Abstract
The heterogeneity of vascular smooth muscle cells (SMCs) is related to their different developmental origins such as the neural crest and mesoderm. Derivation of SMCs from different origins will provide valuable in vitro models for the investigation of vascular development and diseases. From the perspective of regenerative medicine and tissue engineering, an expandable cell source of SMCs is required for the construction of tissue-engineered blood vessels. In this study, we developed a robust protocol to derive neural crest stem cells (NCSCs) from human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). NCSCs derived from ESCs and iPSCs were expandable with similar cell doubling times. NCSCs were capable of differentiating into neural and mesenchymal lineages. TGF-β1 induced the expression of SMC markers calponin-1, SM22α, and smooth muscle myosin heavy chain and resulted in the assembly of smooth muscle α-actin, calponin-1, and SM22α into stress fibers. This work provides a basis for using iPSCs to study SMC biology and deriving vascular cells for tissue engineering.
Key Words: Smooth muscle cells, Neural crest, Embryonic stem cells, Induced pluripotent stem cells
Introduction
Diseased blood vessels exhibit multiple cell phenotypes that are responsible for the accumulation of lipids, cell proliferation, cell migration, matrix synthesis, and calcification [Ross, 1999]. The heterogeneity of vascular smooth muscle cells (SMCs) is an important factor in the development of vascular diseases [Regan et al., 2000; Li et al., 2001]. The heterogeneity of SMCs can be attributed to different origins of SMCs during development, and the neural crest (NC) is one of the major sources of SMCs [Bergwerff et al., 1998; Hirschi and Majesky, 2004; Nakamura et al., 2006; Majesky, 2007]. A genetic approach that used a Wnt1-Cre transgenic line crossed with a floxed stop Rosa26 reporter line (R26R) showed that murine NC contributes SMCs in the ascending and arch portions of the aorta, the ductus arteriosus, the innominate and right subclavian arteries, and the right and left common carotid arteries [Jiang et al., 2000]. It is suggested that lineage-dependent differences in vascular smooth muscle properties may play a role in the heterogeneous patterns of disease progression and recurrence among different human arterial beds [DeBakey and Glaeser, 2000]. However, NC-derived SMCs are not readily available.
For tissue engineering applications, expandable cell sources for the construction of tissue-engineered vascular grafts (TEVGs) are desirable. Several methods have been developed to construct TEVGs using endothelial cells, SMCs, and/or fibroblasts [Weinberg and Bell, 1986; Niklason et al., 1999; L'Heureux et al., 2006]. However, adult human autologous cells are not easily expandable ex vivo and thus hinder the feasibility of these approaches. Induced pluripotent stem cells (iPSCs) generated from somatic cells make it possible to derive a variety of autologous cells for therapies [Takahashi et al., 2007; Park et al., 2008a; Soldner et al., 2009; Yamanaka, 2009], including NC stem cells (NCSCs) and vascular cells. NCSCs are multipotent stem cells that have the potential to differentiate into cell types of all three germ layers [Stemple and Anderson, 1992; Crane and Trainor, 2006; Lee et al., 2007] and can be used to generate SMCs and many other cell types.
In this study, we established a novel protocol to effectively differentiate iPSCs into NCSCs and to further derive SMCs from NCSCs. SMCs of NC origin are not only useful for investigations on SMC biology but they are also a valuable cell source for tissue engineering and blood vessel regeneration.
Materials and Methods
Culture of Human Embryonic Stem Cells and iPSCs
An undifferentiated human embryonic stem cell (ESC) line (H1; WiCell Research Institute; passages 30–50) and an iPSC line (BJ1-iPS1, derived from skin fibroblasts; passages 23–55) [Park et al., 2008b] were maintained as described previously [Lam et al., 2008; Kurpinski et al., 2010]. Briefly, cells were cocultured with mitotically inactivated mouse embryonic fibroblasts (MEFs) in ESC maintenance medium consisting of Knockout Dulbecco's Modified Eagle's Medium (DMEM)/F-12 medium supplemented with Knockout serum replacer, 1% non-essential amino acids, 1 mM L-glutamine (all from Invitrogen Corp.), 0.1 mM β-mercaptoethanol (Sigma-Aldrich Corp.), and 4 ng/ml human basic fibroblast growth factor (bFGF) (R&D Systems, Inc.). Undifferentiated iPSCs and human ESCs were characterized with positive staining of Oct3/4, Nanog, SSEA-3, and TRA-1-60.
