Abstract
Nitric oxide (NO) is an essential signaling molecule in plants. However little is known about signaling pathways regulating NO levels in plants. Recently we reported a NO overproducing mutant of tomato that had extremely short roots (shr) at seedling stage. The scavenging of NO restored root elongation in the shr mutant providing us with a convenient bioassay to analyze the signaling pathway upstream of NO production. The application of previously reported pharmacological inhibitors of ubiquitin-proteasome signaling caused a drastic reduction in NO levels and restored root elongation in the mutant. Since these pharmacological inhibitors specifically inhibit mammalian IKK/NFκB signaling, we propose that a pathway functionally similar to IKK/NFκB pathway regulates NO levels in tomato.
Key words: nitric oxide, root growth, short root mutant (shr), pharmacological inhibitors, NFκB, tomato (Solanum lycopersicum)
Nitric oxide (NO) is a bioactive gaseous molecule that functions as an essential signaling molecule in plants.1–3 NO has been shown to be involved in the signaling of growth, development and adaptive responses to multiple stresses in plants.4–9 In plants, NO is assumed to be synthesized by several mechanisms of which two distinct pathways are a nitrite pathway likely via nitrate reductase10 and an arginine pathway via a nitric oxide synthase (NOS) like enzyme.2,11–13 Several plant hormones and light appear to regulate NO levels in plants.1 NO brings about its biological functions through its action on multiple downstream signaling pathways that includes generation of cGMP, cADPR and elevation of cytosolic calcium.1 Though diverse responses regulate NO formation,1 little is known about the molecular mechanisms regulating production of NO in plants. In mammals, NO is produced by action of three different NOS, namely constitutively expressed endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS).14,15 The induction of iNOS is considered to be regulated by several mechanisms including transcriptional regulation by the IκB kinase (IKK)/nuclear factor κB (NFκB) pathway.16–19 NFκB is an inducible transcription factor that regulates the expression of a variety of genes such as adhesion factors, chemokines and cytokines, in addition to inducible enzymes such as iNOS. The studies on mechanism of NFκB activation have indicated that NFκB is sequestered in the cytoplasm of cells by the inhibitory IκB proteins. In response to a variety of stimuli and developmental responses, IκB is rapidly phosphorylated, ubiquitinated and degraded via proteasome, releasing NFκB for translocation into the nucleus to initiate transcription of genes such as of iNOS. IKK is the convergence point in most signaling pathways activated by many stimuli leading to the phosphorylation and degradation of IκB.
NO being a vital molecule for plant development, it is possible that its cellular level is regulated by a pathway similar to mammalian IKK/NFκB signaling pathway, which possibly regulates biosynthesis of NOS like enzyme(s). It has been shown that Arabidopsis NIM1/NPR1 protein and AKR2 protein regulating pathogenesis related-1 (PR-1) gene expression involved in systemic acquired resistance (SAR) in plants have high homology to mammalian IB-like proteins,20,21 and both of these proteins exert their regulatory function through interaction with other proteins. Though NFκB-like proteins have not been reported from plants, gene annotation studies have revealed that glutathione peroxidase,22 phenylalanine-ammonia lyase (PAL),23 and PR-1,24 genes have motifs in their promoter elements that are analogous to NFκB-like protein binding sites. However, at the moment there is no conclusive evidence available of the existence of a signaling pathway similar to that established for IKK/NFκB in animal systems that regulates NO level in plants.
Enhanced NO Levels Causes Shortening of Root in the shr Mutant Seedlings
To elucidate the pathway regulating NO formation in plants we analyzed a tomato mutant that had extremely short roots (shr) at the seedling stage. Mutant plants showed elevated NO levels in all organs compared to the wild type, with significantly higher NO levels at the root tip.25 Higher NO level in the shr mutant was also associated with increase in NOS activity in all organs. Though the shr mutant showed reduction in overall growth of adult plants, the most striking effect was the extreme reduction of primary root length of seedlings. Fascinatingly, application of NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) that is known to reduce endogenous NO levels, as well as NG-nitroL-argininemethyl ester (L-NAME) an inhibitor of NOS activity stimulated root elongation along with significant decrease in NO levels in the shr root tips. These results provided compelling evidence that short root phenotype of the mutant seedlings is related to the overproduction of NO and restoration of root elongation can serve as an elegant bioassay to decipher the mechanism(s) regulating NO level in plants.
