Abstract
Rho of Plants (ROP) small G proteins function at discrete domains of the plasma and possibly endo membranes. ROPs are synthesized as soluble proteins and their attachment to membranes and partitioning in membrane microdomains are facilitated by the posttranslational lipid modifications prenylation and/or S-acylation. Based on their amino acid sequences, ROPs can be classified into two major subgroups: type-I ROPs terminate with a canonical CaaX box motif and are prenylated primarily by geranyl-geranyltransferase-I (GGT-I) and to a lesser extent by farnesyltransferase (FT). Type-II ROPs terminate with a plant specific GC-CG box domain and are attached to the plasma membrane by stable S-acylation. In addition, type-I and possibly also type-II ROPs undergo activation dependent transient S-acylation in the G-domain and consequent partitioning into lipid rafts. Surprisingly, although geranylgeranylation is required for the membrane attachment of type-I ROPs and the γ subunits of heterotrimeric G proteins, Arabidopsis mutants lacking GGT-I function have a mild phenotype compared to wild type plants. The mild phenotype of the ggt-I mutants suggested that farnesylation by FT may compensate for the loss of GGT-I function and that possibly the prenylated type-I and S-acylated type-II ROPS have some overlapping functions. In a paper recently published in Plant Physiology1 we examined the role of the prenyl group type in type-I ROP function and membrane interaction dynamics and the functional redundancy between type-I and type-II ROPs. This study complements a second paper in which we examined the role of G-domain transient S-acylation in the membrane interaction dynamics and signaling by type-I ROPs.2 Together these two studies provide a framework for realizing the role of prenylation and S-acylation in subcellular targeting, membrane interaction dynamics and signaling by ROP GTPases.
Key words: prenylation, S-acylation, ROP, FRAP, membrane targeting, lipid rafts
The subcellular distribution of Rho superfamily small G proteins orchestrates cytoskeletal organization, vesicle trafficking and cell wall modifications at specific subcellular domains.3–6 Rhos are synthesized as soluble protein precursors in the cytoplasm and their attachment to membranes depends on posttranslational lipid modifications. Lipid modifications of Rho proteins in concert with their C-terminal polybasic domains affect subcellular targeting, membrane interaction dynamics, conformation and interaction with other proteins. Lipid modifications may also provide a link between metabolic and Rho-regulated signaling pathways. Hence, identification of the lipid modifications of Rho proteins and elucidation of their effects on subcellular distribution, membrane interaction dynamics and activity is essential for realizing the cellular function of these proteins.
Rho of plants (ROPs), also known as RACs, are master regulators of cell polarity and signaling.5,7,8 A classification method based on the amino acid sequence divided ROPs into two major subgroups designated type-I and type-II.9 All type-I ROPs terminate with a CaaX box motif and are prenylated mainly by C20 geranylgeranyl moieties and to a much lesser degree by C15 farnesyl moieties.10 Type-II ROPs are not prenylated and attach to the membrane by S-acylation of cysteines that are part of a conserved domain designated the GC-CG box, in which the acylated cysteines are separated by a stretch of five or six aliphatic residues and flanked by glycines.11–13 S-acylation, often referred to as palmitoylation, involves the attachment of palmitate (C16:0) or stearate (C18:0) to cysteine residues via a reversible thioester linkage.14–16 It has been shown that the composition and structure of the GC-CG box is required for stable association with the membrane.13 In addition, the type-I ROP At-ROP6 (ROP6), and possibly other type-I and type-II ROPs, undergo activation-dependent transient S-acylation on two highly conserved cysteine residues in the catalytic G-domain (C21 and C158 in ROP6).