Derivation and Characterization of NCSCs from Human ESCs and iPSCs
To derive NCSCs, iPSCs and ESCs were detached by collagenase IV (1 mg/ml) and dispase (0.5 mg/ml) and grown as embryo body (EB)-like floating cell aggregates in ESC maintenance medium without bFGF for 5 days. The cell aggregates were allowed to adhere to CELLstart™ matrix- (Invitrogen) coated dishes in a serum-free neural induction medium (NCSC medium) consisting of Knockout DMEM/F12, StemPro neural supplement (Invitrogen), 1% GlutaMAX™-I (Invitrogen), 20 ng/ml bFGF, and 20 ng/ml epidermal growth factor (EGF). After 7 more days, the colonies with rosette structures were mechanically harvested, cultured in suspension in NCSC medium for 1 week, replated onto CELLStart-coated dishes, and cultured for 3 more days. Cells were dissociated into single cells using TryplE Select (Invitrogen) and cultured in NCSC medium as a monolayer and further purified by cloning, subculture, and/or flow-activated cell sorting (FACS) to obtain homogeneous populations that were positive for NC markers. NCSCs were characterized by immunostaining for nestin, the low affinity nerve growth factor (NGF) receptor p75, vimentin, HNK1, AP2, and Slug. Homogeneity was analyzed by flow cytometry analysis of p75. The cells were maintained in NCSC medium, with 50% of the cultural medium changed every other day, and were passaged weekly.
NCSCs could also be cultured as neurospheres in suspension. For sphere formation assay, cells were dissociated and plated onto 6-well ultra-low-attachment plates (Costar; Corning) and cultured in NCSC medium. Neurospheres formed 2 days after being cultured in suspension and increased in size over time. The spheres were fixed in paraformaldehyde and cryosectioned into 10-μm-thick sections as the sphere sizes reached approximately 200 μm. The sections were immunostained with NC markers to confirm the NC identity.
Multipotency and Differentiation Potential of iPSC-NCSCs
Cell differentiation into neural cells and mesenchymal cells was carried out using the protocols described previously [Wang et al., 2004; Lee et al., 2007; Wang et al., 2011]. To induce NCSC differentiation into peripheral neurons, NCSCs were cultured for 2 weeks in N2 medium supplemented with brain-derived neurotrophic factor (BDNF; R&D Systems) (10 ng/ml), NGF (R&D Systems) (10 ng/ml), glial cell-derived neurotrophic factor (GDNF; R&D Systems) (10 ng/ml), and 1 mM dibutyryl-cAMP (Sigma-Aldrich). To induce NCSC differentiation into Schwann cells, NCSCs were cultured for 2 weeks in N2 medium supplemented with ciliary neurotrophic factor (CNTF; R&D Systems) (10 ng/ml), bFGF (10 ng/ml), 1 mM dibutyryl-cAMP, and neuregulin (20 ng/ml; R&D Systems).
For osteogenic differentiation, NCSCs were seeded at a low density (103 cells/cm2) on tissue culture-treated dishes and maintained for 4 weeks in αMEM medium containing 10% fetal bovine serum (FBS), 10 mM β-glycerol phosphate, 0.1 μM dexamethasone, and 200 μM ascorbic acid (all from Sigma-Aldrich). Then, the culture was fixed in 4% paraformaldehyde and stained with Alizarin Red (2% in water, pH to 4.2 using 10% ammonium hydroxide) for calcified matrix.
For chondrogenic differentiation, NCSCs were cultured as cell pellets (<1 mm in diameter) in suspension for 4 weeks in αMEM medium containing 10% FBS, 10 ng/ml transforming growth factor (TGF)-β3 (R&D Systems), 0.1 μM dexamethasone, and 200 μM ascorbic acid. The cell pellets were embedded in OCT compound (TissueTek, Inc.), cryosectioned, and fixed in 4% paraformaldehyde. The cross sections of the pellets were immunostained for collagen II or stained for glycosaminoglycans using Alcian Blue (pH 2.5).
