Abstract
Although the role of Ca2+ influx channels in oxidative stress signaling and cross-tolerance in plants is well established, little is known about the role of active Ca2+ efflux systems in this process. In our recent paper,17 we reported Potato Virus X (PVX)-induced acquired resistance to oxidative stress in Nicotiana benthamiana and showed the critical role of plasma membrane Ca2+/H+ exchangers in this process. The current study continues this research. Using biochemical and electrophysiological approaches, we reveal that both endomembrane P2A and P2B Ca2+-ATPases play significant roles in adaptive responses to oxidative stress by removing excessive Ca2+ from the cytosol, and that their functional expression is significantly altered in PVX-inoculated plants. These findings highlight the crucial role of Ca2+ efflux systems in acquired tolerance to oxidative stress and open up prospects for practical applications in agriculture, after in-depth comprehension of the fundamental mechanisms involved in common responses to environmental factors at the genomic, cellular and organismal levels.
Key words: cytosolic calcium, reactive oxygen species, cross-tolerance, calcium pump
The phenomenon of cross-tolerance to a variety of biotic and abiotic stresses is well-known.1,2 Some of the demonstrated examples include the correlation between oxidative stress tolerance and pathogen resistance.3–5 At the mechanistic level, changes in cytosolic Ca2+ levels [Ca2+]cyt, have long been implicated as a quintessential component of this process.6 The rise in [Ca2+]cyt is proven to be essential for the development of the oxidative burst required for triggering the activation of several plant defense reactions.7,8 The observed elevation in H2O2 level is believed to result from Ca2+-dependent activation of the NADPH oxidase,8 which then causes a further increase in [Ca2+]cyt via a positive feedback mechanism. This process is further accomplished by defense gene activation, phytoalexin synthesis and eventual cell death.9 Downstream from the stimulus-induced [Ca2+]cyt elevation, cells possess an array of proteins that can respond to a message. Such proteins include calmodulin (CaM),10 Ca2+-dependent protein kinases11 and CaM binding proteins.12 Of note is that when Ca2+ channels are blocked, biosynthesis of ROS is prevented.13
While the role of Ca2+ influx channels in oxidative stress signaling and cross-tolerance in plants is well established, little is known about the involvement of active Ca2+ efflux systems in this process. In contrast, in animal systems the essential role of re-establishing [Ca2+]cyt to resting levels is widely reported. A sustained increase in [Ca2+]cyt in the alveolar macrophage is thought to be the consequence of membrane Ca2+-ATPase dysfunction.14 In endothelial cells, inhibition of the Ca2+/Na+ electroneutral exchanger of the mitochondria was named as one of the reasons for [Ca2+]cyt increases.15 A significant loss of the plasma membrane Ca2+-ATPase (PMCA) activity was reported in brain synapses in response to oxidative stress,16 suggesting that PMCA may be a downstream target of oxidative stress.
In our recently published paper17 we reported the phenomenon of Potato Virus X (PVX)-induced acquired resistance to oxidative stress in Nicotiana benthamiana plants and showed the critical role of plasma membrane Ca2+/H+ exchangers in this process. Nonetheless, questions remain, is this transporter the only active Ca2+ efflux system involved in this process?
In addition to Ca2+/H+ exchangers, active Ca2+ extrusion could also be achieved by Ca2+-ATPases. Two major types of Ca2+-ATPases that differ substantially in their pharmacology and sensitivity to CaM are known.18 Type P2A pumps (also called ER-type or ECA19,20) are predominantly ER-localized,19 although they are also present at other endomembranes (e.g., tonoplast and Golgi). Four members of this group have been identified in the Arabidopsis genome (named AtECAs 1 to 4).18,21 These pumps lack an N-terminal autoregulatory domain, are insensitive to CaM and suppressed by cyclopropiazonic acid (CPA).19 P2B (or ACA) pumps contain an autoinhibitory N-terminal domain that possesses a binding site for Ca2+-CaM.18 Ten members are known in Arabidopsis (termed AtACA1, 2, 4 and 7 to 13).21 Plant P2B pumps are located at the plasma membrane20 as well as in inner membranes such as tonoplast (e.g., ACA4), ER (e.g., ACA2) and plastids.18,19 These pumps probably constitute the basis for precise cytosolic Ca2+ regulation; as the Ca2+ concentration increases, CaM is activated and binds to the autoinhibitory domain of the Ca2+ pump. This results in the activation of the pump.
