Abstract
Understanding plant response to wind is complicated as this factor entails not only mechanical stress, but also affects leaf microclimate. In a recent study, we found that plant responses to mechanical stress (MS) may be different and even in the opposite direction to those of wind. MS-treated Plantago major plants produced thinner more elongated leaves while those in wind did the opposite. The latter can be associated with the drying effect of wind as is further supported by data on petiole anatomy presented here. These results indicate that plant responses to wind will depend on the extent of water stress. It should also be recognized that the responses to wind may differ between different parts of a plant and between plant species. Physiological research on wind responses should thus focus on the signal sensing and transduction of both the mechanical and drought signals associated with wind, and consider both plant size and architecture.
Key words: biomechanics, leaf anatomy, phenotypic plasticity, plant architecture, signal transduction thigmomorphogenesis, wind
Wind is one of the most ubiquitous environmental stresses, and can strongly affect development, growth and reproductive yield in terrestrial plants.1–3 In spite of more than two centuries of research,4 plant responses to wind and their underlying mechanisms remain poorly understood. This is because plant responses to mechanical movement themselves are complicated and also because wind entails not only mechanical effects, but also changes in leaf gas and heat exchange.5–7 Much research on wind has focused primarily on its mechanical effect. Notably, several studies that determine plant responses to mechanical treatments such as flexing, implicitly extrapolate their results to wind effects.8–10 Our recent study11 showed that this may lead to errors as responses to wind and mechanical stimuli (in our case brushing) can be different and even in the opposite direction. In this paper, we first separately discuss plant responses to mechanical stimuli, and other wind-associated effects, and then discuss future challenges for the understanding of plant responses to wind.
It is often believed that responses to mechanical stress (thigmomorphogenesis) entail the production of thicker and stronger plant structures that resist larger forces. This may be true for continuous unidirectional forces such as gravity, however for variable external forces (such as wind loading or periodic flooding) avoiding such mechanical stress by flexible and easily reconfigurable structures can be an alternative strategy.12–14 How plants adapt or acclimate to such variable external forces depends on the intensity and frequency of stress and also on plant structures. Reduced height growth is the most common response to mechanical stimuli.15,16 This is partly because such short stature increases the ability of plants to both resist forces (e.g., real-locating biomass for radial growth rather than elongation growth), and because small plants experience smaller drag forces (Fig. 1). Some plant species show a resistance strategy in response to mechanical stress by increasing stem thickness1,10 and tissue strength.7 But other species show an avoidance strategy by a reduction in stem or petiole thickness and flexural rigidity in response to MS.11,15–18 These different strategies might be associated with plant size and structure. Stems of larger plants such as trees and tall herbs are restricted in the ability to bend as they carry heavy loads7,10,19 (Fig. 1). Conversely short plants are less restricted in this respect and may also be prone to trampling for which stress-avoidance would be the only viable strategy.18,20 Systematic understanding of these various responses to mechanical stress remains to be achieved.
Figure 1.
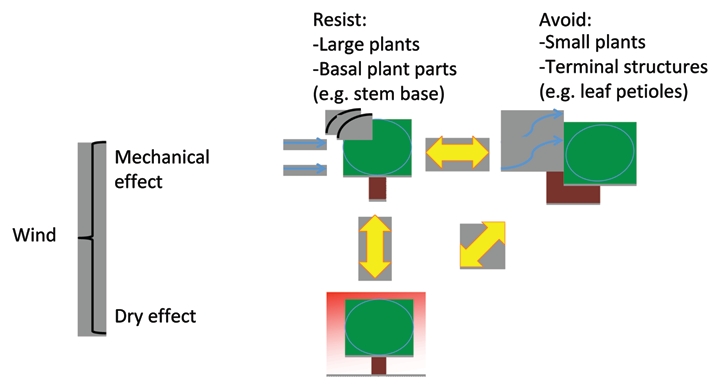
A graphical representation of how wind effects can be considered to entail both a drying and a mechanical effect. Adaptation or acclimation to the latter can be through a force resistance strategy or a force avoidance strategy, the benefit of which may depend on the size and architecture of plants as well as the location of a given structure within a plant.