For adipogenic differentiation, NCSCs were grown to confluence and exposed to 1 mM dexamethasone, 10 μg/ml insulin, and 0.5 mM isobutylxanthine (all from Sigma-Aldrich) in αMEM medium containing 10% FBS for 4 weeks. Cells were fixed in 4% paraformaldehyde and stained with Oil Red O (0.5% in 85% propylene glycol) for lipids and fat.
Derivation of SMCs from iPSC-NCSCs
To derive SMCs with NC origin, iPSC-NCSCs were seeded at a low density (103 cells/cm2) on tissue culture dishes for 3 weeks in DMEM (high glucose) medium containing 5% FBS and 10 ng/ml TGF-β1 (R&D Systems). Cells were fixed in 4% paraformaldehyde, and immunostaining was performed for smooth muscle α-actin (SMA), calponin-1 (CNN1), SM22α, and smooth muscle myosin heavy chain (SM-MHC).
Immunostaining, Microscopy, and Flow Cytometry Analysis
Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min, followed by permeabilization with 0.5% Triton X-100 in PBS for 10 min. For immunostaining, the specimens were incubated with respective primary antibodies for 2 h and with appropriate secondary antibodies for 1 h. Nuclei were stained by DAPI (Invitrogen) in blue. The following antibodies were used: p75, nestin, SM22α, and SM-MHC (all from Abcam, Inc.); HNK1, SMA, and S100β (all from Sigma-Aldrich); vimentin (from Dako North America, Inc.); peripherin and collagen-II (both from Millipore Corp.); CNN1 (from Epitomics); alkaline phosphatase (ALP) and AP2 (both from Developmental Studies Hybridoma Bank), and Slug (from Santa Cruz Biotechnology).
The fluorescently stained samples were imaged using a Nikon fluorescence microscope, a Zeiss microscope, or a Leica confocal microscopy system. For confocal microscopy, multiple z-section images (0.3 μm per section) were collected for a given specimen. The optical sections were subsequently projected onto a single plane to create an overall image of the specimen.
Flow cytometry analysis was performed as described previously. Briefly, cells were detached by EDTA treatment, and the nonspecific binding sites on the cell surface were blocked for 30 min by incubation with 1% bovine serum albumin (BSA) (Sigma-Aldrich). The samples were incubated for 30 min with a primary antibody against the surface marker, followed by staining with a fluorescence-labeled secondary antibody (Molecular Probes, Inc.) for 30 min. As negative controls, the samples were incubated with nonspecific antibody with the same isotype as the respective primary antibody, followed by incubation with the same secondary antibody. Dead cells were excluded by 7-amino-actinomycin D (7-AAD) (Invitrogen) staining. The expression of the markers was quantified using a Beckman-Coulter EPICS XL flow cytometer.
Results
Derivation and Characterization of NCSCs from iPSCs and Human ESCs
A schematic diagram of the protocol for NCSC derivation is shown in figure 1a. Undifferentiated ESCs or iPSCs were maintained and expanded on a mouse feeder layer in media supplemented with bFGF (fig. 1b). After the treatment with collagenase and dispase, ESC or iPSC colonies were collected, with feeders left behind (fig. 1c). ESC and iPSC colonies were cultured as cell aggregates in suspension (fig. 1d) and then allowed to adhere to form rosette structures (fig. 1e) for NCSC isolation. The colonies with rosette structures were mechanically harvested and cultured in suspension in NCSC medium for 7 more days. The cells that aggregated in clusters or spheres (fig. 1f) survived and grew, while nonneural single cells died in suspension. The neural aggregates were allowed to attach, dissociated into single cells, and plated as a monolayer in fresh NCSC medium (fig. 1g).
Fig. 1.
Procedure to derive NCSCs from human ESCs and iPSCs. a A schematic diagram of the protocol was described. b Human ESCs and iPSCs were cultured on MEFs. c ESC and iPSC colonies were detached by collagenase and dispase while MEFs were left behind. The asterisk indicates the area occupied by the ESC/iPSC colony. d EB-like cell aggregates were cultured in suspension. e Rosette colony formed after EB-like cell aggregates attached to the CELLstart-coated surface. f Rosette colonies were mechanically harvested and cultured in suspension. The arrow indicates dead nonneural cells in suspension. g iPSC-derived NCSCs were cultured as a monolayer. Scale bar = 100 μm.