Inhibitors of Mammalian IKK/NFκB Pathway Rescues shr Seedling Phenotype
Presently the molecular nature of the components regulating NO level in plants remains to be determined. Although there is no priori knowledge about pathway regulating NO synthesis in plants, it is reasonable to assume that the signaling pathway regulating NO synthesis in plants may perhaps have some commonality to that observed in mammalian system. For example in animals, the signaling function of NO is mediated via a pathway involving soluble guanylyl cyclases that generate cGMP and phosphodiesterases that hydrolyze cGMP.26 Though sequences homologous to mammalian guanylyl cyclases and phosphodiesterases have not been reported in plants, results obtained with use of pharmacological inhibitors provide evidence for cGMP function in NO-triggered signaling pathway27 such as pollen tube growth28 and root development.29 The reduction in endogenous NO levels coupled with stimulation of root elongation in the shr mutant seedlings upon application of pharmacological inhibitors provides an ideal system for further elucidating the pathway(s) regulating NO synthesis in plants.
In mammals NO levels are regulated by IKK/NFκB signal transduction pathway via NFκB activation, a transcription factor associated with increased iNOS expression. We examined the likely operation of this pathway by monitoring the rescue of shr root elongation25 using a series of pharmacological inhibitors specifically blocking this pathway.30 In fact in plants, the existence of NOS like enzymic activity was first proposed based on reduction of NO levels on application of mammalian NOS inhibitors.1 Therefore we reasoned that upstream signal transduction components regulating NO levels in plants might also show similar sensitivity to pharmacological inhibitors used in mammalian systems. Our studies revealed that the shortening of the root in the shr mutant can be reversed by application of inhibitors that can cause a reduction in endogenous NO levels.
NFκB, a family of transcriptional factors, is the key partner in the IKK/NFκB signaling pathway of mammalian system. A range of inhibitors is known that blocks different aspects of the action of NFκB.30 One of the potent inhibitor of NFκB activity is glucocorticoid dexamethasone that induces increase in levels of IκBα resulting in reduced amounts of NFκB that translocates to the nucleus, thus inhibiting iNOS gene transcription and promoter activation by decreasing NFκB DNA binding activity.31 Pyrrolidine dithiocarbamate (PDTC) is another widely used NFκB inhibitor that acts by hampering NFκB binding to DNA.30 The reported stimulation of the shr root elongation by two diverse inhibitors of NFκB action suggests the existence of molecules with similar properties and affinity to these inhibitors in plant systems.
It is known that IKK phosphorylates mammalian IκBα proteins, and subsequently phosphorylated IκBα protein is targeted for polyubiquitination and subsequent degradation by the proteasome, thereby releasing NFκB. Application of a IKK-2 inhibitor V that selectively blocks IκBα phosphorylation and thereby prevents the induction of NFκB nuclear translocation,30 also stimulated root elongation in shr seedlings. Similar results were also obtained with application of RO106-9920, a cell-permeable highly selective irreversible inhibitor of IκBα ubiquitination that blocks IκB degradation and NFκB activation.32 It is known that peptide aldehyde N-carbobenzoxyl-L-leucinyl-L-leucinyl-L-norleucinal (MG-132) acts as a specific inhibitor of proteasome blocking IκBα degradation and NFκB activation.30 As expected use of proteasome inhibitor MG-132 stimulated shr root elongation. In essence, the amelioration of root phenotype by above inhibitors point toward occurrence of a signaling pathway in plants, which perhaps operates in a fashion similar to IKK/NFκB signaling pathways of mammals.
