, the second a chicken muscle extract hydrolyzed with an Aspergillus protease. The blood pressure of SHR was significantly lowered after ingestion of both extracts. A reduction in blood pressure occurred after 1 h and lasted until 4 h post-ingestion. The purification of ACE inhibitory peptides from Aspergillus protease- and gastric protease-hydrolyzed chicken breast muscle resulted in the identification of four peptides displaying strong ACE inhibitory effects. The peptide expressing the strongest ACE inhibitory activity referred to as P4 had amino acid sequence GFHypGTHypGLHypGF. The IC50 value of this peptide was determined as 42.4 µM, whose sequence along with the others identified was homologous to that of collagen [79]. Interestingly, an immediate decrease in the blood pressure of SHR was observed after intravenous administration of the P4 peptide (30 mg per kilogram of body weight). However, blood pressure returned to base pressure 60 min post administration, suggesting that the P4 peptide may not act as a long-term vasodepressor in vivo [36]. It was also demonstrated that the P4 peptide is a non-competitive inhibitor of ACE. The presence of an aromatic amino acid at the antepenultimate position and phenylalanine at the C-terminal end of the peptide are responsible for the ACE inhibitory effect of the peptide. This supports the theory that the ACE inhibitory activity of peptides is based on the presence of hydrophobic amino acids located at the C-terminal end of the peptide.
ACE inhibitory peptides were also detected following the hydrolysis of chicken collagen by an Aspergillus oryzae protease. The resulting hydrolysate was further hydrolyzed with four proteases, namely protease FP, protease A, amino G and protease N. Four peptides displaying ACE inhibitory activity were identified along with their individual IC50 values, GAHypGLHypGP (IC50 29.4 µM), GAHypGPAGPGGIHypGERG (IC50 45.6 µM), GLHypGSRGERGERGLHypG (IC50 60.8 µM) and finally GIHypGERGPVGPSG (IC50 43.4 µM). The chicken collagen hydrolysates were fed to SHR and a reduction in blood pressure was detected 2 h after administration of the hydrolysates with the greatest reduction occurring after 6 h. Following continuous long-term administration of chicken collagen hydrolysates to SHR a significant reduction in blood pressure was observed. It is therefore proposed that low molecular weight chicken collagen hydrolysates show long-term hypotensive effects in vivo and have potential as antihypertensive therapeutic agents.
3.2.4. Antihypertensive Peptides from Miscellaneous Sources
The first identified ACE inhibitory peptide from beef hydrolysate was reported to be a hexapeptide with the amino acid sequence VLAQYK; this peptide had an IC50 value of 32.06 µM and was isolated from the sacroplasmic proteins of beef rump [25]. Feeding this hexapeptide to SHR resulted in a significant reduction in SBP in addition to lower total and LDL cholesterol blood concentrations [88]. It was proposed that the hexapeptide VLAQYK is a potent ACE inhibitor with potential use in clinical applications and functional food products. Subsequently, the same research group explored the possibility that beef sacroplasmic protein hydrolysates may contain other peptides capable of ACE inhibitory activity. This was part of a larger investigation to determine if antihypertensive peptides were capable of exhibiting other bioactivities, namely antimicrobial and antiproliferative [10]. Four peptides were found to exhibit strong ACE inhibitory activity, the strongest of which was from the Alcalase hydrolyzed hydrolysate. Other bioactivities associated with these peptides will be discussed later. A summary of ACE inhibitory meat derived peptides is given in Table 1.
Table 1.
ACE inhibitory peptides from meat of domestic animals. Adapted with permission from American Chemical Society [3], Copyright 2005.
| Source | Amino Acid Sequence | Parent Protein | Enzyme | IC50 (µM) | SHR a | Ref. |
|---|---|---|---|---|---|---|
| pig | ITTNP | myosin | thermolysin | 549 | ∆ 21.0 mmHg | [23,66] |
| MNPPK | myosin | synthesized | 945.5 | ∆ 23.4 mmHg | [23,66] | |
| MNP | myosin | synthesized | 66.6 | ∆ 19.6 mmHg | [23,66] | |
| PPL | myosin | synthesized | >1000 | ∆ 24.7 mmHg | [23,66] | |
| NPP | myosin | synthesized | 290.5 | ∆ 17.6 mmHg | [23,66] | |
| TNP | myosin | synthesized | 207.4 | ∆ 11.1 mmHg | [23,66] | |
| RMLGQTPTK | troponin | pepsin | 34 | nt | [50,85] | |
| RMLGQTP | troponin | pepsin | 503 | nt | [50] | |
| VKKVLGNP | myosin | pepsin | 28.5 | ∆ 24.0 mmHg b | [84] | |
| KRVITY | myosin | pepsin | 6.1 | ∆ 23.0 mmHg b | [83] | |
| VKAGF | actin | pepsin | 20.3 | ∆ 17.0 mmHg b | [83] | |
| KRQKYDI | troponin T | pepsin | 26.2 | ∆ 9.9 mmHg b | [24] | |
| KAPVA | titin | pepsin + pancreatin | 46.56 | nt | [86] | |
| PTPVP | titin | pepsin + pancreatin | 256.41 | nt | [86] | |
| ER | muscle | pepsin + pancreatin | 667 | nt | [86] | |
| KLP | muscle | pepsin + pancreatin | 500 | nt | [86] | |
| RPR | muscle | pepsin + pancreatin | 382 | nt | [86] | |
| chicken | LKA | creatine kinase | thermolysin | 8.5 | nt | [89] |
| LKP | aldolase | thermolysin | 0.32 | ∆ 75 mmHg c | [89] | |
| LAP | muscle | thermolysin | 3.2 | ∆ 40.0 mmHg c | [89] | |
| FQKPKR | myosin | thermolysin | 14 | nt | [89] | |
| FKGRYYP | creatine kinase | thermolysin | 0.55 | ∆ 0 mmHg c | [89] | |
| IKW | muscle | thermolysin | 0.21 | ∆ 50.0 mmHg c | [89] | |
| GFXGTXGLXGF | muscle | Aspergillus protease + gastric proteases | 42.4 | ∆ 20 mmHg d | [36,79] | |
| GAXGLXGP | collagen | Aspergillus protease + protease FP, A, G, N | 29.4 | nt | [37] | |
| GAXGPAGPGGIXGERGLXG | collagen | Aspergillus protease + protease FP, A, G, N | 45.6 | nt | [37] | |
| GLXGSRGERGERGLXG | collagen | Aspergillus protease + protease FP, A, G, N | 60.8 | nt | [37] | |
| GIXGSRGERGPVGPSG | collagen | Aspergillus protease + protease FP, A, G, N | 43.4 | nt | [37] | |
| cow | VLAQYK | muscle | thermolysin + proteinase A | 32.06 | + | [25,88] |
| GFHI | muscle | proteinase K | 64.3 | nt | [10] | |
| DFHING | muscle | alcalase | 50.5 | nt | [10] | |
| FHG | muscle | thermolysin + proteinase A | 52.9 | nt | [10] | |
| GLSDGEWQ | muscle | thermolysin + proteinase A | 117 | nt | [10] | |
| GPV | skin gelatin | alcalase + pronase E + collagenase | 4.67 | nt | [34] | |
| GPL | skin gelatin | alcalase + pronase E + collagenase | 2.55 | nt | [34] |
a The maximum decrease in systolic blood pressure in SHR upon oral administration of peptide at 1 mg/kg; b The maximum decrease in systolic blood pressure in SHR upon oral administration of peptide at 10 mg/kg; c The maximum decrease in systolic blood pressure in SHR upon intravenous administration of peptide at 10 mg/kg; d The maximum decrease in systolic blood pressure in SHR upon intravenous administration of peptide at 30 mg/kg; nt: Not tested in spontaneously hypertensive rats.
