Abstract
Astoma (pl. stomata) is the pore formed by two guard cells found predominantly in the leaf epidermis. Plants control stomatal aperture (opening and closing) and/or number (density) to regulate carbon dioxide (CO2) uptake and water loss (transpiration), which is necessary to optimize plant growth, development, and fitness in response to various environmental conditions. Recently, we identified Arabidopsis GT2-LIKE 1 (GTL1) as a transcriptional repressor of STOMATAL DENSITY AND DISTRIBUTION 1 (SDD1), a negative regulator of stomatal density. GTL1 directly interacts with the SDD1 promoter regulating stomatal density, transpiration and water use efficiency (WUE). Here we discuss potential GTL1 orthologs in other plant species.
Key words: GTL1, transcription factor, SDD1, water use efficiency, drought, stomatal density
The plant epidermal surface consists of pavement cells and stomata, and regulates gas exchange of carbon dioxide (CO2) and water (H2O) vapor between the leaf and air.1 Plants have physiological and developmental mechanisms controlled by genetic pathways to regulate stomatal aperture and/or number (density) to optimize fitness by controlling WUE in various environmental conditions, particularly under water deficit.1 Stomatal density in leaves is controlled by two genetic pathways: the stomatal cell lineage pathway (basal pathway) that results in the development of guard cells from post-protodermal cells and the negative regulatory pathway that inhibits cells adjacent to stomata from developing into stomata.2,3 The interaction and competition between stomatal cell lineage and negative regulatory pathways is finely controlled to determine stomatal density for environmental adaptation.4,5
The basal pathway is regulated by several basic-helix-loop-helix (bHLH) transcription factors such as SPEECHLESS (SPCH), MUTE, and FAMA, which specifically control the three developmentally distinct steps: asymmetric cell division that produces a meristemoid and a larger sister cell, transition of a meristemoid into a guard mother cell, and guard cell differentiation.2,3 Each step is also regulated by the interaction of INDUCER OF CBF EXPRESSION 1 (ICE1)/SCREAM (SCRM 1) or ICE2/SCRM2.6
STOMATAL DENSITY AND DISTRIBUTION 1 (SDD1) that encodes a subtilisin-like serine protease is the furthest upstream genetic determinant in the negative regulatory pathway.7,8 SDD1 processes propeptides to ligands that activate receptors such as TOO MANY MOUTHS (TMM) and ERECTA (ER), which transduces the signal to the mitogen-activated protein kinase (MAPK) pathway and suppresses stomatal development.
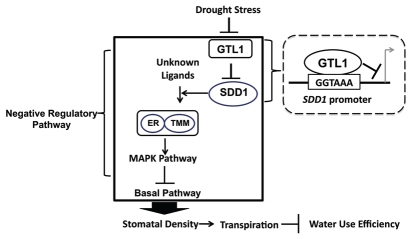
We recently identified the GTL1 transcription factor as a determinant that regulates SDD1 expression and stomatal density9 (Fig. 1). Loss-of GTL1 function results in reduction of transpiration with similar biomass accumulation and net CO2 assimilation, which improves integrated and instantaneous WUE, and drought tolerance.9 Similar level of net CO2 assimilation in gtl1 plants, despite their lower stomatal conductance, may indicate that net CO2 assimilation is still at a maximum rate in gtl1 plants under our conditions, or that gtl1 plants have a higher carboxylation efficiency of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco).1 The determination of any effects of GTL1 on CO2 assimilation requires further investigation. Reduced transpiration in gtl1 plants is attributable to the reduction of stomatal density in abaxial leaves.9 GTL1 protein directly interacts with the SDD1 promoter to repress its expression.9 Taken together, our results indicate that GTL1 is a transcriptional repressor of SDD1 that negatively regulates stomatal density, which is necessary for fine-tuning of stomatal density to regulate WUE (Fig. 1). We postulate that GTL1 receives an environmental signal and transduces this signal to the regulation of stomatal development for an adaptive response that prevents excessive water loss.
Figure 1.
Model of how the GTL1 transcription factor regulates stomatal development in Arabidopsis. GTL1 binds to the GT3 box (GGTAAA) in the SDD1 promoter and transrepresses SDD1 expression. SDD1 presumably processes unknown ligands for the TMM-ER receptor complexes to activate the MAPK pathway, thereby repressing the basal pathway. Repression or induction of SDD1 expression increases or decreases stomatal density, respectively. GTL1 fine-tunes SDD1 expression to optimize water use efficiency.
