Abstract
Recent observations suggest that the adventitial layer of blood vessels exhibits properties resembling a stem/progenitor cell niche. Progenitor cells have been isolated from the adventitia of both murine and human blood vessels with the potential to form endothelial cells, mural cells, osteogenic cells, and adipocytes. These progenitors appear to cluster at or near the border zone between the outer media and inner adventitia. In the mouse, this border zone region corresponds to a localized site of sonic hedgehog signaling in the artery wall. This brief review will discuss the emerging evidence that the tunica adventitia may provide a niche-like signaling environment for resident progenitor cells and will address the role of the adventitia in growth, remodeling, and repair of the artery wall.
Key Words: Vascular development, Stem cell, Progenitor cell niche
The Adventitia: New Concepts and Novel Functions
The tunica adventitia, the outer layer of most blood vessel walls, has historically been regarded as a loosely organized collection of fibroblasts, perivascular nerves, and microvessels embedded in a collagen-rich extracellular matrix (ECM). Recent studies, however, suggest a more complex and dynamic picture of the adventitia that emphasizes critical roles played by interacting adventitial cell types in growth, inflammation, repair, and disease of the artery wall. We now know that normal adventitia contains resident macrophages, mast cells, T cells, B cells, and dendritic cells and is a major site for immune surveillance and innate immune responses [Galkina et al., 2006; Mayranpaa et al., 2009; Tieu et al., 2009; Zhou et al., 2010, Swedenborg et al., 2011] (fig. 1). The adventitia is also an active compartment for inflammatory cell trafficking into and out of the vessel wall and maintains a microvascular network, the vasa vasorum, that provides the principle means for this trafficking [Heistad et al., 1981; Moulton et al., 2003; Galkina et al., 2006; Ritman and Lerman, 2007; Drinane et al., 2009]. The adventitia signals to the media and plays important roles in the control of lumen size by short-term regulation of medial smooth muscle contraction and by long-term control of inward and outward wall remodeling processes [Gutterman, 1999; Smith et al., 1999; Rey and Pagano, 2002; Haurani and Pagano, 2007; Korshunov et al., 2007; Tang et al., 2008]. Upon this background, rapidly accumulating evidence that the adventitia contains resident vascular progenitor cells and that it may also possess stem/progenitor cell niche-like activity to maintain these resident progenitors is particularly interesting [Hu et al., 2004; Zengin et al., 2006; Pasquinelli et al., 2007; Hoshino et al., 2008; Passman et al., 2008; Campagnolo et al., 2010]. The new picture that is emerging of the adventitia is one of a dynamic environment of interacting cell types with unexpected roles in the control of vascular wall growth, remodeling, and disease.
Fig. 1.
The adventitia. Schematic view of a large elastic artery in cross section showing organization of the major cell types into layers around a central lumen (top). Int = Intima; Med = media; Adv = adventitia. A segment of the vessel wall (inset) is expanded to show the constituent cell types in the normal, uninjured arterial adventitia (bottom). The left side shows a histological cross section of postnatal day 2 thoracic aorta from a Ptc1-LacZ transgenic mouse stained with nuclear fast red. Note that Shh signaling, as indicated by Ptc1-LacZ activity (blue), is confined to the adventitial layer. The right side shows a false color rendering of the same tissue shown at left depicting CD68+ cells (macrophages, orange), Sca1+ progenitor cells (red), adventitial fibroblasts (green), and T cells (purple). Also present in variable numbers are perivascular nerves and associated glial cells, microvessels of the vasa vasorum network, and adipocytes (see also fig. 2).