4. Antihypertensive Peptides from Fish Sources
ACE inhibitory peptides from fish sources were first identified in sardine meat over twenty years ago [90]. Since then ACE inhibitory peptides have been found in various fish species, including shellfish, tuna, bonito, salmon and sardine [49,72,73,74,75,91].
There have been several reports of crude fish protein hydrolysates containing ACE inhibitory peptides. Following the hydrolysis of purified catfish protein with the commercial enzyme Protamex® ACE inhibitory peptides were released, with the majority being found in the soluble protein fraction [92]. A crude enzyme extract from the viscera of sardine was used to hydrolyze the protein contained within the head and viscera of the fish species know as sardinelle. The resulting hydrolysate contained a high concentration of peptides displaying low hydrophobicity with molecular masses between 200 and 600 Da [93]. Pacific Hake fish protein subjected to simulated gastrointestinal digestion was reported to contain ACE inhibitory activity. Most inhibitory peptides were reported to be short chained, polar and containing few hydrophobic amino acids in their sequence [94].
Antihypertensive Peptides from Fish By-Products
A major issue for food producers is discarded by-products of food processing. Adding value to waste streams is very appealing to food producers, as the by-products are usually incorporated into low economic value products such as animal feed. The frame protein of Alaskan Pollock which is normally discarded as an industrial by-product was mined for ACE inhibitory peptides [95]. Frame protein was first hydrolyzed with pepsin and then separated into five fractions depending on molecular weight, the most active ACE inhibitory peptides were found in the fraction <1 kDa. From this fraction, a novel peptide was isolated with an amino acid sequence FGASTRGA and an IC50 value of 14.7 µM.
The normally discarded yellowfin sole frame protein was identified as another source of ACE inhibitory peptides [96]. Using α-chymotrypsin, a peptide with a molecular mass of 1.3 kDa was isolated with the corresponding amino acid sequence MIFPGAGGPEL. It was demonstrated that this peptide reduced the SBP of SHR over 9 h when administrated at 10 mg per kilogram of animal body weight. The SBP of SHR was reduced by 22 mmHg at 3 h, a reduction which was comparable to the group treated with Captopril. Reduction in SBP remained after 9 h, suggesting that MIFPGAGGPEL is an effective antihypertensive agent in vivo. Frame protein hydrolysate derived from tuna frame protein was investigated for novel ACE inhibitory peptides [51]. Six proteases were used, with pepsin hydrolysates displaying the highest ACE inhibitory activity. Consecutive purification steps resulted in the isolation of an ACE inhibitory peptide of 21 amino acids in length. The peptide with the amino acid sequence GDLGKTTTVSNWSPPKYKDTP had an IC50 value of 11.28 µM and actively decreased the SBP of SHR for up to 9 h after oral administration, with a maximum decrease of 21 mmHg after 6 h.
Following oral administration of a thermolysin hydrolysate from chum salmon muscle, the SBP of SHR was significantly reduced when compared to that of the controls. The greatest reduction in SBP occurred after 4 h suggesting that short chain peptides were responsible for the blood pressure lowering effect, since reduction in blood pressure occurred soon after administration. Indeed, a reduction in SBP 6 to 8 h after administration is associated with peptides of greater chain length, because larger peptides are converted from substrate to true ACE inhibitors in vivo [74]. The SBP of all test groups returned to initial levels after 24 h, demonstrating that hydrolysates of chum salmon are temporary hypotensors. The amino acid sequences of the six di-peptides identified from the thermolysin digest of chum salmon were WA, VW, WM, MW, IW, and LW. The IC50 values of these six peptides were determined as 277.3, 2.5, 98.6, 9.8, 4.7 and 17.4 µM, respectively. Interestingly, it was found that a change in the amino acid sequence of some dipeptides resulted in a change in the inhibitory mechanism as well as a change in the reported IC50 value [97]. For example, the peptide MW has an IC50 value of 9.8 µM and is a non-competitive inhibitor of ACE. When the amino acid sequence was changed to WM, the IC50 value increased to 98.6 µM and mode of inhibition changed to competitive, indicating that the amino acid composition of peptides as well as position of amino acids in the peptide sequence contributes to the ACE inhibitory activity of peptides.
The pepsin hydrolysate of Bigeye tuna dark muscle was reported to contain the ACE inhibitory peptide WPEAAELMMEVDP, with a molecular mass of 1581 Da and an IC50 value of 21.6 µM [49]. Employing Lineweaver-Burk plots, the authors determined that the peptide forms enzyme-substrate-inhibitor and enzyme-inhibitor complexes to lower the efficiency of ACE in vitro. Administration of this peptide to SHR resulted in a maximum decrease in SBP between 3 and 6 h. The SBP of test animals remained lower than control animals (~15 mmHg) after 10 h proving that WPEAAELMMEVDP is an effective hypotensor in vivo.
Following the hydrolysis of shark meat with the protease SM98011 from Bacillus sp. SM98011, four ACE inhibitory peptides were identified [98]. Three of the peptides were reported as being novel ACE inhibitory peptides, the amino acid sequences of these peptides were EY, FE and CF, with IC50 values of 1.98, 2.68 and 1.45 µM, respectively.
Fujita et al. [72] developed a thermolysin hydrolysate from “Katsuo-bushi”, a traditional Japanese food processed from dried bonito. This hydrolysate was administered to thirty hypertensive and borderline hypertensive human subjects in a small scale clinical trial. The hydrolysate contained the previously reported ACE inhibitory peptide LKPNM [75]. Following eight weeks of administration of the dried bonito hydrolysate, the SBP of subjects was reduced by 12.55 ± 1.5 mmHg. This clearly demonstrates that the thermolysin hydrolsate of dried bonito is sufficiently well absorbed in humans to effectively reduce the SBP of hypertensive and borderline hypertensive subjects in vivo. The hydrolysate has been approved as Foods for Specified Health Use (FOSHU) by the Ministry of Health and Welfare in Japan [72]. A summary of ACE inhibitory fish derived peptides is given in Table 2.
Table 2.
ACE inhibitory peptides derived from meat of fish. Adapted with permission from American Chemical Society [3], Copyright 2005.