Wind often enhances water stress by reducing leaf boundary layers and reduces plant temperature by transpiration cooling. The latter effect may be minor,11 but the former could significantly affect plant development. Anten et al. (2010) compared phenotypic traits and growth of Plantago major that was grown under mechanical stimuli by brushing (MS) and wind in the factorial design. Both MS and wind treatments reduced growth and influenced allocation in a similar manner. MS plants, however, had more slender petioles and narrower leaf blades while wind exposed plants exhibited the opposite response having shorter and relatively thicker petioles and more round-shaped leaf blades. MS plants appeared to exhibit stress avoidance strategy while such responses could be compensated or overridden by water stress in wind exposure.11 A further analysis of leaf petiole anatomy (Fig. 2) supports this view. The vascular fraction in the petiole cross-section was increased by wind but not by MS, suggesting that higher water transport was required under wind. Our results suggest that drying effect of wind can at least to some extent override its mechanical effect.
Figure 2.
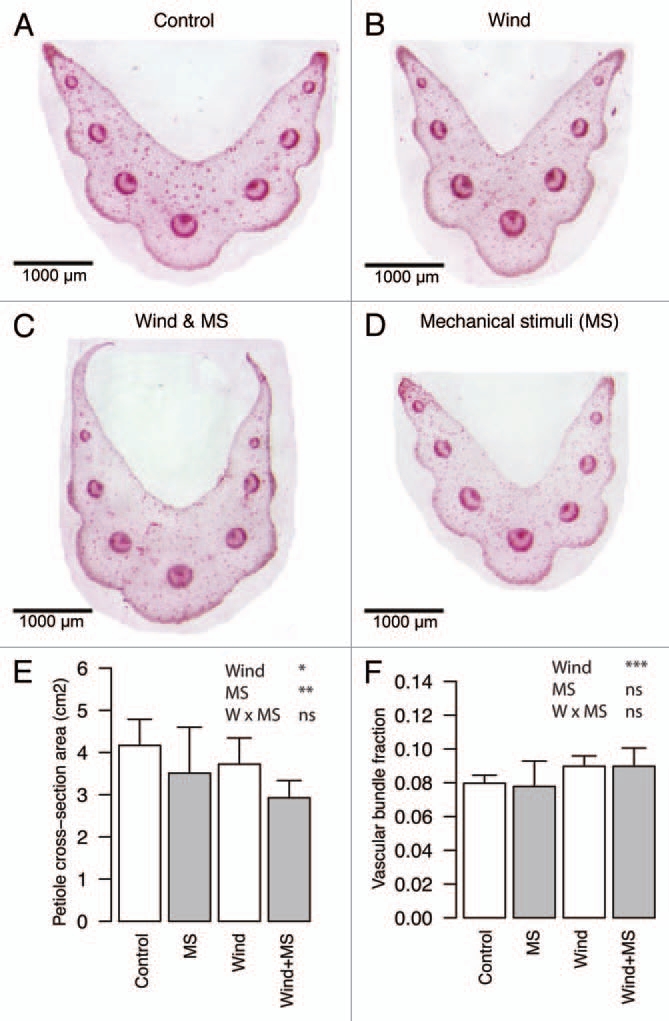
Representative images of petiole cross-sections of Plantago major grown in 45 days in continuous wind and/or mechanical stimuli (A–D). Petiole cross-section area (E) and vascular bundle fraction in the cross-section of petiole (F). mean + SD (n = 12) are shown. Significance levels of ANOVA; ***p < 0.001, **p < 0.01, *p < 0.05, ns p > 0.05.
Physiological knowledge on plant mechanoreception and signal transduction has been greatly increased during the last decades. Plants sense mechanical stimuli through membrane strain with stretch activated channels21 and/or through some linker molecules connecting the cell wall, plasma membrane and cytoskeleton.4,22,23 This leads to a ubiquitous increase in intracellular Ca2+ concentration. The increased Ca2+ concentration is sensed by touch induced genes (TCHs),24,25 which activates downstream transduction machineries including a range of signaling molecules and phytohormones, consequently altering physiological and developmental processes.26 Extending this knowledge to understand plant phenotypic responses to wind however remains a challenge. As responses to wind have been found to differ among parts of a plant (e.g., terminal vs. basal stem) and also across species, physiological studies should be extended to the whole-plant as integrated system rather than focusing on specific tissue level. Furthermore to understand the general mechanism across species, it is required to study different species from different environmental conditions. Advances in bioinformatics, molecular and physiological research will facilitate cross-disciplinary studies to disentangle the complicated responses of plants to wind.
Acknowledgments
This study was partly supported by Grant-in-Aid for from JSPS for Young Research Fellows (Y.O.).