In our recent study,17 we found no significant difference between the purified plasma membranes fractions isolated from control and UV-treated tobacco plants (with or without PVX inoculation) either in the Ca2+-ATPase activity or in the Ca2+-ATPase expression level and its ability to bind CaM. This suggests that the plasma membrane P2B type pumps (the only pump type known to be expressed at the plasma membrane) play no major role in removing excess Ca2+ from the cytosol under oxidative stress conditions. This led to an obvious question: what about endomembrane Ca2+-ATPases?
To address this issue, microsomal membrane fractions were isolated from tobacco leaves in a manner previously described for plasma membrane fractions17 (Fig. 1A). Western blot and CaM overlay assays were then made to investigate the role of endomembrane P2B Ca2+-ATPases in our reported phenomena of acquired resistance. The results show that the expression of the P2B Ca2+ pumps in PVX-inoculated plants is significantly higher than in control plants (Fig. 1B), correlating well with the CaM overlay assay (Fig. 1C). As no difference was observed for the P2B Ca2+-ATPase expression levels in the plasma membranes,17 the observed difference in the microsomal fractions of PVX-infected plants must be due to an increased expression of endomembrane P2B Ca2+-ATPases. Given the fact that Ca2+ pumps have a high affinity for calcium, the observed increase in endomembrane P2B-type Ca2+-ATPases expression in PVX-inoculated plants may be advantageous for more efficient Ca2+ removal from the cytosol into internal organelles.
Figure 1.
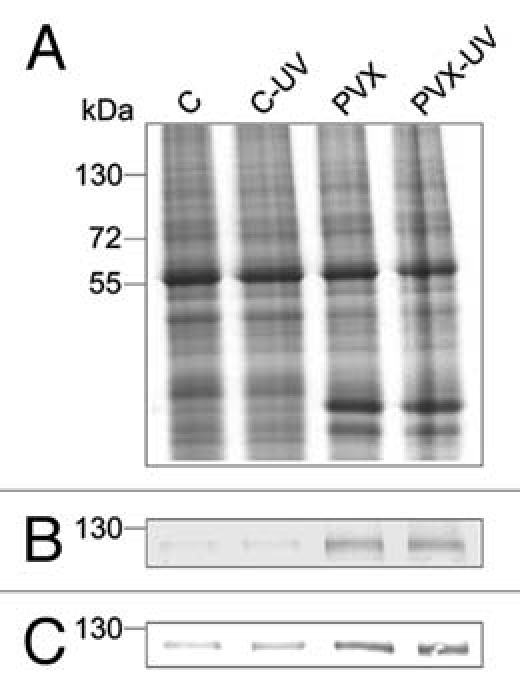
Expression of P2B Ca2+ in purified microsomal fractions from tobacco leaves. Measurements were undertaken C = mock controls; C-UV = mock controls treated with UV-light; PVX = PVX infected plants; PVX-UV = PVX inoculated plants treated with UV-light. (A) Coomassie Brilliant Blue-stained gel; (B) Protein blot immunostained with a non isoform-specific polyclonal antibody for P2B Ca2+-ATPases; (C) CaM overlay assay.
To decipher the possible role of P2A Ca2+-ATPases in acquired resistance, a series of electrophysiological experiments were conducted using inhibitors of P2A-type Ca2+-ATPases, such as thapsigargin (TG)22 and cyclopiazonic acid (CPA).23 Ion-selective Ca2+ microelectrodes were prepared as described elsewhere in reference 24 and 25, and net Ca2+ fluxes were measured from tobacco mesophyll tissue following previously described protocols.17 Leaf pre-treatment for 2 h in either of these inhibitors dramatically suppressed the net Ca2+ efflux measured from tobacco mesophyll cells 2 h after UV light exposure (Fig. 2). Given the specificity of TG and CPA inhibitors for P2A-type Ca2+-ATPases, these results strongly support a hypothesis that both endomembrane P2A and P2B Ca2+-ATPases play significant roles in plant adaptive responses to oxidative stress. This is achieved by removing excess Ca2+ from the cytosol.
Figure 2.