The Adventitia Contains Resident Progenitor Cells
Hu et al. [2004] reported that adult ApoE–/– mice maintained clusters of stem cell antigen-1 (Sca1, also called Ly6A/E)-positive cells in the aortic root adventitia that differentiated to a smooth muscle cell (SMC)-like cell type when removed from the adventitia and exposed to PDGF-BB in vitro. Howson et al. [2005] reported the isolation of pericyte progenitor cells from rat aorta that formed neurosphere-like colonies in stem cell media when maintained in suspension culture. Zengin et al. [2006] reported that a ‘vasculogenic zone’ could be identified in adult human arteries that contained CD34+ progenitor cells with the ability to form vascular structures in arterial ring explant cultures in vitro as well as promote the formation of microvessels in a transplantable tumor model in vivo. This ‘vasculogenic zone’ was localized to a region between the media and the adventitia in human blood vessels that was highly reminiscent of the position in which Sca1+ progenitor cells were found in the aortic root of wild type and ApoE–/– mice [Hu et al., 2004]. Pasquinelli et al. [2007] reported finding two distinct progenitor cell populations located between the media and the adventitia in human thoracic aortas and femoral arteries. One population was comprised of CD34+ cells and the other contained c-kit+ cells. When isolated and placed in cell culture in the presence of vascular endothelial growth factor (VEGF), some of these adventitial progenitor cells acquired an endothelial cell-like phenotype and formed capillary-like vascular structures in vitro [Pasquinelli et al., 2007]. Passman et al. [2008] reported that progenitor cells with the cell surface marker profile CD34+, Sca1+, c-kit–, Flk1+, and CD140b+ were found clustered in a zone of sonic hedgehog (Shh) signaling in the adventitial layer of the artery wall. Using both patched-1 lacZ (Ptc1lacZ/+) and Ptc2lacZ/+ reporter mice, the zone of Shh signaling was found to be almost entirely restricted to the adventitial layer [Passman et al., 2008]. Shh protein itself was concentrated in the adventitia close to the border between the media and adventitia. At E18.5, Shh–/– embryos had greatly diminished numbers of adventitial Sca1+ progenitor cells (referred to as AdvSca1 cells) in the aortic root [Passman et al., 2008]. Exposure of isolated AdvSca1 cells in vitro to Shh or the Hh signaling antagonist cyclopamine provided evidence that Hh signaling mediates mitogenic and survival responses in these progenitor cells [Passman et al., 2008; Regan, 2010]. Mouse aortic AdvSca1 cells appear to be a diverse population of progenitor cells with heterogeneous potentials for cell differentiation. For example, when freshly isolated from adult mouse thoracic aorta and placed into serum-containing culture medium ex vivo, roughly 50% of the isolated AdvSca1 cells differentiated into SMCs and about 25% proliferated and maintained Sca1 expression and progenitor cell properties (i.e. self-renewal), while the remaining 25% lost expression of Sca1 but did not acquire detectable levels of SMC or endothelial cell marker expression [Passman et al., 2008; Majesky et al., 2011]. These phenotypes resemble the intrinsic heterogeneity that has been described for the satellite stem/progenitor cell populations in skeletal muscle [Le Grand et al., 2009; Biressi and Rando, 2010]. When stimulated with bone morphogenic protein-2 (BMP2) a small percentage (∼0.1–0.5%) of AdvSca1 cells became osteogenic while stimulation with insulin, isobutylmethylxanthine, and dexamethasone [Spiegelman and Green, 1980] produced a similarly small percentage of adipocytes [Passman et al., 2008]. Likewise, Campagnolo et al. [2010] isolated a population of CD34+/CD31– cells from the adventitia and perivascular zone of human saphenous veins from patients undergoing coronary artery bypass surgery [Campagnolo et al., 2010]. These CD34+ progenitor cells could be cloned in vitro and possessed multilineage potential to form osteoblasts, adipocytes, pericytes, and SMCs under selective differentiation-promoting conditions in culture. When transplanted into ischemic hind limbs of immunodeficient mice, human saphenous vein-derived progenitor cells adopted a pericyte-like phenotype, formed N-cadherin-mediated cell-cell contacts with endothelial cells, stimulated angiogenesis, and improved blood flow recovery in this model [Campagnolo et al., 2010]. Finally, Zorzi et al. [2010] demonstrated that rat thoracic aorta contains adventitial macrophage-like cells that can stimulate angiogenesis in an aortic ring assay. A subset of these cells acquired an endothelial cell phenotype when cultured in the presence of VEGF and formed capillary-like vascular structures in a matrigel angiogenesis assay in vitro. In summary, these findings suggest that the adventitia maintains multiple types of progenitor cells that appear to act in concert as part of a coordinated healing response to vascular injury.