Prenylation of CaaX box proteins is catalyzed by two distinct prenyltransferases, farnesyltransferase (FT) and geranylgeranyltransferase-I (GGT-I).17 FT and GGT-I are heterodimeric proteins that share a common α subunit and have distinct β subunits that determine substrate specificity. In Arabidopsis, the FT β subunit (ERA1), the GGT-I β subunit (GGT-Ib) and the FT/GGT-I common α subunit (PLURIPETALA) mutants are viable. The viability of the FT and GGT-I mutants enabled us to explore: (1) how the type of prenyl group affects the function of ROPs and (2) the functional redundancy between prenylated type-I ROPs and S-acylated type-II ROPs. The results of this study were summarized in a paper that was published in the February 2011 issue of Plant Physiology.1
At the heart of our study were two techniques that enabled us to link between the prenylation and/or S-acylation status and membrane interaction dynamics. Gas chromatography coupled mass spectrometry (GC-MS) was used to identify the lipid moieties released from purified recombinant proteins that were expressed in transgenic plants18 (Sorek et al. Methods in Molecular Biology, In Press). Using GC-MS, we were able to identify the prenyl and acyl groups that modify ROP6 and AGG1 in wt and GGT-I β subunit mutant (ggt-Ib) mutant plants. The second technique is a specialized form of Fluorescence Recovery After Photobleaching (FRAP) called “FRAP beam-size analysis.” This technique, which is based on comparing the recovery rates in FRAP experiments conducted with two different laser beam sizes, characterizes the membrane interaction dynamics of GFP-tagged proteins by comparing the relative contribution of lateral diffusion in the membrane versus exchange with the cytoplasm to the fluorescence recovery process (Fig. 1A).19 FRAP beam size analysis is conducted on live cells and measures the membrane interactions of the proteins in their native milieu, resulting in high sensitivity which can reveal changes in protein-membrane interaction dynamics that cannot be detected by conventional techniques such as immunoblotting or fluorescence microscopy.
Figure 1.
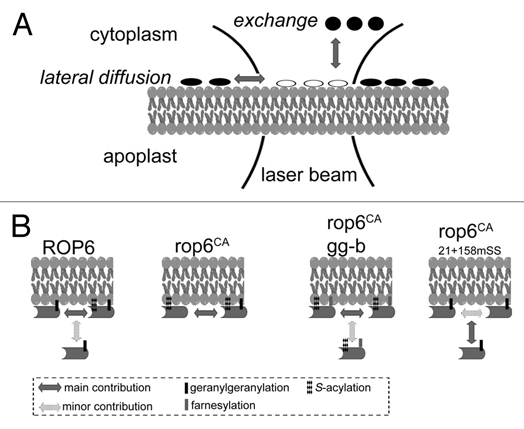
Determination of the role of lipid modifications in ROP membrane dynamics by FRAP beam-size analysis. (A) FRAP beam-size analysis. In this technique,19 a laser beam is focused to a small Gaussian spot on the membrane, collecting the fluorescence via the narrow focus of a confocal setup. After bleaching, the bleached molecules of the protein measured (white ellipses) can be replaced in the bleached spot with fresh fluorescent molecules (black ellipses) by lateral diffusion or by exchange between membrane-bound and cytoplasmic populations. The experiment is repeated with two different laser beam sizes. For lateral diffusion, the recovery time, τ, is directly proportional to the area bleached by the beam. However, for recovery by exchange, τ is independent of the beam size, as it reflects a chemical relaxation rate. (B) The effect of lipid modifications on the membrane interaction dynamics of ROP6. In the wt version of ROP6 (ROP6) the main contribution to fluorescence recovery is by lateral diffusion, although exchange is not negligible. Fluorescence recovery of a constitutively active S-acylated ROP6 (rop6CA) takes place by pure lateral diffusion. Fluorescence recovery of rop6CA in ggt-Ib background (rop6CA gg-b) demonstrated a mixed contribution of lateral diffusion and exchange. However, the contribution of the exchange was minor. The fluorescence recovery of the non S-acylated constitutively active ROP6 (rop6CA21 + 158mSS) takes place primarily by exchange and to a lesser extent by lateral diffusion.
We combined the identification of the lipid modifications and the measurements of membrane interaction dynamics with functional analysis of ROPs in mammalian and plant cells. In mammalian cells, activated membrane-bound Racs induce formation of actin-rich lamellipodia and membrane ruffles. This phenomenon enabled to examine the functional conservation between plant ROPs and mammalian Racs, and to explore whether the mechanism responsible for membrane targeting and function of type-II ROPs is unique to plants. In plants, the structure of leaf epidermis pavement is affected by ROP activation status. Together, the expression of ROP and ROP mutants in mammalian cells and transgenic Arabidopsis provided means to link ROP function with their lipid modifications and membrane interaction dynamics.
When expressed in mammalian cells, constitutively active ROP6 induced formation of actin-rich lamellipodia and membrane ruffles, similar to Rac proteins. Interestingly, a dominant negative ROP6 accumulated in cytoplasmic aggregates and was not detected in the plasma membrane. In these aggregates the dominant negative ROP6 mutant might have been in complex with RhoGDI and/or RhoGEFs. In contrast to ROP6, the type-II ROP At-ROP9 remained dispersed in the cytoplasm and did not induce visible phenotypic changes in the cells. Taken together, these data indicated that type-I ROPs are functionally related to Rac proteins in metazoans and can interact with their effectors regardless of the structural differences in the insert region and the switch-II domain.20,21 The data further confirmed that the mechanism associated with S-acylation and membrane targeting of type-II ROPs is unique to plants.
