Abstract
Autophagy has an important function in cellular homeostasis. In recent years autophagy has been implicated in plant basal immunity and assigned negative (“anti-death”) and positive (“pro-death”) regulatory functions in controlling cell death programs that establish sufficient immunity to microbial infection. We recently showed that Arabidopsis mutants lacking the autophagy-associated (ATG) genes ATG5, ATG10 and ATG18a are compromised in their resistance towards infection with necrotrophic fungal pathogens but display an enhanced resistance towards biotrophic bacterial invaders. Thus, the function of autophagy as either being pro-death or anti-death depends critically on the lifestyle and infection strategy of invading microbes. Here we show that ATG7 contributes to resistance to fungal pathogens. Genetic inactivation of ATG7 results in elevated susceptibility towards the necrotrophic fungal pathogen, Alternaria brassicicola, with atg7 mutants developing spreading necrosis accompanied by production of reactive oxygen intermediates. Likewise, treatment with the fungal toxin fumonisin B1 causes spreading lesion formation in the atg7 mutant. We conclude that ATG7-dependent autophagy constitutes an “anti-death” (“pro-survival”) plant mechanism to control the containment of cell death and immunity to necrophic fungal infection.
Key words: autophagy, ATG7, basal immunity, fungal resistance, arabidopsis
Plants have evolved a bipartite plant immune system to cope with microbial infections. The first layer of defense relies on the recognition of pathogen-associated molecular patterns (PAMP) by pattern-recognition receptors (PAMP-triggered immunity, PTI).1,2 To overcome this defense strategy, successful pathogens deliver so-called effector proteins into plant cells to modify host cellular processes and to suppress immune responses to enhance virulence. The presence or activities of these microbial effectors is sensed by plant resistance proteins and triggers the second layer of defense, the effector-triggered immunity (ETI).1,2 In contrast to PTI, ETI is most often accompanied by programmed host cell death (PCD) at the site of attempted microbial invasion; however the molecular basis of this apoptosis-like hypersensitive response (HR) is largely unknown.
In recent years evidence accumulated that a non-apoptotic form of cell death called autophagy is not only involved in animal PCD and innate immunity3 but is also an important component in the plant basal immune response.4 Generally, autophagy (auto, meaning “self” and phagy, “to eat”) is a cytoplasmic bulk degradation process in which cellular components are targeted to lysosomal or vacuolar degradation. This process is ubiquitous in eukaryotic organisms and is considered to aid cellular survival, differentiation, development and homeostasis by nutrient recycling or removal of damaged or toxic materials.5–7
Autophagy in Plant InnateImmunity—Pathogen Life Style Makes the Difference
Autophagy has a key role in protecting animal organisms against diverse pathologies, including microbial infection.3 Numerous autophagy (ATG)-associated genes have been identified in metazoans and yeast, most of which also have counterparts in plants.8–10 These proteins regulate the formation of double-membrane-coated autophagosomes and subsequent fusion of these vesicles with lysosomal or vacuolar compartments for cargo degradation.
Recent reports have shown that plant autophagy is essential for the restriction, and conversely, also for the promotion of HR-PCD at the site of pathogen infection.11–14 In tobacco mosaic virus (TMV)-infected Nicotiana benthamiana, knock-down of ATG6 and ATG7 genes resulted in spreading of N-mediated HR to adjacent uninfected tissue.12 Likewise, Arabidopsis plants silenced for ATG6 and atg5 mutant plants were not able to contain effector-triggered HR-PCD upon infection with avirulent Pseudomonas syringae pv. tomato (Pto) DC3000 bacteria carrying the AvrRpm1 effector protein.13,14 Additionally, the disease-associated cell death induced by the infection of virulent Pto DC3000 bacteria was also partially mis-regulated in AtATG6-silenced plants.13 Notably, it was also reported that autophagy-deficiency does not result in unrestricted HR-PCD but is rather required for HR-PCD execution.11 Mutants in ATG7 and ATG9 did not develop the typical HR after infection with avirulent strains of Pto DC3000 expressing either the effector protein AvrRps4 or AvrRpm1. Likewise, atg7 and atg9 mutant genotypes also did not respond with an HR to infection with an avirulent isolate of the oomycete Hyaloperonospora arabidopsidis, indicating that autophagy may have a death-promoting function in some plant-pathogen interactions.