| Source | Amino acid sequence | Parent protein | Enzyme | IC50 (µM) | SHR a | Ref. |
|---|---|---|---|---|---|---|
| bonito | IKPLNY | muscle | thermolysin | 43 | nt | [75] |
| IVGRPRHQG | actin | thermolysin | 2.4 | nt | [75] | |
| IWHHT | actin | thermolysin | 5.8 | nt | [75] | |
| ALPHA | actin | thermolysin | 10 | nt | [75] | |
| FQP | actin | thermolysin | 12 | nt | [75] | |
| LKPNM | muscle | thermolysin | 2.4 | ∆ 23.0 mmHg b | [72,75] | |
| IY | actin | thermolysin | 2.31 | ∆ 19.0 mmHg b | [72,75] | |
| DYGLYP | muscle | thermolysin | 62 | nt | [75] | |
| LKP | muscle | thermolysin | 0.32 | ∆ 18.0 mmHg b | [72] | |
| IWHHT | actin | thermolysin | 3.5 | ∆ 26.0 mmHg b | [72] | |
| IKP | muscle | thermolysin | 6.9 | ∆ 20.0 mmHg b | [72] | |
| IVGRPR | actin | thermolysin | 300 | ∆ 25.0 mmHg b | [89] | |
| salmon | WA | muscle | thermolysin | 277.3 | nt | [74] |
| VW | muscle | thermolysin | 2.5 | nt | [74] | |
| WM | muscle | thermolysin | 96.6 | nt | [74] | |
| MW | muscle | thermolysin | 9.9 | nt | [74] | |
| IW | muscle | thermolysin | 4.7 | nt | [74] | |
| LW | muscle | thermolysin | 17.4 | nt | [74] | |
| sardine | MF | muscle | alcalase | 44.7 | nt | [73] |
| RY | muscle | alcalase | 51 | nt | [73] | |
| MY | muscle | alcalase | 193 | nt | [73] | |
| LY | muscle | alcalase | 38.5 | nt | [73] | |
| YL | muscle | alcalase | 82 | nt | [73] | |
| IY | muscle | alcalase | 10.5 | nt | [73] | |
| VF | muscle | alcalase | 43.7 | nt | [73] | |
| GRP | muscle | alcalase | 20 | nt | [73] | |
| RFP | muscle | alcalase | 330 | nt | [73] | |
| AKK | muscle | alcalase | 3.13 | nt | [73] | |
| RVY | muscle | alcalase | 205.6 | nt | [73] | |
| GWAP | muscle | alcalase | 3.86 | nt | [73] | |
| KY | muscle | alcalase | 1.63 | nt | [73] | |
| VY | muscle | alcalase | 10 | ∆ 7.0 mmHg | [99,100] | |
| tuna | PTHIKWGD | muscle | acid | nd | nt | [101] |
| GDLGKTTTVSNWSPPKYKDTP | frame protein | pepsin | 11.28 | ∆ 21.0 mmHg | [51] | |
| WPEAAELMMEVDP | dark muscle | pepsin | 21.6 | ∆ 18.0 mmHg | [49] | |
| alaska pollack | GPL | skin | alcalase + pronase + collagenase | 2.65 | nt | [102] |
| GPM | skin | alcalase + pronase + collagenase | 17.13 | nt | [102] | |
| FGASTRGA | frame protein | pepsin | 14.7 | nt | [95] | |
| yellowfin sole | MIFPGAGGPEL | frame protein | α chymotrypsin | 28.7 | ∆ 22.0 mmHg | [96] |
| shark | EY | muscle | protease SM98011 | 1.98 | nt | [98] |
| FE | muscle | protease SM98012 | 2.68 | nt | [98] | |
| CF | muscle | protease SM98013 | 1.45 | nt | [98] |
a The maximum decrease in systolic blood pressure in SHR upon oral administration of peptide at 10 mg/kg;
b The maximum decrease in systolic blood pressure in SHR upon oral administration of peptide at 60 mg/kg; nt: Not tested in SHR; nd: IC50 of peptide was not determined.
5. Antioxidant Peptides
Antioxidants are known to be beneficial to human health as they may protect the body against molecules known as reactive oxygen species (ROS), which can attack membrane lipids, protein and DNA. This in turn can be a causative factor in many diseases such as cardiovascular disease, diabetes, cancer and Alzheimer’s disease. Lipid oxidation can cause deterioration of food quality and a reduction in the shelf-life of a food product, while the consumption of foods containing lipid oxidation products has been linked to various diseases, including cancers, diabetes and cardiovascular disease [103,104,105,106].
The use of natural antioxidants in foodstuffs is appealing because of the potential health risk associated with synthetic antioxidants in vivo [107,108,109,110]. Antioxidant peptides have been found in numerous foodstuffs including milk [111], wheat [16], potato [22] and fungi [112]. In the past number of years, a great deal of research has focused on antioxidant peptides sourced from fish, while the research on antioxidant peptides from the hydrolysates of domesticated animal muscle is limited. There are a number of methods available to measure antioxidant potential of various food components based on the ability of potential antioxidants to scavenge ROS, free radicals or prevent oxidation in model systems, some of which are discussed below.
5.1. Antioxidant Peptides from Fish Sources
The hydrolysates of mackerel were found to contain peptides exhibiting antioxidant activity in vitro [113]. Peptides released by the hydrolysis of mackerel with Protease N inhibited the autoxidation of linoleic acid, quenched the free radical α,α-diphenyl-β-picrylhydrazyl (DPPH) and reduced Fe3+ to Fe2+. The strongest antioxidant fraction of mackerel protein hydrolysate contained small peptides and free amino acids and had a molecular weight of 1400 Da.
Alaska Pollack frame protein (APF) was found to contain the peptide LPHSGY, released after hydrolysis of APF by a crude enzyme extract contained within mackerel intestine. This peptide was responsible for the quenching of 35% of available hydroxyl radicals at a peptide concentration of 53.6 µM. The authors proposed that a correlation existed between the observed antioxidant activity and the molecular weight of the peptides, with greater antioxidant activity associated with peptides of low molecular weight (<1 kDa) [114].
The antioxidant potential of Tilapia (common name for cichlid fish) protein hydrolysates was also demonstrated [115]. Hydrolysates generated with the enzymes Cyrotin-F, Protease A, Protease N, Flavourzyme and Neutrase showed significant ability to scavenge ROS and reduce ferric ions. The ability to effectively scavenge ROS was associated with the low molecular weight peptides present in the hydrolysates. Low molecular weight peptides were also responsible for the observed antioxidant activity in the hydrolysates of Silver carp following hydrolysis by Alcalase and Flavourzyme [116]. In previous studies, it was noted that increasing the degree of hydrolysis produced greater numbers of low molecular weight peptides, which was associated with an increase in the radical scavenging ability of salmon muscle hydrolysate [97]. This phenomenon, however, was not observed in the hydrolysates of catfish protein isolate hydrolyzed with Protamex [92], where the ability of the hydrolysates to scavenge DPPH radicals or reduce Fe3+ decreased with increasing degree of hydrolysis. An increase in the number of low molecular weight peptides did; however, result in an increase in the time required for lipid peroxidation products to accumulate in a model muscle washed system. This suggests that antioxidant ability of peptides in vitro depends on peptide size, amino acid composition of the peptide and presence of free amino acids within the hydrolysate.
The peptides from Alcalase- and Flavourzyme-digested Yellow Stripe Trevally were shown to prevent oxidative damage to DNA using the Fenton reaction. The possible reason for this was that hydrolysates chelated Fe2+ thus preventing it from reacting with H2O2 and forming hydroxyl radicals [117].
Seven antioxidant peptides were identified in the hydrolysates of proteins recovered from the waste stream of sardinelle processing [118]. The amino acid sequences of the seven peptides were LARL, GGE, LHY, GAH, GAWA, PHYL and GALAAH. All peptides were <600 Da and were deemed to be novel antioxidant peptides. It was reported that the peptide LHY displayed the highest DPPH radical scavenging activity, with an ability to scavenge 63% of available DPPH radical present at a peptide concentration of 150 µg/mL.