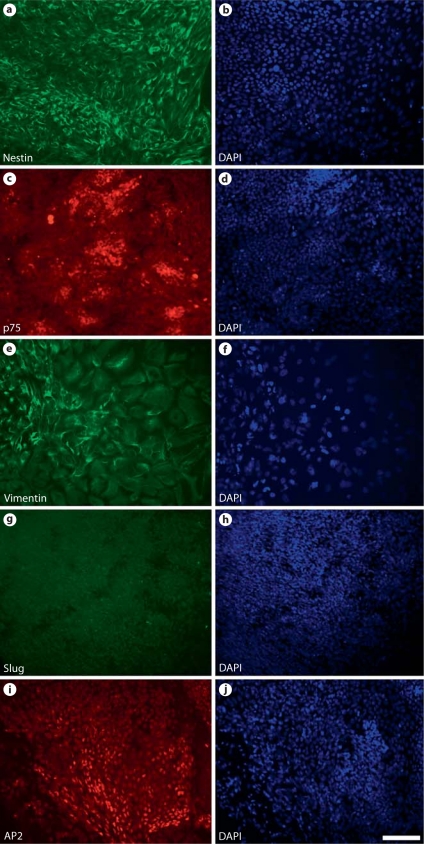
At the rosette stage of ESC and iPSC differentiation, four types of colonies were observed, i.e. colonies with a rosette structure, colonies with differentiated neurons, colonies with differentiated flat nonneural cells, and a small number of undifferentiated ESC/iPSC colonies. Rosette-containing colonies accounted for the NCSC derivation efficiency during the ESC/iPSC differentiation process. Immunostaining for NC markers showed that the majority of cells in the colonies with rosette structures were positive for NC markers nestin (fig. 2a, b), p75 (fig. 2c, d), vimentin (fig. 2e, f), Slug (fig. 2g, h), and AP2 (fig. 2i, j). The percentage of rosette-containing colonies for H1 and BJ1-iPS1 cells is summarized in figure 3a. H1 and BJ1-iPS1 cell lines had similar efficiencies in forming rosettes.
Fig. 2.
Characterization of the cells in the rosette-containing colonies. The majority of cells in the colonies with rosette structures were positive for NC markers nestin (a, b), p75 (c, d), vimentin (e, f), Slug (g, h), and AP2 (i, j). Nuclei were stained using DAPI (blue in the online version). Scale bar = 100 μm.
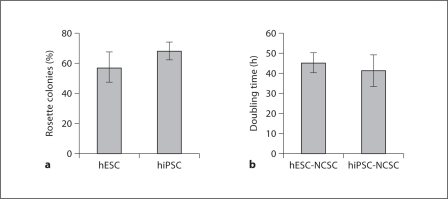
Fig. 3.
Rosette-forming capability of human ESCs and iPSCs and proliferation rate of derived NCSCs. a Percentage of rosette-containing colonies in the culture of ESCs and iPSCs. Eighty to 120 total colonies were counted from each culture. b Doubling time of NCSCs derived from ESCs and iPSCs. Values are reported as means ± standard deviation. n = 3.
Interestingly, in the colonies with differentiated flat nonneural cells, many of the cells stained positive for SMA (data not shown). In addition, when ESCs and iPSCs were allowed to spontaneously differentiate, the majority of the cells that migrated out of the colonies were also SMA positive (data not shown). However, it was not clear whether these myofibroblasts or SMC precursors were derived from the NC or the mesoderm.
The adherent NCSCs were expandable and could be maintained in NCSC medium. The doubling time of ESC-NCSCs and iPSC-NCSCs during expansion is characterized and summarized in figure 3b. There was no significant difference between ESC-NCSCs and iPSC-NCSCs in terms of doubling time.
During cell maintenance and expansion, NCSCs became more homogeneous. As shown in figure 4, iPSC- (BJ1-iPS1 cell line) derived NCSCs homogeneously expressed NCSC markers nestin (fig. 4a, b), p75 (fig. 4c, d), vimentin (fig. 4e, f), and HNK1 (fig. 4g, h). The homogeneity was further confirmed by flow cytometry analysis of p75 expression (fig. 4i, j).