Analysis of the prenylation of ROP6 and the Gγ subunit AGG1 by GC-MS demonstrated that both proteins were farnesylated in the ggt-Ib background. Both confocal imaging and immunoblots with anti-ROP specific antibodies showed that relative to wt plants, increased amounts of ROPs could be detected in the cytoplasm at steady state. Interestingly, recombinant ROP6, which was purified from the soluble protein extracts of ggt-Ib plants, was farnesylated and S-acylated (Fig. 1B). Hence, unlike H-ras, where transient S-acylation of hypervariable domain cysteines promotes stable interaction with the plasma membrane,19 the transient S-acylation of G-domain cysteines in ROP6 only promotes stable membrane association in conjunction with geranylgeranylation.
The FRAP beam-size analysis revealed that the type of prenyl group had little effect on membrane interaction dynamics (Fig. 1B). In line with the FRAP results, the type of prenyl group had no effect on the distribution of ROPs in the membrane between detergent soluble and insoluble (lipid rafts) fractions. Furthermore, leaf epidermis pavement cells of ggt-Ib mutants were only slightly less polar compared to wt cells, indicating that the prenyl group had little effect on ROP signaling in cell polarity.1 The minor differences in cell polarity that were observed are likely due to the smaller fraction of ROPs in the plasma membrane rather than a direct effect on their function. In contrast, mutations that abolished ROP G-domain transient S-acylation strongly affected ROP membrane interaction dynamics (Fig. 1B) and partitioning into lipid rafts, and compromised ROP function in regulation of cell polarity.2
The data described above indicate that prenylation is required for membrane targeting of ROPs and is therefore crucial for their function. Farnesylated ROPs are still targeted to the plasma membrane and remain functional, although their interaction with the membrane is weaker. The recovery of farnesylated and S-acylated ROPs from the soluble protein extracts prepared from ggt-Ib mutant plants is compatible with two alternative scenarios. One scenario is that the prenyl group is embedded in the lipid bilayer and that the greater hydrophobicity of geranyl-geranyl in the C-terminal hypervariable domain together with the proximal poly-basic domain are required for stable association with the membrane. An alternative explanation is that the prenyl moiety is associated with the plasma membrane through an adaptor with higher affinity for geranylgeranyl compared to farnesyl. Existence of such factor/s is compatible with the identification of Galectin-1 and Galectin-3 as adaptors for H-ras and K-ras membrane anchoring, respectively.22 Given that cells have proteins that specifically recognize the isoprenylated cysteine, including CaaX proteases, Isoprenyl cysteine Carboxy Methyl Transferase (ICMT) and RhoGDI, the existence of membrane adaptors for geranylgeranylated ROPs is not unlikely.
ROPs have been implicated to play a role in cell polarity and development.5,23 pluripetala (plp) FT/GGT-I α-subunit mutant plants display severe growth and developmental alterations characterized by pronounced expansion of the shoot apical meristem (SAM), a strongly retarded growth rate, and almost complete male sterility.24 We observed that the epidermal cells of plp leaves are bigger and less polar then in wt plants. Interestingly, the differences were much more pronounced in the adaxial (closer to the stem) epidermal cells compared to abaxial (away from the stem) cells. The stronger change in cell polarity at the adaxial side might reflect a greater contribution of type-I ROPs to cell growth on this side of the leaf. Nevertheless, possible contributions by other prenylated proteins cannot be ignored. Expression of constitutively active At-ROP11 (rop11CA) in plp induced a novel phenotype. The leaf epidermis abaxial cells expressing the rop11CA mutant were rectangular or cubical, differing in their morphology from abaxial plp pavement cells. rop11CA expression in plp also induced ectopic symmetric and asymmetric cell divisions resulting in increased number of stomata, a phenotype that was not observed when either rop11CA or other constitutively active ROP mutants were expressed in wt plants. The establishment of the adaxial and abaxial identities takes place shortly after emergence of leaf primordia from the SAM and is regulated by hormonal, transcriptional and posttranscriptional networks.25 The plp phenotype and the effect of the constitutively active rop11CA on cell division in the plp background suggest that the expression of type-I and type-II ROPs, and perhaps more importantly, the equilibrium between their GTP (ON) and the GDP (OFF) bound states, might be coordinated by the signaling network that regulates adaxial/abaxial cell fates.