Antioxidant peptides were generated from the hydrolysis of tuna dark muscle by-product by the commercial enzymes orientase (OR) and protease XXІІІ (PR) [119]. Antioxidant activity was determined by measuring the DPPH radical scavenging capacity and the ability of hydrolysates to inhibit or delay lipid peroxidation of linoleic acid. From the OR enzyme digest, a purified antioxidant peptide with the amino acid sequence LPTSEAAKY was identified as having the capacity to scavenge 79.6% of available DPPH radicals, while also inhibiting lipid peroxidation of linoleic acid for 7.13 days at a peptide concentration of 100 µg/mL. Following the hydrolysis of tuna dark muscle by-product with the enzyme PR, the peptide with amino acid sequence PMNYMVT was demonstrated to scavenge 85.2% of available DPPH radicals, while also inhibiting oxidation of linoleic acid for 7.89 days at a peptide concentration of 100 µg/mL. Furthermore, when compared to the controls (butyl hydroxyanisol (BHA) and α-tocopherol employed in determination of lipid peroxidation inhibition, and L-ascorbic acid in determination of DPPH radical scavenging ability), both peptides displayed similar if not higher activity. Therefore, it is clear that the peptides generated during the hydrolysis of tuna dark muscle by-product are effective in vitro antioxidants with potential for incorporation into foodstuffs as natural antioxidants.
Hoki skin gelatin was hydrolyzed by the digestive enzymes pepsin, trypsin and α-chymotrypsin with the tryptic hydrolysates displaying the highest levels of antioxidant activity [120]. A single purified peptide, with the amino acid sequence HGPLGPL was identified as being responsible for the observed antioxidant activity of the tryptic hydrolysate. When the peptide HGPLGPL was added to a linoleic acid peroxidation system, inhibition of lipid peroxidation was significantly greater than that of the control α-tocopherol and as effective as the synthetic antioxidant butylated hydroxytoluene (BHT). Furthermore, the peptide HGPLGPL increased the expression of antioxidative enzymes in human hepatoma cells in vitro.
5.2. Antioxidant Peptides from Meat Sources
Peptides derived from porcine myofibrillar proteins using the proteases Papain and Actinase E represent the first report of antioxidant peptides from the myofibrillar proteins of edible meat [121]. Following digestion, these crude hydrolysates inhibited peroxidation of linoleic acid, DPPH scavenging and metal chelating activities. Purification of the peptides responsible for the antioxidant activity in the Papain hydrolysate resulted in the identification of five peptides: DSGVT (actin), IEAEGE (unknown), DAQEKLE (tropomyosin), EELDNALN (tropomyosin) and VPSIDDQEELM (myosin heavy chain).
A cocktail of enzymes including pepsin, papain and proteases from the bovine pancreas and bacterial proteases from Streptomyces and Bacillus polymyxa, were used to hydrolyze porcine collagen [122]. This resulted in the release of the antioxidant peptide QGAR, which was identified as residues 72–75 and 180–183 of the α1 chain of collagen. It was proposed that the QG amino acid residues may be a contributing factor to radical scavenging activity.
Plasma proteins represent another valuable but under-utilized protein source. Indeed, following hydrolysis with Alcalase, porcine plasma hydrolysates (PPH) were found to be effective inhibitors of lipid oxidation, as well as effective DPPH scavengers, metal chelating and reducing agents [123]. Increasing the degree of hydrolysis increased antioxidant activity and the most active fractions were <3 kDa. The peptide with MW 441 Da and amino acid sequence HNGN was identified as the active antioxidant peptide.
Chicken essence, a traditional product consumed in China was shown to possess various antioxidant activities including the inhibition of linoleic acid autoxidation, DPPH radical scavenging activity, reducing power and the ability to chelate metal ions [124]. From the extract of chicken essence, two peptides were identified that displayed inhibition of the autoxidation of linoleic acid in a model system. The first peptide with the amino acid sequence HVTEE had an induction period of 2.49 days while the second peptide with the amino acid sequence PVPVEGV had an induction period of 6.50 days.
A hydrolysate displaying strong antioxidant activity was produced following the digestion of venison with papain where two peptides responsible for the activity were identified, i.e., (APVPH І) MQIFVKTLTG and (APVPH ІІ) DLSDGEQGVL [7]. The peptide APVPH І displayed the greater free radical scavenging activity of the two peptides with IC50 values of 4.47, 7.82, 22.02 and 8.62 µM per mL for hydroxyl, DPPH, superoxide and peroxyl radicals, respectively, lower than the control IC50 value of vitamin C.
6. Antimicrobial and Antiproliferative Peptides
Antimicrobial peptides (AMPs) have been identified in a range of foods to date, with peptides released from milk proteins being the most plentiful source of AMPs. Indeed, AMPs have been found in a host of different milk proteins including the caseins [125,126] and lactoferrin [127,128]. While AMPs from the protein of muscle foods are less well documented, there has been one report of AMPs from a bovine meat source [10]. In this study, the antimicrobial and human cancer cell cytotoxic effects of four previously identified ACE inhibitory peptides, were evaluated. The four peptides GFHI, DFHING, FHG and GLSDGEWQ were assayed for antimicrobial activity against six pathogenic bacteria, three Gram-positive (Bacillus cereus, Listeria monocytogenes and Staphylococcus aureus) and three Gram-negative (Salmonella typhimurium, Escherichia coli and Pseudomonas aeruginosa). The peptide GLSDGEWQ inhibited the growth of S. typhimurium, B. cereus, E. coli and L. monocytogenes. This was the only peptide that inhibited the growth of both Gram-positive and Gram-negative pathogens. GFHI and FHG inhibited the growth of the pathogen P. aeruginosa.
A cysteine rich antimicrobial peptide was produced from the digestion of oyster muscle using a combination of Alcalase and bromelin [26]. The peptide referred to as CgPep33 inhibited the growth of the pathogenic bacteria E. coli, P. aeruginosa, B. subtilis and S. aureus in addition to inhibiting the growth of the fungi Botrytis cinerea and Penicillium expansum. Although the sequence of the CgPep33 peptide was not determined, the principal amino acids present in the active fraction were C, L, E, D, F, Y, I and G residues. Jang et al. [10] investigated the cytotoxic effect of four AMPs from a bovine meat source, using the cell lines breast adenocarcinoma (MCF-7), stomach adenocarcinoma (AGS) and lung carcinoma (A549) cells. The peptide GFHI possessed the strongest cytotoxic effect on MCF-7 cells and also decreased the cell viability of AGS cells, while the peptide GLSDGEWQ strongly inhibited the proliferation of AGS cells.
Peptides isolated from anchovy sauce were capable of inducing apoptosis in a human lymphoma cell line (U937) [129]. The peptide of interest was characterized as being hydrophobic and having a molecular weight of 440.9 Da [130]. Protein hydrolysates from different fish species were investigated for their antiproliferative activity on two human breast cancer cell lines (MCF-7/6 and MDA-MB-231) [131]. Hydrolysates of blue whiting, cod, plaice and salmon resulted in the significant inhibition of growth in both MCF-7/6 and MDA-MB-231 cell lines.
Recently, the hydrolysate of tuna dark muscle by-product was examined for potential antiproliferative activity by exposure to the human breast cancer cell line MCF-7 [132]. Peptide fractions within the molecular weight range of 400 and 1400 Da exhibited the strongest antiproliferative activity. In these fractions two antiproliferative peptides were identified, i.e., LPHVLTPEAGAT from papain hydrolysate and PTAEGVYMVT from Protease XXIII. The IC50 value of each peptide was determined to be 8.1 and 8.8 µM, respectively. This demonstrates once again the potential of meat products and meat by-products as valuable sources of bioactive peptides for incorporation into functional foods.