Recently, we conducted a comparative analysis including three Arabidopsis atg genotypes (atg5, atg10, atg18-1, in Col-0 background) to assess the role of autophagy in plant disease-associated cell death and basal immunity.15 We showed that genetic inactivation of ATG5, ATG10 and ATG18a resulted in enhanced fungal growth of the necrotrophic fungus Alternaria brassicicola, accompanied by a spreading necrosis and increased production of reactive oxygen species (ROS). In contrast, atg5, atg10 and atg18a mutants showed reduced bacterial growth rates when infected with the virulent biotrophic bacterium Pseudomonas syringae pv. tomato DC3000 (PtoDC3000), suggesting that autophagy may support infection by biotrophic bacteria. The increased resistance towards Pto infection was solely based on subtly elevated SA levels in the atg mutants and all other assayed innate immune responses proved to be unaltered in the autophagy-deficient plants.
Based on the above described reports we concluded that autophagy controls plant basal immunity to microbial infection in a pathogen lifestyle-dependent manner in either a positive or negative way.16 Moreover, during ETI the function of autophagy as death-promoting or death-containing cellular mechanism seems to be dependent on the nature of the immune receptor involved.11
ATG7 Functions in Immunity Towards Necrotrophic Pathogens
During autophagy cytoplasm containing cellular components is sequestered within double-membrane vesicles called autophagosomes that are subsequently transported to lytic compartments, such as the lysosome or the vacuole.17 At the final step of autophagosome formation two ubiquitin-like conjugates are required. The first is the phosphatidylethanolamine (PE)-conjugated ATG8 which is produced by the ATG7 (E1-like) and ATG3 (E2-like) enzymes. In the second conjugation system, ATG5 is conjugated to ATG12, again relying on ATG7 E1-like activity, followed by ATG10 E2-like processing.4,18 Mutations in the E1-like enzyme ATG7, which in the Arabidopsis genome is encoded by a single gene (At5g45900), results in accelerated, pre-mature senescence and in hypersensitivity to nutrient-limiting growth conditions.19,20 Moreover, ATG7 has been implicated in plant immune responses towards viral and bacterial infections.11,12
Here we investigated if ATG7 is also involved in the plant immune response towards infection with Alternaria brassicicola, a necrotrophic fungal pathogen that causes black spot disease on cruciferous plants.21 Wild type plants of the ecotype Wassilewskija (Ws), atg7 mutants and corresponding complemented atg7/ATG7 plants19 were infected with spore preparations of A. brassicicola and disease symptom development was determined. Necrotic spots on wild type Ws plants appeared approximately 3–4 days after infection (data not shown) and were restricted to inoculations sites up to day 11 (Fig. 1A). However, atg7 mutation resulted in spreading necrosis beyond infection loci, in increased lesion size and increased disease indices when compared to infected wild-type or atg7/ATG7 complemented plants (Fig. 1B and C). Cell death progression, as detected by trypan-blue staining and cell death-associated production of reactive oxygen intermediates, as detected by diaminobenzidine-staining, spread beyond fungal infection loci in the atg7 mutant line, but was limited to infection sites in control plants (Fig. 1D). Likewise, necrotic cell death progression induced by the fungal toxin fumonisin B1 was enhanced in atg7 mutants (Fig. 1E). Thus, our previous observation using atg5, atg10 and atg18a mutant genotypes15 could be extended to the atg7 mutant. As the genetic background of the atg7 mutant was the ecotype Ws, we further introduced a novel ecotype in our studies in addition to the previously used Col-0 ecotype (atg5, atg10 and atg18a).15
Figure 1.
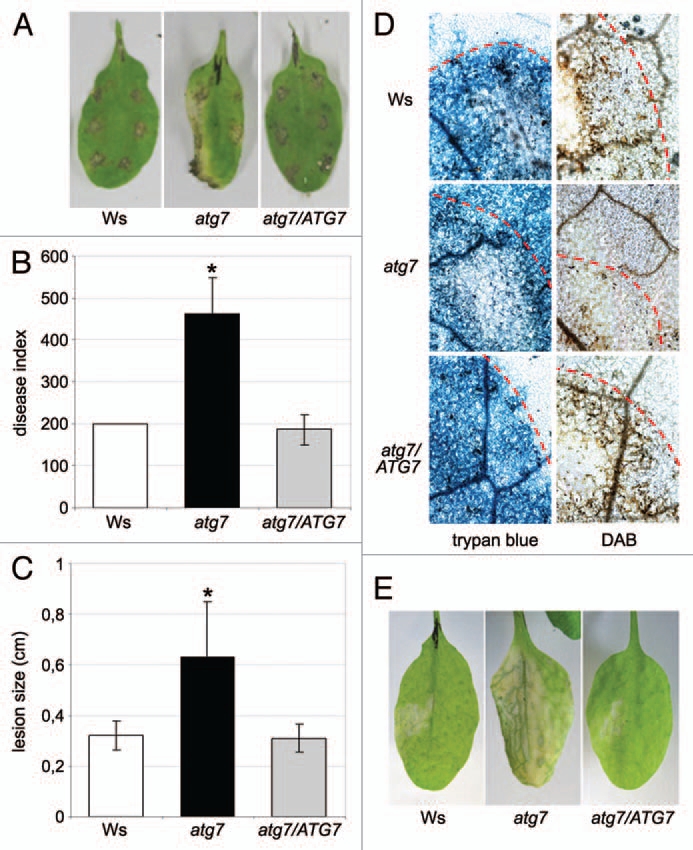