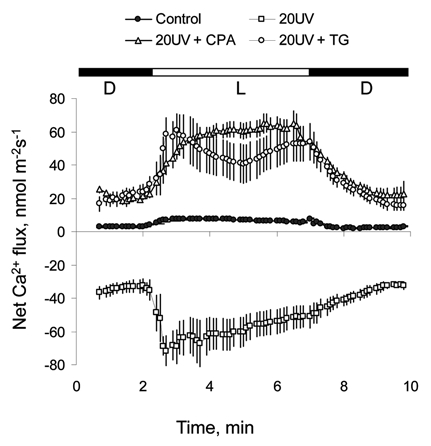
Effect of known Ca2+-ATPase blockers on light-induced Ca2+ flux kinetics after 20 min of UV-C treatment. Leaf mesophyll segments were pre-treated in either 5 µM TG (thapsigargin) or 50 µM CPA (cyclopiazonic acid) for 1–1.5 h prior to exposure to UV-C light. Net Ca2+ fluxes were measured 2 h after the end of UV treatment. These were compared with two controls: (1) no pre-treatment/no UV exposure (closed circles) and (2) no pre-treatment/20 min UV exposure (open squares). Mean ± SE (n = 4 to 7).
Combining these results with our previously reported observations in reference 17, the following model is proposed (Fig. 3). Oxidative stress (such as UV) causes increased ROS production in leaf chloroplasts, leading to the elevated [Ca2+]cyt. Several Ca2+ efflux systems are involved in restoring basal cytosolic Ca2+ levels. Two of these, the plasma membrane Ca2+/H+ exchanger17 and endomembrane P2A and P2B Ca2+-ATPases (as reported in this study) are upregulated in PVX inoculated plants and contribute to the improved tolerance to oxidative stress. Overall, these findings highlight the potential role of Ca2+ efflux systems in virus-induced tolerance to oxidative stress in plants. This is consistent with our previous reports on the important role of Ca2+ efflux systems in biotic stress tolerance26 and brings forth possibilities for genetic engineering of more tolerant plants by targeting expression and regulation of active Ca2+ efflux systems at either the plasma or endomembranes.
Figure 3.
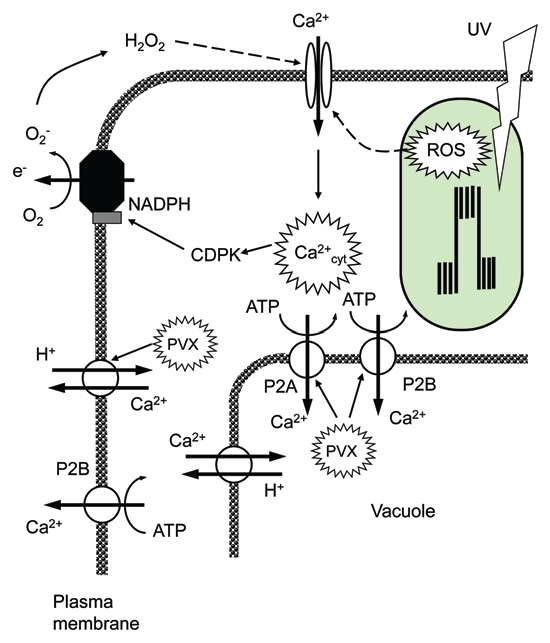
The proposed model of oxidative stress signaling and the role of Ca2+-efflux systems in acquired resistance and plant adaptation to oxidative stress.
Overall, a better adaptation of virus-infected plants to a short wave UV irradiation as compared to uninfected controls may suggest that infection triggers common defense mechanisms that could be efficient against secondary unrelated stresses. This observation may lead to the development of novel strategies to protect plants against complex environmental stress conditions.
Acknowledgments
This work was supported by an ARC Discovery grant (DP 1094663) to S. Shabala.