7. Conclusion
The increase in food related diseases, such as cardiovascular disease, diabetes, cancer and obesity has led consumers to demand food products that not only offer nutritional value but also functional and health benefits. Meat and fish derived peptides have been shown to exhibit antihypertensive effects in vivo, along with antioxidant capabilities and other bioactivities such as antimicrobial and antiproliferative activities in vitro. In spite of this; however, very few food products containing meat or fish-derived bioactive peptides are available commercially, yet these bioactives harbor huge potential. For example, Katsuobushi Oligopeptide LKPNM, which is found in blood pressure-lowering capsules, is derived from dried bonito and converted to the active form by digestive enzymes following ingestion [133]. The commercial chicken-meat extract known as “Brand’s Essence of Chicken” (BEC; Cerebos Pacific Ltd., Singapore) also claims to contain antihypertensive components [134]. These two examples show the potential of fish and meat bioactive peptides for use as functional ingredients in foods. However, the ability of bioactive peptides to exert a physiological effect in vivo is dependent on the bioavailability of the peptide. This factor is dependent on the resistance of the peptide to hydrolysis by peptidases of both the intestinal tract and serum, and its ability to be absorbed across the intestinal epithelium [61]. Therefore, when identifying bioactive peptides for the development of meat and fish-based nutraceutical products, this fact should be taken into account.
Acknowledgements
JT Ryan is in receipt of a Teagasc Walsh Fellowship. This work was funded through the Department of Agriculture, Fisheries and Food under the National Development Plan 2007–2013 and partly by Science Foundation Ireland (SFI).
References
- 1.Korhonen H., Pihlanto A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006;16:945–960. [Google Scholar]
- 2.Friedman M. Nutritional value of proteins from different food sources: A review. J. Agric. Food Chem. 1996;44:6–29. [Google Scholar]
- 3.Vercruysse L., van Camp J., Smagghe G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food Chem. 2005;53:8106–8115. doi: 10.1021/jf0508908. [DOI] [PubMed] [Google Scholar]
- 4.Vermeirssen V., Camp J.V., Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2007;92:357–366. doi: 10.1079/bjn20041189. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann K., Cheung B.W.Y., Schroder H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008;19:643–654. doi: 10.1016/j.jnutbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Arihara K. Strategies for designing novel functional meat products. Meat Sci. 2006;74:219–229. doi: 10.1016/j.meatsci.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Kim E.K., Lee S.J., Jeon B.T., Moon S.H., Kim B., Park T.K., Han J.S., Park P.J. Purification and characterisation of antioxidative peptides from enzymatic hydrolysates of venison protein. Food Chem. 2009;114:1365–1370. [Google Scholar]
- 8.Matsui T., Matsumoto K., Mahmud T.H.K., Arjumand A. Advances in Phytomedicine. Vol. 2. Elsevier; Oxford, UK: 2006. Antihypertensive peptides from natural resources; pp. 255–271. [Google Scholar]
- 9.Cinq-Mars C.D., Hu C., Kitts D.D., Li-Chan E.C.Y. Investigations into inhibitor type and mode, simulated gastrointestinal digestion, and cell transport of the angiotensin I-Converting enzyme-inhibitory peptides in pacific hake (Merluccius productus) fillet hydrolysate. J. Agric. Food Chem. 2008;56:410–419. doi: 10.1021/jf072277p. [DOI] [PubMed] [Google Scholar]
- 10.Jang A., Jo C., Kang K.S., Lee M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008;107:327–336. [Google Scholar]
- 11.Mellander O. The physiological importance of the casein phosphopeptide calcium salts. II. Peroral calcium dosage of infants. Acta Soc. Med. Ups. 1950;55:247–255. [PubMed] [Google Scholar]
- 12.Rutherfurd-Markwick K.J., Moughan P.J. Bioactive peptides derived from food. J. AOAC Int. 2005;88:955–966. [PubMed] [Google Scholar]
- 13.Hayes M., Ross R.P., Fitzgerald G.F., Stanton C. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part I: Overview. J. Biotechnol. 2007;2:426–434. doi: 10.1002/biot.200600246. [DOI] [PubMed] [Google Scholar]
- 14.Korhonen H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods. 2009;1:177–187. [Google Scholar]
- 15.Hernandez-Ledesma B., Quiros A., Amigo L., Recio I. Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int. Dairy J. 2007;17:42–49. [Google Scholar]
- 16.Zhu K.X., Zhou H.M., Qian H.F. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006;41:1296–1302. [Google Scholar]
- 17.You L.J., Zhao M.M., Cui C., Zhao H.F., Yang B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Inn. Food Sci. Emerg. Technol. 2009;10:235–240. [Google Scholar]
- 18.Rizzello C.G., Losito I., Gobbetti M., Carbonara T., de Bari M.D., Zambonin P.G. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J. Dairy Sci. 2005;88:2348–2360. doi: 10.3168/jds.S0022-0302(05)72913-1. [DOI] [PubMed] [Google Scholar]
- 19.Hayes M., Ross R.P., Fitzgerald G.F., Hill C., Stanton C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006;72:2260–2264. doi: 10.1128/AEM.72.3.2260-2264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai J.S., Chen T.J., Pan B.S., Gong S.D., Chung M.Y. Antihypertensive effect of bioactive peptides produced by protease-facilitated lactic acid fermentation of milk. Food Chem. 2008;106:552–558. [Google Scholar]
- 21.Li G.H., Le G.W., Shi Y.H., Shrestha S. Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004;24:469–486. [Google Scholar]
- 22.Pihlanto A., Akkanen S., Korhonen H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum) Food Chem. 2008;109:104–112. doi: 10.1016/j.foodchem.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Arihara K., Nakashima Y., Mukai T., Ishikawa S., Itoh M. Peptide inhibitors for angiotensin I-converting enzyme from enzymatic hydrolysates of porcine skeletal muscle proteins. Meat Sci. 2001;57:319–324. doi: 10.1016/s0309-1740(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 24.Katayama K., Anggraeni H.E., Mori T., Ahhmed A.A., Kawahara S., Sugiyama M., Nakayama T., Maruyama M., Mugurumat M. Porcine skeletal muscle troponin is a good source of peptides with angiotensin-I converting enzyme inhibitory activity and anti hypertensive effects in spontaneously hypertensive rats. J. Agric. Food Chem. 2008;56:355–360. doi: 10.1021/jf071408j. [DOI] [PubMed] [Google Scholar]
- 25.Jang A., Lee M. Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Sci. 2005;69:653–661. doi: 10.1016/j.meatsci.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z.Y., Dong S.Y., Xu J., Zeng M.Y., Song H.X., Zhao Y.H. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control. 2008;19:231–235. doi: 10.1016/j.foodcont.2007.03.004. [DOI] [Google Scholar]
- 27.Hayes M., Stanton C., Slattery H., O’Sullivan O., Hill C., Fitzgerald G.F., Ross R.P. Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors. Appl. Environ. Microbiol. 2007;73:4658–4667. doi: 10.1128/AEM.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudoh Y., Matsuda S., Igoshi K., Oki T. Antioxidative peptide from milk fermented with Lactobacillus delbrueckii subsp. bulgaricus IFO13953. J. Jpn. Soc. Food Sci. Technol. 2001;48:44–50. doi: 10.3136/nskkk.48.44. [DOI] [Google Scholar]
