Abstract
Effects of water deficit and/or abscisic acid (ABA) were investigated on early seedling growth of Medicago truncatula, and on glutamate metabolism under dark conditions. Water deficit (simulated by polyethylene glycol, PEG), ABA and their combination resulted in a reduction in growth rate of the embryo axis, and also in a synergistic increase of free amino acid (AA) content. However, the inhibition of water uptake retention induced by water deficit seemed to occur in an ABA-independent manner. Expression of several genes involved in glutamate metabolism was induced during water deficit, whereas ABA, in combination or not with PEG, repressed them. The only exception came from a gene encoding 1-pyrroline-5-carboxylate synthetase (P5CS) which appeared to be induced in an ABA-dependent manner under water deficit. Our results demonstrate clearly the involvement of an ABA-dependent and an ABA-independent regulatory system, governing growth and glutamate metabolism under water deficit.
Key words: abscisic acid, amino acid metabolism, water deficit, glutamate, Medicago truncatula, seedlings
To counter the effects of unfavorable environmental conditions, young seedlings and plants have developed complex cellular signaling mechanisms which require distinct physiological and metabolic adjustments, such as sugar, amino acid or amine accumulation1 through different pathways. The phytohormone abscisic acid (ABA) has been reported to be rapidly produced and accumulated under different environmental stresses, and responses mediated by this hormone lead to the induction of complex tolerance mechanisms to osmotic stress.2 However, it has been shown that the drought-inducible genes were governed by both ABA-dependent and ABA-independent regulatory systems,3 but it is not entirely clear how water deficit and exogenous ABA could affect and regulate plant nitrogen metabolism when applied simultaneously.
ABA-Dependent and Independent Modulation of Growth and Water Content during Water Deficit Stress
An initial characterization of the response of M. truncatula seedlings to water deficit (PEG), ABA and their combination was performed by measuring some physiological parameters in darkness. Water deficit and ABA induced similarly a reduction of the growth rate of the embryo axis (Table 1), showing that this water deficit-induced reduction was mediated through an ABA-dependent pathway. However, the combined effect of exogenous ABA and PEG treatment induced a synergistic reduction of growth rate, indicating the potential existence of an ABA-dependent and an ABA-independent pathway. The water content rate of the embryo axis was similarly affected as growth rate, with one exception: while both, PEG and ABA caused an inhibition of longitudinal axis growth, the increase of water content rate was only weakly affected by ABA, but was strongly decreased by PEG or PEG plus ABA (Table 1), suggesting that PEG effect on water content was not mediated by endogenous ABA.
Table 1.
Effects of ABA or/and water deficit on rates of growth and water content in M. truncatula seedlings
| H2O | PEG | ABA | PEG + ABA | |
| Growth rate (cm·axis−1·day−1) | 2.06 ± 0.05 | 1.42 ± 0.15 | 1.30 ± 0.06 | 1.09 ± 0.09 |
| Water content rate (mg·axis−1·day−1) | 7.82 ± 0.37 | 3.70 ± 0.22 | 6.32 ± 0.49 | 3.24 ± 0.82 |
After 46 h of germination (T = 0 h), seedlings were incubated with H2O, PEG (−0.25 MPa), ABA (10 µM) or both PEG and ABA in darkness during three days. Each data is the mean of three independent experiments ± SE.
Nitrogen Metabolism Responses to Water Deficit and/or Exogenous ABA
Plant metabolism is highly flexible and has the potential to adjust to changes of environmental conditions. In order to discern whether water deficit and ABA effects on seedling growth are due to metabolic changes, free amino acid (AA) accumulation in M. truncatula seedlings was quantified. Water deficit or ABA induced a small but significant increase of total free AA in embryo axes, and an even stronger increase took place when ABA and water deficit were applied simultaneously (Fig. 1). This synergistic accumulation of AA suggests that ABA-dependent signaling pathways act in concert with ABA-independent signaling pathways to induce appropriate metabolite responses. ABA might trigger AA mobilisation for maintaining seedling growth, but also for a potential stress recovery; whereas water deficit signals might be necessary for a complete metabolic adjustment to moderate water deficit stress. Similar observations have been reported with high salinity on carbohydrate metabolism.4
Figure 1.