References
- 1.Sorek N, Gutman O, Bar E, Abu-Abied M, Feng X, Running MP, et al. Differential Effects of Prenylation and S-Acylation on Type-I and Ii Rops Membrane Interaction and Function. Plant Physiol. 2010;155:706–720. doi: 10.1104/pp.110.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorek N, Segev O, Gutman O, Bar E, Richter S, Poraty L, et al. An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Curr Biol. 2010;20:914–920. doi: 10.1016/j.cub.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Yalovsky S, Bloch D, Sorek N, Kost B. Regulation of membrane trafficking, cytoskeleton dynamics and cell polarity by ROP/RAC GTPases. Plant Physiol. 2008;147:1527–1543. doi: 10.1104/pp.108.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z. Small GTPases: versatile signaling switches in plants. Plant Cell. 2002;14:375–388. doi: 10.1105/tpc.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mucha E, Fricke I, Schaefer A, Wittinghofer A, Berken A. Rho proteins of plants—Functional cycle and regulation of cytoskeletal dynamics. Eur J Cell Biol. 2011 doi: 10.1016/j.ejcb.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Nibau C, Wu HM, Cheung AY. RAC/ROP GTPases: “hubs” for signal integration and diversification in plants. Trends Plant Sci. 2006;11:309–315. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Winge P, Brembu T, Bones AM. Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol Biol. 1997;35:483–495. doi: 10.1023/a:1005804508902. [DOI] [PubMed] [Google Scholar]
- 10.Sorek N, Poraty L, Sternberg H, Bar E, Lewinsohn E, Yalovsky S. Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Mol Cell Biol. 2007;27:2144–2154. doi: 10.1128/MCB.02347-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Ivanchenko M, Vejlupkova Z, Quatrano RS, Fowler JE. Maize ROP7 GTPase contains a unique, CaaX box-independent plasma membrane targeting signal. Plant J. 2000;24:79–90. doi: 10.1046/j.1365-313x.2000.00855.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavy M, Bracha-Drori K, Sternberg H, Yalovsky S. A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell. 2002;14:2431–2450. doi: 10.1105/tpc.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavy M, Yalovsky S. Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J. 2006;46:934–947. doi: 10.1111/j.1365-313X.2006.02749.x. [DOI] [PubMed] [Google Scholar]
- 14.Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem Sci. 2011;36:245–253. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Greaves J, Gorleku OA, Salaun C, Chamberlain LH. Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases. J Biol Chem. 2010;285:24629–24638. doi: 10.1074/jbc.M110.119289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salaun C, Greaves J, Chamberlain LH. The intracellular dynamic of protein palmitoylation. J Cell Biol. 2010;191:1229–1238. doi: 10.1083/jcb.201008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid TS, Terry KL, Casey PJ, Beese LS. Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J Mol Biol. 2004;343:417–433. doi: 10.1016/j.jmb.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Sorek N, Yalovsky S. Analysis of protein S-acylation by gas chromatography-coupled mass spectrometry using purified proteins. Nat Protoc. 2010;5:834–840. doi: 10.1038/nprot.2010.33. [DOI] [PubMed] [Google Scholar]
- 19.Henis YI, Rotblat B, Kloog Y. FRAP beam-size analysis to measure palmitoylation-dependent membrane association dynamics and microdomain partitioning of Ras proteins. Methods. 2006;40:183–190. doi: 10.1016/j.ymeth.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Berken A, Wittinghofer A. Structure and function of Rho-type molecular switches in plants. Plant Physiol Biochem. 2008;46:380–393. doi: 10.1016/j.plaphy.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Sormo CG, Leiros I, Brembu T, Winge P, Os V, Bones AM. The crystal structure of Arabidopsis thaliana RAC7/ROP9: the first RAS superfamily GTPase from the plant kingdom. Phytochemistry. 2006;67:2332–2340. doi: 10.1016/j.phytochem.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Belanis L, Plowman SJ, Rotblat B, Hancock JF, Kloog Y. Galectin-1 is a novel structural component and a major regulator of h-ras nanoclusters. Mol Biol Cell. 2008;19:1404–1414. doi: 10.1091/mbc.E07-10-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarsky V, Cvrckova F, Potocky M, Hala M. Exocytosis and cell polarity in plants—exocyst and recycling domains. New Phytol. 2009;183:255–272. doi: 10.1111/j.1469-8137.2009.02880.x. [DOI] [PubMed] [Google Scholar]
- 24.Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, et al. Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci USA. 2004;101:7815–7820. doi: 10.1073/pnas.0402385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidner CA, Timmermans MC. Mixing and matching pathways in leaf polarity. Curr Opin Plant Biol. 2007;10:13–20. doi: 10.1016/j.pbi.2006.11.013. [DOI] [PubMed] [Google Scholar]