- 29.Pihlanto A., Virtanen T., Korhonen H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. Int. Dairy J. 2010;20:3–10. [Google Scholar]
- 30.Arihara K., Ohata M. Functional Properties of Bioactive Peptides Derived from meat Proteins. In: Toldra F., editor. Advanced Technologies for Meat Processing. Springer; New York, NY, USA: 2006. pp. 245–274. [Google Scholar]
- 31.Hammes W.P., Haller D., Ganzle M.G. Fermented Meat. In: Farnworth E.R., editor. Handbook of Fermented Functional Foods. CRC; New York, NY, USA: 2003. pp. 251–269. [Google Scholar]
- 32.Ondetti M.A., Rubin B., Cushman D.W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 33.Iroyukifujita H., Eiichiyokoyama K., Yoshikawa M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000;65:564–569. [Google Scholar]
- 34.Kim S.-K., Byun H.-G., Park P.-J., Shahidi F. Angiotensin I converting enzyme inhibitory peptides purified from bovine skin gelatin hydrolysate. J. Agric. Food Chem. 2001;49:2992–2997. doi: 10.1021/jf001119u. [DOI] [PubMed] [Google Scholar]
- 35.Qian Z.J., Jung W.K., Lee S.H., Byun H.G., Kim S.K. Antihypertensive effect of an angiotensin I-converting enzyme inhibitory peptide from bullfrog (Rana catesbeiana Shaw) muscle protein in spontaneously hypertensive rats. Process Biochem. 2007;42:1443–1448. [Google Scholar]
- 36.Saiga A., Okumura T., Makihara T., Katsuda S.I., Morimatsu F., Nishimura T. Action mechanism of an angiotensin I-converting enzyme inhibitory peptide derived from chicken breast muscle. J. Agric. Food Chem. 2006;54:942–945. doi: 10.1021/jf0508201. [DOI] [PubMed] [Google Scholar]
- 37.Saiga A., Iwai K., Hayakawa T., Takahata Y., Kitamura S., Nishimura T., Morimatsu F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008;56:9586–9591. doi: 10.1021/jf072669w. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira S.H., Bartelt D.C., Greene L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–2593. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 39.Vermeirssen V., van Camp J., Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J. Biochem. Biophys. Methods. 2002;51:75–87. doi: 10.1016/s0165-022x(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 40.Shalaby S.M., Zakora M., Otte J. Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates. J. Dairy Res. 2006;73:178–186. doi: 10.1017/S0022029905001639. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Fandino R., Otte J., van Camp J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006;16:1277–1293. [Google Scholar]
- 42.Brown N.J., Vaughan D.E. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 43.Folkow B., Johansson B., Mellander S. The comparative effects of angiotensin and noradrenaline on consecutive vascular sections. Acta Physiol. Scand. 1961;53:99–104. doi: 10.1111/j.1748-1716.1961.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 44.Biron P., Koiw E., Nowaczynski W., Brouillet J., Genest J. The effects of intravenous infusions of valine-5 angiotensin II and other pressor agents on urinary electrolytes and corticosteroids, including aldosterone. J. Clin. Invest. 1961;40:338–347. doi: 10.1172/JCI104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padfield P.L., Morton J.J. Effects of angiotensin II on arginine-vasopressin in physiological and pathological situations in man. J. Endocrinol. 1977;74:251–259. doi: 10.1677/joe.0.0740251. [DOI] [PubMed] [Google Scholar]
- 46.Proud D., Kaplan A.P. Kinin formation: Mechanisms and role in inflammatory disorders. Annu. Rev. Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- 47.Vanhoutte P.M. Endothelium and control of vascular function. State of the art lecture. Hypertension. 1989;13:658–667. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- 48.Ahhmed A.M., Muguruma M. A review of meat protein hydrolysates and hypertension. Meat Sci. 2010;86:110–118. doi: 10.1016/j.meatsci.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 49.Qian Z.-J., Je J.-Y., Kim S.-K. Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of bigeye tuna dark muscle, thunnus obesus. J. Agric. Food Chem. 2007;55:8398–8403. doi: 10.1021/jf0710635. [DOI] [PubMed] [Google Scholar]
- 50.Katayama K., Tomatsu M., Kawahara S., Yamauchi K., Fuchu H., Kodama Y., Kawamura Y., Muguruma M. Inhibitory profile of nonapeptide derived from porcine troponin C against angiotensin I-converting enzyme. J. Agric. Food Chem. 2004;52:771–775. doi: 10.1021/jf0350865. [DOI] [PubMed] [Google Scholar]
- 51.Lee S.-H., Qian Z.-J., Kim S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010;118:96–102. [Google Scholar]
- 52.Wijesekara I., Kim S.K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs. 2010;8:1080–1093. doi: 10.3390/md8041080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuller M.F., Tomé D. In vivo determination of amino acid bioavailability in humans and model animals. J. AOAC Int. 2005;88:923–934. [PubMed] [Google Scholar]
- 54.Balimane P.V., Chong S., Morrison R.A. Current methodologies used for evaluation of intestinal permeability and absorption. J. Pharmacol. Toxicol. Methods. 2000;44:301–312. doi: 10.1016/s1056-8719(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 55.Ekmekcioglu C. A physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002;76:225–230. [Google Scholar]
- 56.Pauletti G.M., Gangwar S., Siahaan T.J., Jeffrey A., Borchardt R.T. Improvement of oral peptide bioavailability: Peptidomimetics and prodrug strategies. Adv. Drug Deliv. Rev. 1997;27:235–256. doi: 10.1016/s0169-409x(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 57.DeSesso J.M., Jacobson C.F. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol. 2001;39:209–228. doi: 10.1016/s0278-6915(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 58.Greger J.L. Using animals to assess bioavailability of minerals: Implications for human nutrition. J. Nutr. 1992;122:2047–2052. doi: 10.1093/jn/122.10.2047. [DOI] [PubMed] [Google Scholar]
- 59.Foltz M., Cerstiaens A., van Meensel A., Mols R., van der Pijl P.C., Duchateau G.S.M.J.E., Augustijns P. The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides. 2008;29:1312–1320. doi: 10.1016/j.peptides.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 60.Sun H., Liu D., Li S., Qin Z. Transepithelial transport characteristics of the antihypertensive peptide, Lys-Val-Leu-Pro-Val-Pro, in human intestinal Caco-2 cell monolayer. Biosci. Biotechnol. Biochem. 2009;73:293–298. doi: 10.1271/bbb.80473. [DOI] [PubMed] [Google Scholar]
- 61.Vermeirssen V., Camp J.V., Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004;92:357–366. doi: 10.1079/bjn20041189. [DOI] [PubMed] [Google Scholar]
- 62.Cinq-Mars C.D., Hu C., Kitts D.D., Li-Chan E.C.Y. Investigations into inhibitor type and mode, simulated gastrointestinal digestion, and cell transport of the angiotensin I-converting enzyme-inhibitory peptides in pacific hake (Merluccius productus) fillet hydrolysate. J. Agric. Food Chem. 2007;56:410–419. doi: 10.1021/jf072277p. [DOI] [PubMed] [Google Scholar]
- 63.Bailey C.A., Bryla P., Malick A.W. The use of the intestinal epithelial cell culture model, Caco-2, in pharmaceutical development. Adv. Drug Deliv. Rev. 1996;22:85–103. doi: 10.1016/S0169-409X(96)00416-4. [DOI] [Google Scholar]