Abbreviation
- MS
mechanical stress
References
- 1.Biddington NL. The effects of mechanically-induced stress in plants—a review. Plant Growth Regul. 1986;4:103–123. [Google Scholar]
- 2.Cleugh HA, Miller JM, Bohm M. Direct mechanical effects of wind on crops. Agroforest Syst. 1998;41:85–112. [Google Scholar]
- 3.Bang C, Sabo JL, Faeth SH. Reduced wind speed improves plant growth in a desert city. PLoS ONE. 2010;5:11061. doi: 10.1371/journal.pone.0011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telewski FW. A unified hypothesis of mechanoperception in plants. Am J Bot. 2006;93:1466–1476. doi: 10.3732/ajb.93.10.1466. [DOI] [PubMed] [Google Scholar]
- 5.Grace J. Plant responses to wind. New York: Academic Press; 1977. [Google Scholar]
- 6.Ennos AR. Wind as an ecological factor. Trends Ecol Evol. 1997;12:108–111. doi: 10.1016/s0169-5347(96)10066-5. [DOI] [PubMed] [Google Scholar]
- 7.Smith VC, Ennos AR. The effects of air flow and stem flexure on the mechanical and hydraulic properties of the stems of sunflowers Helianthus annuus L. J Ex Bot. 2003;54:845–849. doi: 10.1093/jxb/erg068. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe MJ. Thigmomorphogenesis: The response of plant growth and development to mechanical stimulation. Planta. 1973;114:143–157. doi: 10.1007/BF00387472. [DOI] [PubMed] [Google Scholar]
- 9.Niklas KJ. Effects of vibration on mechanical properties and biomass allocation pattern of Capsella bursapastoris (cruciferae) Ann Bot. 1998;82:147–156. [Google Scholar]
- 10.Anten NPR, Pawlowski M, von Wettberg E, Huber H. Interactive effects of spectral shading and mechanical stress on the expression and costs of shade avoidance. Am Nat. 2009;173:241–255. doi: 10.1086/595761. [DOI] [PubMed] [Google Scholar]
- 11.Anten NPR, Alcalá-Herrera R, Schieving F, Onoda Y. Wind and mechanical stimuli differentially affect leaf traits in Plantago major. New Phytol. 2010;188:554–564. doi: 10.1111/j.1469-8137.2010.03379.x. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright SA, Biggs WD, Currey JD, Gosline JM. Mechanical design in organisms. London: Edward Arnold Limited; 1976. [Google Scholar]
- 13.Vogel S. Drag and reconfiguration of broad leaves in high winds. J Exp Bot. 1989;40:941–948. [Google Scholar]
- 14.Bouma TJ, De Vries MB, Low E, Peralta G, Tanczos IC, van de Koppel J, et al. Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology. 2005;86:2187–2199. [Google Scholar]
- 15.Jaffe MJ, Telewski FW, Cooke PW. Thigmomorphogenesis: On the mechanical properties of mechanically perturbed bean plants. Physiol Plantarum. 1984;62:73–78. doi: 10.1111/j.1399-3054.1984.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Schieving F, Stuefer JF, Anten NPR. The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Ann Bot. 2007;99:121–130. doi: 10.1093/aob/mcl230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordero RA. Ecophysiology of Cecropia schreberiana saplings in two wind regimes in an elfin cloud forest: growth, gas exchange, architecture and stem biomechanics. Tree Physiol. 1999;19:153–163. doi: 10.1093/treephys/19.3.153. [DOI] [PubMed] [Google Scholar]
- 18.Paul-Victor C, Rowe N. Effect of mechanical perturbation on the biomechanics, primary growth and secondary tissue development of inflorescence stems of Arabidopsis thaliana. Ann Bot. 2011;107:209–218. doi: 10.1093/aob/mcq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson PR. Stem form of young Larix as influenced by wind and pruning. Forest Science. 1965;11:412–424. [Google Scholar]
- 20.Niklas KJ. Differences between Acer saccharum leaves from open and wind-protected sites. Ann Bot. 1996;78:61–66. [Google Scholar]
- 21.Jaffe MJ, Leopold AC, Staples RC. Thigmo responses in plants and fungi. Am J Bot. 2002;89:375–382. doi: 10.3732/ajb.89.3.375. [DOI] [PubMed] [Google Scholar]
- 22.Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell. 2009;21:2341–2356. doi: 10.1105/tpc.109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutand C. Mechanosensing and thigmomorphogenesis, a physiological and biomechanical point of view. Plant Sci. 2010;179:168–182. [Google Scholar]
- 24.Braam J, Davis RW. Rain-, wind- and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 25.Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 26.Chehab EW, Eich E, Braam J. Thigmomorphogenesis: a complex plant response to mechano-stimulation. J Ex Bot. 2009;60:43–56. doi: 10.1093/jxb/ern315. [DOI] [PubMed] [Google Scholar]