GTL1 Orthologs in Other Plant Species
GTL1 is a member of the GT2 transcription factor family containing two DNA-binding domains.10–12 The Basic Local Alignment Search Tool (BLAST) in Phytozome (www.phytozome.net) was used to identify GTL1 orthologs in the plant species Arabidopsis thaliana (At, Arabidopsis), Carica papaya (Cp, papaya), Cucumis sativus (Cs, Cucumber), Populus trichocarpa (Pt, Poplar), Prunus persica (Pp, Peach), Solanum lycopersicum (Sl, Tomato), Sorghum bicolor (Sb, Sorghum) and Vitis vinifera (Vv, Grape). We identified GTL1 orthologs similar to AtGTL1 based on the amino acid sequence identity and the existence of two DNA-binding domains (Table 1). The most similar AtGTL1 orthologs identified were in poplar (Pt01s45870), peach (Pp001704), grape (Vv01019722001), and cucumber (Cs193210). Interestingly, monocots such as rice, maize and sorghum also have GTL1 orthologs that are more similar to AtGTL1 than AtGT2 or AtGTL2. Considering the evidence indicating that stomatal development pathways in monocot and dicot may be similar despite different stomatal morphology,13 we hypothesize that GTL1 orthologs in monocots positively regulate stomatal density and transpiration, and therefore may play a role in regulating WUE. Suppression of GTL1 orthologs in other plant species by tools such as RNAi suppression may provide a means to improve WUE in important economic plants.14
Table 1.
AtGTL 1 othologs in other plant species
| Species | Locus name |
| Poplar | Pt01s31660, Pt01s45870, Pt02s06900, Pt05s21410, Pt5s21420, Pt19s02650, Pt11s15050 |
| Peach | Pp001704, Pp002848, Pp003808, Pp021097 |
| Grape | Vv01019722001, Vv01026588001 |
| Cucumber | Cs193210, Cs204000, Cs250070 |
| Tomato | S14g071360, SI11g005380, SI12g056510 |
| Papaya | Cp21.194, Cp21.195, Cp2611.1 |
| Soybean | Gm2g09050, Gm4g39400, Gm6g15500, Gm10g36950, Gm10g36960, Gm16g28240, Gm20g30640, Gm20g30650 |
| Rice | Os2g43300, Os3g02240.1, Os4g45750, Os10g37240 |
| Maize | Zm016649, Zm063203, Zm113585 |
| Sorgum | Sb1g017120, Sb1g049740, Sb4g033390, Sb6g023980 |
Acknowledgments
This work was supported by a National Science Foundation Award MCB-0424850 and Binational Agricultural Research and Development Awards 103314.
References
- 1.Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV. Regulation of transpiration to improve crop water use. Crit Rev Plant Sci. 2009;28:410–431. [Google Scholar]
- 2.Bergermann DC, Sack FD. Stomatal development. Annu Rev Plant Biol. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- 3.Dong J, Bergmann DC. Stomatal patterning and development. Curr Top Dev Biol. 2010;91:267–297. doi: 10.1016/S0070-2153(10)91009-0. [DOI] [PubMed] [Google Scholar]
- 4.Casson SA, Hetherington AM. Environmental regulation of stomatal development. Curr Opin Plant Biol. 2010;13:90–95. doi: 10.1016/j.pbi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Larkin JC, Brown ML, Schiefelbein J. How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annu Rev Plant Biol. 2003;54:403–430. doi: 10.1146/annurev.arplant.54.031902.134823. [DOI] [PubMed] [Google Scholar]
- 6.Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, et al. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell. 2008;20:1775–1785. doi: 10.1105/tpc.108.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger D, Altmann T. A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 2000;14:1119–1131. [PMC free article] [PubMed] [Google Scholar]
- 8.von Groll U, Berger D, Altmann T. The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell. 2002;19:63–83. doi: 10.1105/tpc.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell. 2010;22:4128–4141. doi: 10.1105/tpc.110.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni M, Dehesh K, Tepperman JM, Quail PH. GT-2: In vivo transcriptional activation activity and definition of novel twin DNA binding domains with reciprocal target sequence selectivity. Plant Cell. 1996;8:1041–1059. doi: 10.1105/tpc.8.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smalle J, Kurepa J, Haegman M, Gielen J, Van Montagu M, Van Der Straeten D. The trihelix DNA-binding motif in higher plants is not restricted to the transcription factor GT-1 and GT-2. Proc Natl Acad Sci USA. 1998;95:3318–3322. doi: 10.1073/pnas.95.6.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu DX. Regulatory mechanism of plant gene transcription by GT-elements and GT-factors. Trends Plant Sci. 1999;4:210–214. doi: 10.1016/s1360-1385(99)01418-1. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Ohashi-Ito K, Bergmann DC. Orthologs of Arabidopsis thaliana bHLH genes and regulation of stomatal development in grasses. Development. 2009;136:2265–2276. doi: 10.1242/dev.032938. [DOI] [PubMed] [Google Scholar]
- 14.Umezawa T, Fujika M, Fujika Y, Yamaguchi-Shinozaki K, Shinozaki K. Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotech. 2006;17:113–122. doi: 10.1016/j.copbio.2006.02.002. [DOI] [PubMed] [Google Scholar]