- 64.Hidalgo I.J., Raub T.J., Borchardt R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 65.Fujita H., Yokoyama K., Yasumoto R., Yoshikawa M. Antihypertensive effect of thermolysin digest of dried bonito in spontaneously hypertensive rat. Clin. Exp. Pharmacol. Physiol. 1995;22:S304–S305. doi: 10.1111/j.1440-1681.1995.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 66.Nakashima Y., Arihara K., Sasaki A., Mio H., Ishikawa S., Itoh M. Antihypertensive activities of peptides derived from porcine skeletal muscle myosin in spontaneously hypertensive rats. J. Food Sci. 2002;67:434–437. [Google Scholar]
- 67.Quirós A., Dávalos A., Lasunción M.A., Ramos M., Recio I. Bioavailability of the antihypertensive peptide LHLPLP: Transepithelial flux of HLPLP. Int. Dairy J. 2008;18:279–286. [Google Scholar]
- 68.Foltz M., Meynen E.E., Bianco V., van Platerink C., Koning T.M.M.G., Kloek J. Angiotensin converting enzyme inhibitory peptides from a lactotripeptide-enriched milk beverage are absorbed intact into the circulation. J. Nutr. 2007;137:953–958. doi: 10.1093/jn/137.4.953. [DOI] [PubMed] [Google Scholar]
- 69.Hata Y., Yamamoto M., Ohni M., Nakajima K., Nakamura Y., Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996;64:767–771. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 70.Seppo L., Jauhiainen T., Poussa T., Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am. J. Clin. Nutr. 2003;77:326–330. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- 71.Jauhiainen T., Vapaatalo H., Poussa T., Kyrönpalo S., Rasmussen M., Korpela R. Lactobacillus helveticus fermented milk lowers blood pressure in hypertensive subjects in 24-h ambulatory blood pressure measurement. Am. J. Hypertens. 2005;18:1600–1605. doi: 10.1016/j.amjhyper.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Fujita H., Yoshikawa M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology. 1999;44:123–127. doi: 10.1016/s0162-3109(99)00118-6. [DOI] [PubMed] [Google Scholar]
- 73.Matsufuji H., Matsui T., Seki E., Osajima K., Nakashima M., Osajima Y. Angiotensin I-converting enzyme inhibitory peptides in an alkaline protease hydrolyzate derived from sardine muscle. Biosci. Biotechnol. Biochem. 1994;58:2244–2245. doi: 10.1271/bbb.58.2244. [DOI] [PubMed] [Google Scholar]
- 74.Ono S., Hosokawa M., Miyashita K., Takahashi K. Isolation of peptides with angiotensin I-converting enzyme inhibitory effect derived from hydrolysate of upstream chum salmon muscle. J. Food Sci. 2003;68:1611–1614. [Google Scholar]
- 75.Yokoyama K., Chiba H., Yoshikawa M. Peptide inhibitors for angiotensin I-converting enzyme from thermolysin digest of dried bonito. Biosci. Biotechnol. Biochem. 1992;56:1541–1545. doi: 10.1271/bbb.56.1541. [DOI] [PubMed] [Google Scholar]
- 76.Hayes M., Stanton C., Fitzgerald G.F., Ross R.P. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part II: Bioactive peptide functions. J. Biotechnol. 2007;2:435–449. doi: 10.1002/biot.200700045. [DOI] [PubMed] [Google Scholar]
- 77.Cushman D.W., Cheung H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 78.Rho S.J., Lee J.-S., Chung Y.I., Kim Y.-W., Lee H.G. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009;44:490–493. [Google Scholar]
- 79.Saiga A., Okumura T., Makihara T., Katsuta S., Shimizu T., Yamada R., Nishimura T. Angiotensin I-converting enzyme inhibitory peptides in a hydrolyzed chicken breast muscle extract. J. Agric. Food Chem. 2003;51:1741–1745. doi: 10.1021/jf020604h. [DOI] [PubMed] [Google Scholar]
- 80.Maruyama S., Mitachi H., Awaya J., Kurono M., Tomizuka N., Suzuki H. Angiotensin I-converting enzyme inhibitory activity of the C-terminal hexapeptide of s1-casein. Agric. Biol. Chem. 1987;51:2557–2561. doi: 10.1271/bbb1961.51.2557. [DOI] [Google Scholar]
- 81.Lawrie R.A., Ledward D.A. Lawrie’s Meat Science. In: Lawrie R.A., editor. Lawrie’s Meat Science. 7th. Woodhead Publishing; Cambridge, UK: 2006. pp. 41–73. [Google Scholar]
- 82.Spudich J.A., Watt S. The regulation of rabbit skeletal muscle contraction. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 83.Muguruma M., Ahhmed A.M., Katayama K., Kawahara S., Maruyama M., Nakamura T. Identification of pro-drug type ACE inhibitory peptide sourced from porcine myosin B: Evaluation of its antihypertensive effects in vivo. Food Chem. 2009;114:516–522. doi: 10.1016/j.foodchem.2008.09.081. [DOI] [Google Scholar]
- 84.Katayama K., Mori T., Kawahara S., Miake K., Kodama Y., Sugiyama M., Kawamura Y., Nakayama T., Maruyama M., Muguruma M. Angiotensin-I converting enzyme inhibitory peptide derived from porcine skeletal muscle myosin and its antihypertensive activity in spontaneously hypertensive rats. J. Food Sci. 2007;72:S702–S706. doi: 10.1111/j.1750-3841.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 85.Katayama K., Tomatsu M., Fuchu H., Sugiyama M., Kawahara S., Yamauchi K., Kawamura Y., Muguruma M. Purification and characterization of an angiotensin I converting enzyme inhibitory peptide derived from porcine troponin C. Anim. Sci. J. 2003;74:53–58. [Google Scholar]
- 86.Escudero E., Sentandreu M.A., Arihara K., Toldrá F. Angiotensin I-converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J. Agric. Food Chem. 2010;58:2895–2901. doi: 10.1021/jf904204n. [DOI] [PubMed] [Google Scholar]
- 87.Gelse K., Pöschl E., Aigner T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 88.Jang A., Cho Y.J., Lee J.I., Shin J.H., Kim I.S., Lee M. The effect of beef peptide on blood pressure and serum lipid concentration of spontaneously hypertensive rat (SHR) J. Anim. Sci. Technol. 2004;46:107–114. [Google Scholar]
- 89.Iroyukifujita H., Eiichiyokoyama K., Yoshikawa M. Classification and antihypertensive activity of angiotensin I converting enzyme inhibitory peptides derived from food proteins. J. Food Sci. 2000;65:564–569. [Google Scholar]
- 90.Suetsuna K., Osajima K. The inhibitory activities against angiotensin I-converting enzyme of basic peptides originating from sardine and hair tail meat. Bull. Jpn. Soc. Sci. Fish. 1986;52:1981–1984. [Google Scholar]
- 91.Hai-Lun H., Xiu-Lan C., Cai-Yun S., Yu-Zhong M., Bai-Cheng Z. Analysis of novel angiotensin-I-converting enzyme inhibitory peptides from protease-hydrolyzed marine shrimp Acetes chinensis. J. Pept. Sci. 2006;12:726–733. doi: 10.1002/psc.789. [DOI] [PubMed] [Google Scholar]
- 92.Theodore A.E., Kristinsson H.G. Angiotensin converting enzyme inhibition of fish protein hydrolysates prepared from alkaline-aided channel catfish protein isolate. J. Sci. Food Agric. 2007;87:2353–2357. doi: 10.1021/jf800185f. [DOI] [PubMed] [Google Scholar]
- 93.Bougatef A., Nedjar-Arroume N., Ravallec-Plé R., Leroy Y., Guillochon D., Barkia A., Nasri M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008;111:350–356. doi: 10.1016/j.foodchem.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 94.Samaranayaka A.G.P., Kitts D.D., Li-Chan E.C.Y. Antioxidative and angiotensin-I-converting enzyme inhibitory potential of a Pacific hake (Merluccius productus) fish protein hydrolysate subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J. Agric. Food Chem. 2010;58:1535–1542. doi: 10.1021/jf9033199. [DOI] [PubMed] [Google Scholar]