A Mammalian IKK/NFκB-Like Signaling Pathway Regulates NO Level in Tomato
Bioassays provide a powerful method to predict and identify the components of several hormonal signaling pathways in plants. Additionally, phenocopying of a mutant phenotype as well as rescue of the phenotype by application of bioactive molecules have been often used for inferring signaling pathways regulating such phenotypes. Ostensibly, stimulation of shr root elongation by pharmacological inhibitors specific for mammalian IKK/NFκB signaling pathway compellingly indicates a likely occurrence of similar pathway(s) in plants. It has been shown that Arabidopsis NIM1/NPR1 protein20 and AKR2 protein regulating pathogenesis related-1 (PR-1),21 gene expression have high homology to mammalian IκB-like proteins, and both of these proteins exert their regulatory function through interaction with other proteins. Though NFκB-like proteins have been not reported from plants, the gene annotation studies have revealed that phenylalanine-ammonia lyase (PAL),23 and PR-1,24 genes have in their promoter elements motifs that are analogous to NFκB-like protein binding sites. Mustard NPR1 protein when expressed in mammalian cell lines bound to NFκB and inhibited its nuclear translocation including downregulation of iNOS.33 Based on the inhibitor data and upregulation of NO level, it appears that shr mutation affects a component upstream of NOS like enzyme. Figure 1 shows a simple model that is consistent with our data25 outlining a putative signaling pathway regulating NO levels in tomato seedlings. Since shr mutant shows upregulation of NO, the mutated gene product is probably a negative regulator, which in its native wild type form restrains NO production in plants. Based on our results we speculate that loss of this regulator either affects a component upstream of IKK/NFκB pathway, or perhaps decreases levels of a protein functionally similar to IκBα thus releasing a NFκB-like effector. In mammalian system where extensive knowledge has accumulated about NO synthesis, several mechanisms have been described that lead to upregulation of NOS activity. For example, human diseases such as asthma is believed to be caused by mutations in genes involved in the NFκB signaling pathway leading to constitutive NFκB activation and iNOS upregulation causing NO accumulation.34 It is plausible that in shr mutant activation of a signaling component functionally similar to mammalian NFκB may be involved in upregulation of NOS activity and NO levels. In summary, we propose that operation of a IKK/NFκB-like signaling pathway regulates NO production in plants. Molecular identification of target proteins constituting this signaling pathway will be the next challenge in future.
Figure 1.
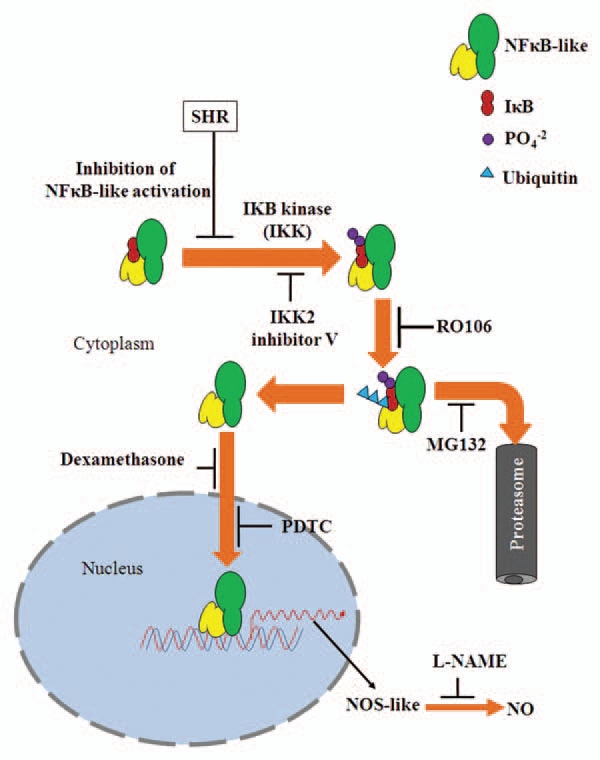
Schematic representation of IKK/NκFB-like signaling pathway regulating NO levels in tomato seedlings. The sites of action of the pharmacological inhibitors as derived from existing mammalian models are indicated. The putative role of native SHR as a repressor of a NFκB-like molecule is also indicated. The mutation in shr presumably results in the removal of this negative regulator thereby resulting in the constitutive transcriptional activation of a NOS-like enzyme. The increase in NOS-like activity leads to increase in NO levels in the mutant resulting in inhibition of root elongation.
Acknowledgments
This work was supported by the International Atomic Energy Agency, Vienna, Austria, and the Department of Biotechnology, New Delhi, India grant (R.S.). S.N. was a recipient of the Young Scientist grant from the Department of Science and Technology, New Delhi, and Research Associateship from the Council of Scientific and Industrial Research, New Delhi. E.V.K. was recipient of fellowships from the Council of Scientific and Industrial Research, New Delhi.