Fig. 4.
Characterization of NCSCs derived from iPSCs cultured as a monolayer. Cells were immunostained uniformly for NCSC markers nestin (a, b), p75 (c, d), vimentin (e, f), and HNK1 (g, h), indicating their NCSC identity. NCSC homogeneity was confirmed by flow cytometry analysis of p75. i Negative control. j p75 staining. Nuclei were stained by DAPI (blue in the online version). Scale bar = 40 μm.
When cultured in suspension in ultra-low-attachment plates in NCSC medium supplemented with bFGF and EGF, both ESC-NCSCs and iPSC-NCSCs formed neural sphere-like aggregates. The cells within the spheres retained the expression of NCSC markers nestin/vimentin (fig. 5a–c) and HNK1/p75 (fig. 5d–f). Furthermore, implantation of ESC-NCSCs and iPSC-NCSCs in nude rats did not result in teratoma formation, suggesting that there were no undifferentiated ESCs or iPSCs in NCSC culture (data not shown).
Fig. 5.
Characterization of NCSCs derived from iPSCs cultured as neurospheres in low-attachment cell culture plates. The cells maintained uniform expression of NCSC markers nestin/vimentin (a–c) and HNK1/p75 (d–f). Nuclei were stained by DAPI (blue in the online version). Scale bar = 100 μm.
Multipotency and Differentiation of iPSC-NCSCs
Next we assessed the differentiation of ESC- and iPSC-derived NCSCs into mesodermal and ectodermal lineages. Representative results for iPSC-NCSC differentiation are shown in figure 6. Neuronal differentiation was induced by the combination of BDNF, GDNF, NGF, and dbcAMP. After 2 weeks, NCSCs differentiated into peripheral neurons (fig. 6a) identified with peripherin (fig. 6b). Schwann cell differentiation was induced in the presence of CNTF, neuregulin 1β, and dbcAMP. After 2 weeks, NCSCs differentiated into Schwann cells (fig. 6c) as assessed by the expression of S100β (fig. 6d).
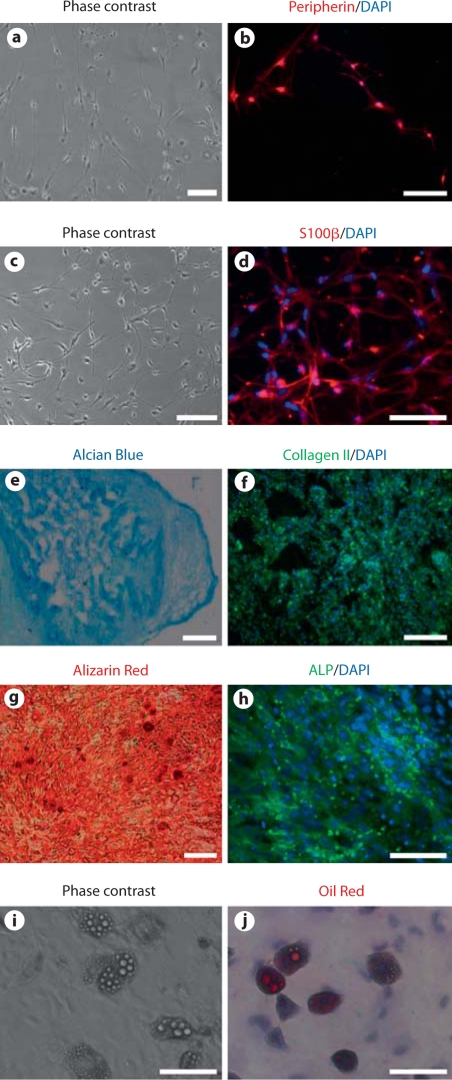
Fig. 6.
In vitro differentiation of iPSC-NCSCs into peripheral neural lineages (peripheral neurons and Schwann cells) and mesenchymal lineages (chondrocytes, osteoblasts, and adipocytes). Peripheral neurons: phase contrast image (a) and immunostaining for peripherin (b). Schwann cells: phase contrast image (c) and immunostaining for S100β (d). Chondrogenic differentiation: Alcian Blue staining for glycosaminoglycans (e) and immunofluorescent staining of collagen II (f). Osteoblastic differentiation: Alizarin Red staining for calcified matrix (g) and immunofluorescent staining of ALP (h). Adipogenic differentiation: phase contrast image (i) and Oil Red staining (j). In all immunofluorescence images, nuclei were stained by DAPI (in blue). Scale bars = 100 μm (a–d, f, h–j) and 200 μm (e, g).