- 95.Je J.-Y., Park P.-J., Kwon J.Y., Kim S.-K. A Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Alaska Pollack (Theragra chalcogramma) Frame Protein Hydrolysate. J. Agric. Food Chem. 2004;52:7842–7845. doi: 10.1021/jf0494027. [DOI] [PubMed] [Google Scholar]
- 96.Jung W.-K., Mendis E., Je J.-Y., Park P.-J., Son B.W., Kim H.C., Choi Y.K., Kim S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006;94:26–32. [Google Scholar]
- 97.Ono S., Hosokawa M., Miyashita K., Takahashi K. Inhibition properties of dipeptides from salmon muscle hydrolysate on angiotensin I-converting enzyme. Int. J. Food Sci. Technol. 2006;41:383–386. [Google Scholar]
- 98.Wu H., He H.L., Chen X.L., Sun C.Y., Zhang Y.Z., Zhou B.C. Purification and identification of novel angiotensin-I-converting enzyme inhibitory peptides from shark meat hydrolysate. Process Biochem. 2008;43:457–461. [Google Scholar]
- 99.Kawasaki T., Seki E., Osajima K., Yoshida M., Asada K., Matsui T., Osajima Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hum. Hypertens. 2000;14:519–523. doi: 10.1038/sj.jhh.1001065. [DOI] [PubMed] [Google Scholar]
- 100.Matsufuji H., Matsui T., Ohshige S., Kawasaki T., Osajima K., Osajima Y. Antihypertensive effects of angiotensin fragments in SHR. Biosci. Biotechnol. Biochem. 1995;59:1398–1401. doi: 10.1271/bbb.59.1398. [DOI] [PubMed] [Google Scholar]
- 101.Kohama Y., Matsumoto S., Oka H., Teramoto T., Okabe M., Mimura T. Isolation of angiotensin-converting enzyme inhibitor from tuna muscle. Biochem. Biophys. Res. Commun. 1988;155:332–337. doi: 10.1016/s0006-291x(88)81089-1. [DOI] [PubMed] [Google Scholar]
- 102.Byun H.-G., Kim S.-K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001;36:1155–1162. doi: 10.1016/S0032-9592(00)00297-1. [DOI] [Google Scholar]
- 103.Ames B.N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 104.Gimenez B., Aleman A., Montero P., Gomez-Guillen M.C. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009;114:976–983. [Google Scholar]
- 105.Cai W., Gao Q.D., Zhu L., Peppa M., He C., Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: Novel mediators of cellular dysfunction. Mol. Med. 2002;8:337–346. [PMC free article] [PubMed] [Google Scholar]
- 106.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993;57:779S–785S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- 107.Pihlanto A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006;16:1306–1314. [Google Scholar]
- 108.Vercruysse L., Smagghe G., Beckers T., Camp J.V. Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis. Food Chem. 2009;114:38–43. doi: 10.1016/j.foodchem.2008.09.011. [DOI] [Google Scholar]
- 109.Valenzuela A., Sanhueza J., Nieto S. Natural antioxidants in functional foods: From food safety to health benefits. Grasas Aceites. 2003;54:295–303. [Google Scholar]
- 110.Moure A., Cruz J.M., Franco D., Domínguez J.M., Sineiro J., Domínguez H., José Núñez M., Parajó J.C. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. [Google Scholar]
- 111.Suetsuna K., Ukeda H., Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000;11:128–131. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 112.Sun J., He H., Xie B.J. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004;52:6646–6652. doi: 10.1021/jf0495136. [DOI] [PubMed] [Google Scholar]
- 113.Wu H.C., Chen H.M., Shiau C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- 114.Je J.Y., Park P.J., Kim S.K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005;38:45–50. doi: 10.1016/j.foodres.2004.07.005. [DOI] [Google Scholar]
- 115.Raghavan S., Kristinsson H.G., Leeuwenburgh C. Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J. Agric. Food Chem. 2008;56:10359–10367. doi: 10.1021/jf8017194. [DOI] [PubMed] [Google Scholar]
- 116.Dong S.Y., Zeng M.Y., Wang D.F., Liu Z.Y., Zhao Y.H., Yang H.C. Antioxidant and biochemical properties of protein hydrolysates prepared from Silver carp (Hypophthalmichthys molitrix) Food Chem. 2008;107:1485–1493. doi: 10.1016/j.foodchem.2007.10.011. [DOI] [Google Scholar]
- 117.Klompong V., Benjakul S., Yachai M., Visessanguan W., Shahidi F., Hayes K. Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis) J. Food Sci. 2009;74:C126–C133. doi: 10.1111/j.1750-3841.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 118.Bougatef A., Nedjar-Arroume N., Manni L., Ravallec R., Barkia A., Guillochon D., Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010;118:559–565. doi: 10.1016/j.foodchem.2009.05.021. [DOI] [Google Scholar]
- 119.Hsu K.-C. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010;122:42–48. [Google Scholar]
- 120.Mendis E., Rajapakse N., Kim S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2004;53:581–587. doi: 10.1021/jf048877v. [DOI] [PubMed] [Google Scholar]
- 121.Saiga A., Tanabe S., Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- 122.Li B., Chen F., Wang X., Ji B.P., Wu Y.N. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2007;102:1135–1143. [Google Scholar]
- 123.Liu Q., Kong B., Xiong Y.L., Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. [Google Scholar]
- 124.Wu H.C., Pan B.S., Chang C.L., Shiau C.Y. Low-molecular-weight peptides as related to antioxidant properties of chicken essence. J. Food Drug Anal. 2005;13:176–183. [Google Scholar]
- 125.Hayes M., Ross R.P., Fitzgerald G.F., Hill C., Stanton C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006;72:2260–2264. doi: 10.1128/AEM.72.3.2260-2264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCann K.B., Shiell B.J., Michalski W.P., Lee A., Wan J., Roginski H., Coventry M.J. Isolation and characterisation of antibacterial peptides derived from the f (164-207) region of bovine [alpha] S2-casein. Int. Dairy J. 2005;15:133–143. [Google Scholar]
- 127.Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- 128.van der Kraan M.I.A., Groenink J., Nazmi K., Veerman E.C.I., Bolscher J.G.M., Nieuw Amerongen A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides. 2004;25:177–183. doi: 10.1016/j.peptides.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 129.Lee Y., Kim J.I.Y., Lee K., Kim K.H., Lee H. Peptides from anchovy sauce induce apoptosis in a human lymphoma cell (U937) through the increase of caspase 3 and 8 activities. Ann. N. Y. Acad. Sci. 2003;1010:399–404. doi: 10.1196/annals.1299.073. [DOI] [PubMed] [Google Scholar]
- 130.Lee Y.G., Lee K.W., Kim J.Y., Kim K.H., Lee H.J. Induction of apoptosis in a human lymphoma cell line by hydrophobic peptide fraction separated from anchovy sauce. BioFactors. 2004;21:63–67. doi: 10.1002/biof.552210112. [DOI] [PubMed] [Google Scholar]
- 131.Picot L., Bordenave S., Didelot S., Fruitier-Arnaudin I., Sannier F., Thorkelsson G., Berge J.P., Guerard F., Chabeaud A., Piot J.M. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006;41:1217–1222. [Google Scholar]
- 132.Hsu K.-C., Li-Chan E.C.Y., Jao C.-L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2010;126:617–622. [Google Scholar]
- 133.Hartmann R., Meisel H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007;18:163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 134.Slim M.-K. Cardiovascular actions of chicken-meat extract in normo- and hypertensive rats. Br. J. Nutr. 2001;86:97–103. doi: 10.1079/bjn2001367. [DOI] [PubMed] [Google Scholar]