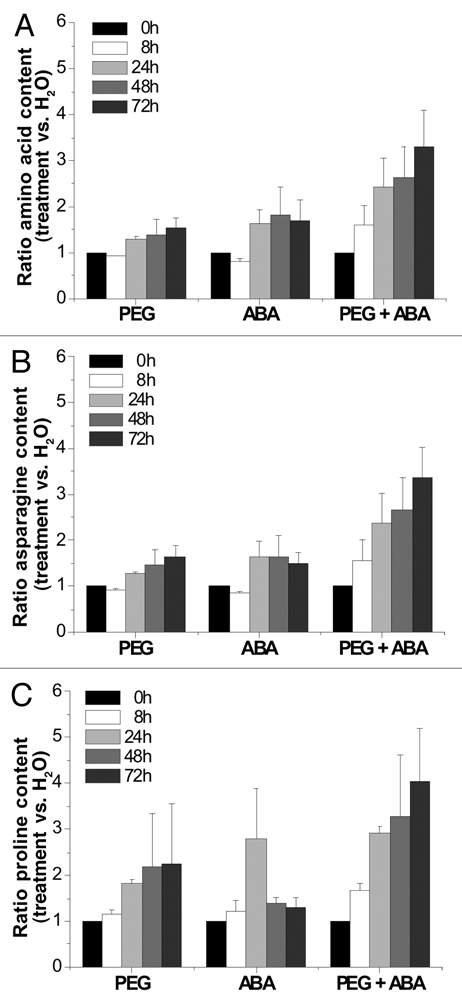
Total free amino acid, asparagine and proline contents of M. truncatula seedlings subjected to exogenous ABA and/or water deficit. All treatments were the same as in Table 1. Total amino acid (A), asparagine (B) and proline (C) contents were expressed as ratio (treatment versus H2O). Each datum point is the mean of two independent experiments ±SE.
Analysis of individual amino acid levels showed that water deficit and ABA had a profound effect on central nitrogen metabolism in a time-dependent manner (Fig. 1). Asparagine, the major amino acid, was strongly accumulated under all treatments and specifically under the combined action of PEG plus ABA. Whereas exogenous ABA induced a transient increase of proline accumulation, water deficit induced a continuous increase of the proline content. Remarkably, a synergistic accumulation of proline content was observed when ABA was combined with PEG. This proline accumulation was correlated with the accumulation of P5CS (1-pyrroline-5-carboxylate synthetase) gene transcripts by ABA under water deficit conditions in M. truncatula seedlings. Similar results have been described in other model species such Arabidopsis5 and Rice6 under different abiotic stresses or with ABA alone, but never under combination.
The predominant route for proline biosynthesis in plants during conditions of osmotic stress occurs through the glutamate pathway. In our system, some genes encoding glutamate metabolism enzymes (e.g., glutamine synthetase, GS1b; glutamate dehydrogenase, GDH3; asparagine synthetase, AS and NADH-GOGAT) in M. truncatula seedlings appears to be upregulated by water deficit through an ABA-independent pathway,7 except P5CS which was upregulated by exogenous ABA and water deficit. Surprisingly, GS activity was increased under the combined effect of ABA supply and water deficit, supporting the idea that GS activity may contribute partially to proline accumulation, as already shown by previous reports relating a potential involvement of GS activity in proline accumulation in tobacco plants grown under salt stress.8
The interactive effects of water deficit stress and the phytohormone ABA in the regulation of nitrogen metabolism in M. truncatula seedlings would suggest the involvement of two separate regulatory systems, an ABA-dependent and an ABA-independent, governing nitrogen metabolic readjustments at different control levels under water deficit. Identification of the ABA-responsiveness of the AA accumulation mechanisms might lead to the identification of novel regulatory components and to new perspectives for improving seedling establishment and plant productivity under environmental constraints.
References
- 1.Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol. 2000;3:217–223. [PubMed] [Google Scholar]
- 3.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Ann Rev Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 4.Kempa S, Krasensky J, Dal Santo S, Kopka J, Jonak C. A central role of abscisic acid in stress-regulated carbohydrate metabolism. PLoS ONE. 2008;3:1–9. doi: 10.1371/journal.pone.0003935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, et al. Correlation between the induction of a gene for 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. 1995;7:751–760. doi: 10.1046/j.1365-313x.1995.07050751.x. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi Y, Yoshiba Y, Sanada Y, Yamaguchi-Shinozaki K, Wada K, Shinozaki K. Characterization of the gene for delta1-pyrroline-5-carboxylate synthetase and correlation between the expression of the gene and salt tolerance in Oryza sativa L. Plant Mol Biol. 1997;33:857–865. doi: 10.1023/a:1005702408601. [DOI] [PubMed] [Google Scholar]
- 7.Planchet E, Rannou O, Ricoult C, Boutet-Mercey S, Maia-Grondard A, Limami AM. Nitrogen metabolism responses to water deficit act through both abscisic acid (ABA)-dependent and independent pathways in Medicago truncatula during post-germination. J Exp Bot. 2011;62:605–615. doi: 10.1093/jxb/erq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugière N, Dubois F, Limami AM, Lelandais M, Roux Y, Sangwan RS, et al. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell. 1999;11:1995–2012. doi: 10.1105/tpc.11.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