atg7 mutants are more susceptible to infection with necrotrophic A. brassicicola and treatment with fumonisin B1. Wild-type Ws plants, atg7 mutants and the corresponding complemented atg7/ATG7 line were drop-inoculated with 106 spores/ml of Alternaria brassicicola. (A) Disease symptoms on representative infected leaves were monitored on day 11 after infection. (B and C) From leaves shown in (A) the disease indices (B) and lesion sizes (C) were determined. Results represent means ± SD (n ≥ 12) and statistical significance compared to the wild-type (p ≤ 0.05, Student's t-test) is indicated by asterisks. (D) Fungal structures and dead plant cells were stained with trypan blue at day 7 after infection (left parts). H2O2 production in infected leaves was detected 7 days post infection using 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining (right parts). Leaves were analyzed by light microscopy. Dotted lines indicate borders of spore inoculation sites. (E) The fungal toxin fumonisin B1 causes spreading necrosis in atg7 mutants. Wild-type Ws plants, atg7 mutants and the atg7/ATG7-complementation line were infiltrated with 10 µM fumonisin B1 on one leaf side and development of necrotic cell death was monitored 6 days after treatment. All shown experiments were performed at least in triplicate with similar results.
Our results are in agreement with a recent report that infection of atg7 mutant lines resulted in a 2–3-fold higher spore production rate of A. brassicicola compared to the respective control plants.22 Likewise, atg7 mutants exhibited enhanced susceptibility to another necrotrophic fungal pathogens, Botrytis cinerea. Additionally, ATG7 gene expression was upregulated upon infection with Botrytis, and atg7 mutants lacked Botrytis-induced formation of autophagosomes.22
These findings are indicative of an essential role of ATG7-dependent autophagy in the plant immune response towards necrotrophic pathogens, most likely by restricting chlorosis and cell death evoked upon infection by these destructive pathogens. Failure to contain necrotic collapse of the host tissue thus favors growth of necrotrophic pathogens on autophagy-deficient mutants.
References
- 1.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 3.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayward AP, Dinesh-Kumar SP. What can plant autophagy do for an innate immune response? Annu Rev Phytopathol. 2011;49:557–576. doi: 10.1146/annurev-phyto-072910-095333. [DOI] [PubMed] [Google Scholar]
- 5.Klionsky DJ. Autophagy. Curr Biol. 2005;15:282–283. doi: 10.1016/j.cub.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Klionsky DJ. The molecular machinery of autophagy: Unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klionsky DJ. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 8.Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen LJ, et al. Autophagy in development and stress responses of plants. Autophagy. 2006;2:2–11. doi: 10.4161/auto.2092. [DOI] [PubMed] [Google Scholar]
- 9.Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16:21–30. doi: 10.1038/cdd.2008.120. [DOI] [PubMed] [Google Scholar]
- 10.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NH, Mattsson O, et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell. 2009;137:773–783. doi: 10.1016/j.cell.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Dinesh-Kumar SP. Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy. 2008;4:20–27. doi: 10.4161/auto.5056. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, et al. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21:2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J. 2011;66:818–830. doi: 10.1111/j.1365-313X.2011.04546.x. [DOI] [PubMed] [Google Scholar]
- 16.Lenz HD, Haller E, Melzer E, Gust AA, Nürnberger T. Autophagy controls plant basal immunity in a pathogenic lifestyle-dependent manner. Autophagy. 2011;7:773–774. doi: 10.4161/auto.7.7.15535. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Klionsky DJ. Autophagosome formation: Core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 18.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 19.Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem. 2002;277:33105–33114. doi: 10.1074/jbc.M204630200. [DOI] [PubMed] [Google Scholar]
- 20.Phillips AR, Suttangkakul A, Vierstra RD. The ATG12-conjugating enzyme ATG10 Is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics. 2008;178:1339–1353. doi: 10.1534/genetics.107.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomma BP. Alternaria spp.: From general saprophyte to specific parasite. Mol Plant Pathol. 2003;4:225–236. doi: 10.1046/j.1364-3703.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- 22.Lai Z, Wang F, Zheng Z, Fan B, Chen Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 2011;66:953–968. doi: 10.1111/j.1365-313X.2011.04553.x. [DOI] [PubMed] [Google Scholar]