Abbreviations
- shr
short root mutant
- NO
nitric oxide
- NOS
nitric oxide synthase
References
- 1.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide, the versatility of an extensive signal molecule. Ann Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 2.Neill SJ, Bright J, Desikan R, Hancock JT, Harrison J, Wilson I. Nitric oxide evolution and perception. J Exp Bot. 2008;59:25–35. doi: 10.1093/jxb/erm218. [DOI] [PubMed] [Google Scholar]
- 3.Besson-Bard A, Pugin P, Wendehenne D. New insights into nitric oxide signaling in plants. Ann Rev Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- 4.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 5.Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- 6.Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao MG, Tian QY, Zhang WH. Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 2007;144:206–217. doi: 10.1104/pp.107.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dangl JL. Plants just say NO to pathogens. Nature. 1998;394:525–526. doi: 10.1038/28958. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci. 1999;4:128–129. doi: 10.1016/s1360-1385(99)01393-x. [DOI] [PubMed] [Google Scholar]
- 11.Corpas FJ, Hayashi M, Mano S, Nishimura M, Barroso JB. Peroxisomes are required for in vivo nitric oxide (NO) accumulation in the cytosol following salinity stress of Arabidopsis plants. Plant Physiol. 2009;151:2083–2094. doi: 10.1104/pp.109.146100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corpas FJ, Palma JM, del Río LA, Barroso JB. Evidence supporting the existence of l-arginine-dependent nitric oxide synthase activity in plants. New Phytol. 2009;184:9–14. doi: 10.1111/j.1469-8137.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta KJ, Fernie AR, Kaiser WM, Van Dongen JT. On the origins of nitric oxide. Trends Plant Sci. 2011;16:160–168. doi: 10.1016/j.tplants.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 15.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Shared principles in NFκB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Silverman N, Maniatis T. NFκB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 18.Natoli G, Saccani S, Bosisio D, Marazzi I. Interactions of NFκB with chromatin, the art of being at the right place at the right time. Nat Immunol. 2005;6:439–445. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z. Ubiquitin signaling in the NFκB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, et al. The Arabidopsis nim1 protein shows homology to the mammalian transcription factor inhibitor IκB. Plant Cell. 1997;9:425–449. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J, Wang J, Zhang H. An ankyrin repeat-containing protein plays a role in both disease resistance and antioxidation metabolism. Plant J. 2002;29:193–202. doi: 10.1046/j.0960-7412.2001.01205.x. [DOI] [PubMed] [Google Scholar]
- 22.Avsian-Kretchmer O, Gueta-Dahan Y, Lev-Yadun S, Gollop R, Ben-Hayyim G. The salt-stress signal transduction pathway that activates the gpx1 promoter is mediated by intracellular H2O2, different from the pathway induced by extracellular H2O2. Plant Physiol. 2004;135:1685–1696. doi: 10.1104/pp.104.041921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desikan R, Reynolds A, Hancock JT, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defense gene expression in Arabidopsis suspension cultures. Biochem J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E. Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J. 1998;16:223–233. doi: 10.1046/j.1365-313x.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- 25.Negi S, Santisree P, Kharshiing EV, Sharma R. Inhibition of the ubiquitin-proteasome pathway alters cellular levels of nitric oxide in tomato seedlings. Mol Plant. 2010;3:854–869. doi: 10.1093/mp/ssq033. [DOI] [PubMed] [Google Scholar]
- 26.Ignarro JL. Biology and Pathobiology. San Diego, CA: Academic Press; 2000. Nitric Oxide; pp. 3–380. [Google Scholar]
- 27.Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP and ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prado AM, Porterfield DM, Feijó JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- 29.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the IAA-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilmore TD, Herscovitch M. Inhibitors of NFκB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 31.DeVera ME, Taylor BS, Wang Q, Shapiro RA, Billiar TR, Geller DA. Dexamethasone suppresses iNOS gene expression by upregulating IκBα and inhibiting NFκB. Am J Physiol. 1997;273:1290–1296. doi: 10.1152/ajpgi.1997.273.6.G1290. [DOI] [PubMed] [Google Scholar]
- 32.Swinney DC, Xu YZ, Scarafia LE, Lee I, Mak AY, Gan QF, et al. A small molecule ubiquitination inhibitor blocks NFκB-dependent cytokine expression in cells and rats. J Biol Chem. 2002;277:23573–23581. doi: 10.1074/jbc.M200842200. [DOI] [PubMed] [Google Scholar]
- 33.Kesanakurti D, Sareddy GR, Babu PP, Kirti PB. Mustard NPR1, a mammalian IκB homologue inhibits NFκB activation in human GBM cell lines. Biochem Biophys Res Comm. 2009;390:427–433. doi: 10.1016/j.bbrc.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 34.Strober W, Murray PJ, Kitani A, Watanabe T. Signaling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]