References
- 1.Bostock RM. Signal crosstalk and induced resistance: Straddling the line between cost and benefit. Annu Rev Phytopathol. 2005;43:545–580. doi: 10.1146/annurev.phyto.41.052002.095505. [DOI] [PubMed] [Google Scholar]
- 2.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Sharma YK, Leont J, Raskin I, Davis KR. Ozone-induced responses in Arabidopsis thaliana: The role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA. 1996;93:5099–5104. doi: 10.1073/pnas.93.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiol. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AbuQamar S, Luo HL, Laluk K, Mickelbart MV, Mengiste T. Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor. Plant J. 2009;58:347–360. doi: 10.1111/j.1365-313X.2008.03783.x. [DOI] [PubMed] [Google Scholar]
- 6.Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–246. doi: 10.1016/s1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- 7.Blumwald E, Aharon GS, Lam CH. Early signal transduction pathways in plant-pathogen interactions. Trends Plant Sci. 1998;3:342–346. [Google Scholar]
- 8.Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 10.Snedden WA, Fromm H. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2001;151:35–66. doi: 10.1046/j.1469-8137.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 11.Harmon AC, Gribskov M, Gubrium E, Harper JF. The CDPK superfamily of protein kinases. New Phytol. 2001;151:175–183. doi: 10.1046/j.1469-8137.2001.00171.x. [DOI] [PubMed] [Google Scholar]
- 12.Reddy VS, Ali GS, Reddy ASN. Genes encoding calmodulin-binding proteins in the Arabidopsis genome. J Biol Chem. 2002;277:9840–9852. doi: 10.1074/jbc.M111626200. [DOI] [PubMed] [Google Scholar]
- 13.Chandra S, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- 14.Hoyal CR, Thomas AP, Forman HJ. Hydroperoxide-induced increases in intracellular calcium due to annexin VI translocation and inactivation of plasma membrane Ca2+-ATPase. J Biol Chem. 1996;271:29205–29210. doi: 10.1074/jbc.271.46.29205. [DOI] [PubMed] [Google Scholar]
- 15.Jornot L, Maechler P, Wollheim CB, Junod AF. Reactive oxygen metabolites increase mitochondrial calcium in endothelial cells: implication of the Ca2+/Na+ exchanger. J Cell Sci. 1999;112:1013–1022. doi: 10.1242/jcs.112.7.1013. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca2+-ATPase. Free Radical Biol Med. 1999;27:810–821. doi: 10.1016/s0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]
- 17.Shabala S, Baekgaard L, Shabala L, Fuglsang A, Babourina O, Palmgren MG, et al. Plasma membrane Ca2+ transporters mediate virus-induced acquired resistance to oxidative stress. Plant Cell Environ. 2011;34:406–417. doi: 10.1111/j.1365-3040.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 18.Geisler M, Axelsen KB, Harper JF, Palmgren MG. Molecular aspects of higher plant P-type Ca2+-ATPases. Biochim Biophys Acta. 2000;1465:52–78. doi: 10.1016/s0005-2736(00)00131-0. [DOI] [PubMed] [Google Scholar]
- 19.Sze H, Liang F, Hwang I, Curran AC, Harper JF. Diversity and regulation of plant Ca2+ pumps: Insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- 20.Bothwell JHF, Ng CKY. The evolution of Ca2+ signalling in photosynthetic eukaryotes. New Phytol. 2005;166:21–38. doi: 10.1111/j.1469-8137.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 21.Axelsen KB, Palmgren MG. Inventory of the super-family of P-type ion pumps in Arabidopsis. Plant Physiol. 2001;126:696–706. doi: 10.1104/pp.126.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kmonickova E, Melkusova P, Harmatha J, Vokac K, Farghali H, Zidek Z. Inhibitor of sarco-endoplasmic reticulum Ca2+-ATPase thapsigargin stimulates production of nitric oxide and secretion of interferon-gamma. Eur J Pharmacol. 2008;588:85–92. doi: 10.1016/j.ejphar.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Zuppini A, Navazio L, Mariani P. Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J Cell Sci. 2004;117:2591–2598. doi: 10.1242/jcs.01126. [DOI] [PubMed] [Google Scholar]
- 24.Shabala S, Newman I. Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: Masking role of the cell wall. Ann Bot. 2000;85:681–686. [Google Scholar]
- 25.Shabala S, Shabala L. Kinetics of net H+, Ca2+, K+, Na+, NH4+ and Cl− fluxes associated with post-chilling recovery of plasma membrane transporters in Zea mays leaf and root tissues. Physiol Plant. 2002;114:47–56. doi: 10.1046/j.0031-9317.2001.1140108.x. [DOI] [PubMed] [Google Scholar]
- 26.Nemchinov LG, Shabala L, Shabala S. Calcium efflux as a component of the hypersensitive response of Nicotiana benthamiana to Pseudomonas syringae. Plant Cell Physiol. 2008;49:40–46. doi: 10.1093/pcp/pcm163. [DOI] [PubMed] [Google Scholar]