Besides the peripheral neural lineages, NCSCs could also differentiate into various mesenchymal lineages. Under specific conditions, iPSC-NCSCs differentiated into chondrocytes as assessed by Alcian Blue staining for glycosaminoglycans (fig. 6e) and immunofluorescent staining for collagen II (fig. 6f), differentiated into osteoblasts as assessed by Alizarin Red staining for calcified matrix (fig. 6g) and immunofluorescent staining for ALP (fig. 6h), and differentiated into adipocytes as assessed by phase contrast and Oil Red staining for lipid droplets (fig. 6i, j). These results demonstrated the multipotency of the iPSC-NCSCs.
Differentiation of iPSC-NCSCs into Smooth Muscle Lineage
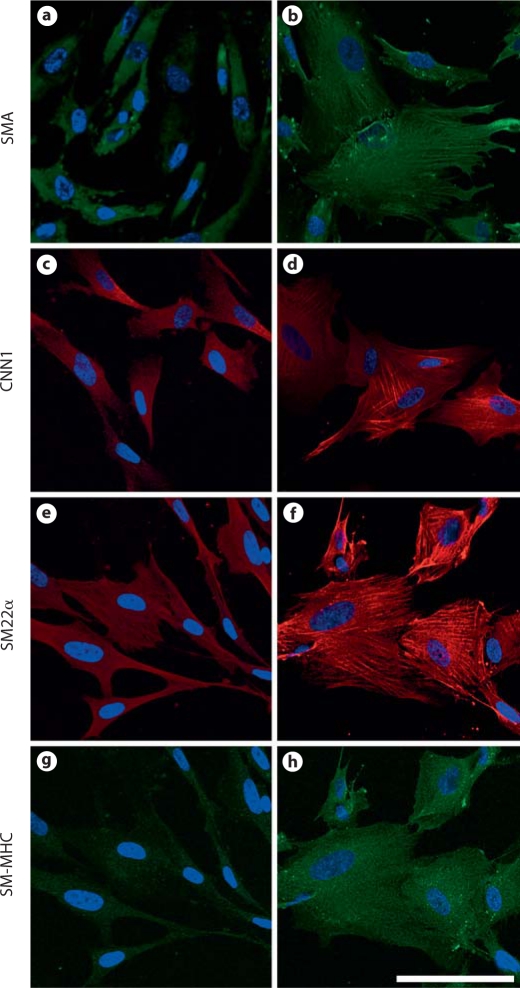
Under general maintenance conditions, iPSC-NCSCs showed low or no expression of smooth muscle markers SMA (fig. 7a), CNN1 (fig. 7c), SM22α (fig. 7e), and SM-MHC (fig. 7g). In addition, none of the SMC contractile markers were detected in stress fibers. After treatment with TGF-β1 (10 ng/ml) for 2 weeks, iPSC-NCSCs showed an increase in cell spreading and a higher expression of SMA (fig. 7b), CNN1 (fig. 7d), SM22α (fig. 7f), and SM-MHC (fig. 7h). Furthermore, TGF-β1 induced the assembly of SMA, CNN1, and SM22α into stress fibers. In TGF-β1-treated samples, SM-MHC showed diffused cytoplasmic staining but was not detected in stress fibers (fig. 7h), suggesting that these differentiated cells did not terminally differentiate into mature SMCs. Image analysis demonstrated that, upon differentiation in the presence of TGF-β1, about 80% of the cells expressed SMA, CNN1, and SM22α, and about 30% of the cells showed a low level of SM-MHC expression. However, only ∼20% of the cells expressed SMA, CNN1, and SM22α assembled into stress fibers, which suggests that other factor(s) are needed to induce the terminal differentiation of SMCs.
Fig. 7.
Differentiation of iPSC-NCSCs into smooth muscle lineage. iPSC-NCSCs were cultured in medium containing 5% FBS in the absence (a, c, e, g) or presence (b, d, f, h) of TGF-β1 (10 ng/ml) for 2 weeks and immunostained for smooth muscle markers SMA (a, b), CNN1 (c, d), SM22α (e, f), and SM-MHC (g, h). Scale bar = 100 μm.
Discussion
Here we developed a robust method to derive NCSCs from pluripotent stem cells and further directed NCSC differentiation into smooth muscle lineage. Since NCSCs are expandable in culture, this approach can provide an unlimited source of SMCs with NC origin. NCSCs and SMCs derived from iPSCs can be used not only for modelling vascular development and vascular diseases with different genetic backgrounds but also for regenerative medicine and tissue engineering applications.
In terms of NCSC derivation efficiency, we did not find a significant difference between human ESC line H1 and iPSC line BJ1-iPS1. Both cell types formed rosette-like structures in approximately 60% of the colonies. The NCSCs derived from H1 and BJ1-iPS1 lines displayed similar characteristics including NC marker expression and differentiation potential. They also displayed similar proliferation rates. However, we did observe a different efficiency of rosette formation in several skin fibroblast-derived iPSCs [Wang et al., 2011]. More ESC and iPSC lines should be compared in order to determine the consistency of NCSC and SMC derivation efficiency from pluripotent stem cells.
Recent studies showed that NCSCs could be isolated from ESCs and purified by sorting for p75+ cells with or without coculturing with stromal cells [Lee et al., 2007; Jiang et al., 2009], and SM22α-positive cells were obtained by sorting for CD73 and NCAM. In this study, we developed a protocol to induce the differentiation of human ESCs and iPSCs into NCSCs in a high yield without coculture. In addition, a homogeneous population of NCSCs could be obtained without sorting in most of the cases. We found that the neural induction medium with StemPro neural supplement, bFGF, and EGF induced the differentiation of pluripotent stem cells into NCSCs with a high yield at the rosette-forming stage. Most of the cells in the rosette structure and on the periphery parts were positive for NC markers nestin, p75, vimentin, Slug, and AP2. After the cells of the rosette-containing colonies were further selected by neurosphere-forming capability and subsequently propagated in NCSC medium, the cells were more homogenous over time and expressed the NC markers.
Among biochemical signaling molecules, TGF-β1 plays an important role in SMC differentiation. TGF-β1 has been shown to upregulate the expression of SMC markers in SMCs [Hautmann et al., 1997; Adam et al., 2000; Hirschi et al., 2002; Liu et al., 2003], MSCs [Kinner et al., 2002; Wang et al., 2004; Kurpinski et al., 2010], ESCs [Kurpinski et al., 2010], and adult NCSC lines [Chen and Lechleider, 2004]. In this study, we showed that TGF-β1 could efficiently induce the differentiation of iPSC-NCSCs into SMC lineage, with the expression of SMC markers SMA, CNN1, SM22α, and SM-MHC. In addition, TGF-β1 promoted the incorporation of SMA, CNN1, and SM22α into the stress fibers. However, only ∼20% of the cells expressed these SMC markers in stress fibers, and SM-MHC had not assembled into the stress fibers at this point. These results suggest that other factors may be needed to drive further maturation of SMCs, which needs further investigation.
Acknowledgements
We thank Julia F. Chu and Rebecca Chen for their technical assistance. This work was supported in part by grants from the National Institutes of Health (EB012240 and HL083900 to S.L.) and postdoctoral training grant TG2-01164 from the California Institute for Regenerative Medicine (to A.W.).
Abbreviations used in this paper
- 7-AAD
7-amino-actinomycin D
- ALP
alkaline phosphatase
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BSA
bovine serum albumin
- CNN1
calponin-1
- CNTF
ciliary neurotrophic factor
- DMEM
Dulbecco's Modified Eagle's Medium
- EB
embryo body
- EGF
epidermal growth factor
- ESC
embryonic stem cell
- FBS
fetal bovine serum
- GDNF
glial cell-derived neurotrophic factor
- iPSCs
induced pluripotent stem cells
- MEFs
mouse embryonic fibroblasts
- NC
neural crest
- NCSCs
neural crest stem cells
- NGF
nerve growth factor
- PBS
phosphate-buffered saline
- SMA
smooth muscle α-actin
- SMCs
smooth muscle cells
- SM-MHC
smooth muscle myosin heavy chain
- TEVGs
tissue-engineered vascular grafts
- TGF
transforming growth factor
References
- Adam P.J., Regan C.P., Hautmann M.B., Owens G.K. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor β control element required for expression of the smooth muscle cell differentiation marker SM22α in vivo. J Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- Bergwerff M., Verberne M.E., DeRuiter M.C., Poelmann R.E., Gittenberger-de Groot A.C. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- Chen S., Lechleider R.J. Transforming growth factor-β-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004;94:1195–1202. doi: 10.1161/01.RES.0000126897.41658.81. [DOI] [PubMed] [Google Scholar]
- Crane J.F., Trainor P.A. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- DeBakey M.E., Glaeser D.H. Patterns of atherosclerosis: effect of risk factors on recurrence and survival-analysis of 11,890 cases with more than 25-year follow-up. Am J Cardiol. 2000;85:1045–1053. doi: 10.1016/s0002-9149(00)00694-9. [DOI] [PubMed] [Google Scholar]
- Hautmann M.B., Madsen C.S., Owens G.K. A transforming growth factor β (TGF-β) control element drives TGF-β-induced stimulation of smooth muscle α-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948–10956. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- Hirschi K.K., Lai L., Belaguli N.S., Dean D.A., Schwartz R.J., Zimmer W.E. Transforming growth factor-β induction of smooth muscle cell phenotpye requires transcriptional and post-transcriptional control of serum response factor. J Biol Chem. 2002;277:6287–6295. doi: 10.1074/jbc.M106649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K.K., Majesky M.W. Smooth muscle stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:22–33. doi: 10.1002/ar.a.10128. [DOI] [PubMed] [Google Scholar]
- Jiang X., Gwye Y., McKeown S.J., Bronner-Fraser M., Lutzko C., Lawlor E.R. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human ESCs. Stem Cells Dev. 2009;18:1059–1070. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Rowitch D.H., Soriano P., McMahon A.P., Sucov H.M. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kinner B., Zaleskas J.M., Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- Kurpinski K., Lam H., Chu J.L., Wang A.J., Kim A., Tsay E., Agrawal S., Schaffer D.V., Li S. Transforming growth factor-β and Notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- Lam H., Patel S., Wong J., Chu J., Lau A., Li S. Localized decrease of β-catenin contributes to the differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2008;372:601–606. doi: 10.1016/j.bbrc.2008.05.116. [DOI] [PubMed] [Google Scholar]
- Lee G., Kim H., Elkabetz Y., Al Shamy G., Panagiotakos G., Barberi T., Tabar V., Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- L'Heureux N., Dusserre N., Konig G., Victor B., Keire P., Wight T.N., Chronos N.A., Kyles A.E., Gregory C.R., Hoyt G., Robbins R.C., McAllister T.N. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Fan Y.S., Chow L.H., Van Den Diepstraten C., van Der Veer E., Sims S.M., Pickering J.G. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res. 2001;89:517–525. doi: 10.1161/hh1801.097165. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sinha S., Owens G.K. A TGF-β control element required for SM α-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem. 2003;278:48004–48011. doi: 10.1074/jbc.M301902200. [DOI] [PubMed] [Google Scholar]
- Majesky M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Colbert M.C., Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- Park I.H., Arora N., Huo H., Maherali N., Ahfeldt T., Shimamura A., Lensch M.W., Cowan C., Hochedlinger K., Daley G.Q. Disease-specific induced pluripotent stem cells. Cell. 2008a;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008b;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Regan C.P., Adam P.J., Madsen C.S., Owens G.K. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Soldner F., Hockemeyer D., Beard C., Gao Q., Bell G.W., Cook E.G., Hargus G., Blak A., Cooper O., Mitalipova M., Isacson O., Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple D.L., Anderson D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Wang D.J., Park J.S., Chu J.S., Krakowski A., Luo K.X., Chen D.J., Li S. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor β1 stimulation. J Biol Chem. 2004;279:43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- Wang A., Tang Z., Park I.H., Zhu Y., Patel S., Daley G.Q., Li S. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32:5023–5032. doi: 10.1016/j.biomaterials.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg C.B., Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]