The Adventitia Provides a Niche-Like Signaling Environment in the Vessel Wall
Studies of stem and progenitor cells in different tissues from various organisms reveal that the survival and function of these cells is largely governed by neighboring cells [Schofield, 1978; Kimble and White, 1981; Spradling, 2001; Scadden, 2006; Jones and Wagers, 2008; Kuang et al., 2008; Hsu et al., 2011]. The neighboring cells and their soluble and ECM products form a cooperative signaling environment that is called a stem/progenitor cell niche [reviewed in Watt and Hogan, 2000; Moore and Lemischka, 2006; Scadden, 2006; Gopinath and Rando, 2008; Jones and Wagers, 2008]. The number of progenitor cells, their proliferative activity, and their low frequency of spontaneous differentiation are determined to a great extent by signaling from neighboring niche cells [Jones and Wagers, 2008]. Examples of a niche environment in mammals include satellite cells within a myofiber in skeletal muscle [Mauro, 1961; Conboy and Rando, 2002; Gopinath and Rando, 2008; Kuang et al., 2008], neural progenitors in a perivascular niche in the subventricular zone (SVZ) of the central nervous system [Shen et al., 2008; Tavazoie et al., 2008], mesenchymal stem cells in various stromal niches including bone matrix [Chen et al., 2007; Crisan et al., 2008], and epidermal stem cells in a hair follicle niche in the skin [Hsu et al., 2011].
The stem cell niche hypothesis was proposed by Schofield [1978], who studied the properties of hematopoietic stem cells (HSCs) in vivo and concluded that: (a) they occupy a defined anatomical location, (b) their self-renewal activity is controlled by adjacent non-HSCs, and (3) displacement of HSCs from neighboring non-HSC cells leads to stem cell differentiation. Assuming these are minimal criteria for what constitutes a stem/progenitor cell niche, the region of adventitia close to the media seems to fulfill the definition of such a niche environment. The inner adventitia has a defined location bordering the external elastic lamina (EEL) that marks the boundary with the outer media. Shh signaling in the adventitia appears to regulate adventitial progenitor cell proliferation, self-renewal, and survival, at least in adventitia surrounding the aortic root and thoracic aorta [Passman et al., 2008; Regan, 2010]. Finally, when removed from the putative aortic adventitial niche by collagenase and elastase digestion and placed in cell culture, about 50% of adventitial progenitor cells spontaneously lose expression of Sca1 and differentiate into SMC-like cells in vitro [Hu et al., 2004; Passman et al., 2008; Majesky et al., 2011]. Differentiation of adventitial progenitor cells into SMCs occurred with similar kinetics even when growth was arrested in the presence or absence of aphidicolin or hydroxyurea (cell cycle inhibitors), suggesting that progenitor cells can directly differentiate into SMCs and that this differentiation does not require cell proliferation [Passman et al., 2008].
In addition to Shh signaling it can be anticipated, based on studies in model organisms including worms, flies, and fish, that other soluble factors will eventually be found to play important roles in the adventitial niche. High on the list of candidates would be Wnt signaling given the roles that have been proposed for this pathway in stem/progenitor cell niches within the intestinal crypt [Pinto et al., 2003; Barker et al., 2007], skin [Gat et al., 1998; Jamora et al., 2003], and hippocampus subgranular zone [Palmer et al., 2000; Lie et al., 2005]. Similar arguments can be made for notch signaling [Conboy and Rando, 2002; Song et al., 2007; Aguirre et al., 2010] and BMP signaling pathways [Kobielak, 2003; Dudley, 2010]. Other studies have shown that specific types of contacts with the ECM provide adhesion, structural organization, and biomechanical inputs that are likely to be important in niche-progenitor cell interactions [Badylak, 2007; Discher et al., 2009; Guilak et al., 2009; Hynes, 2009]. For example, the ECM of the SVZ niche in the mouse brain binds and concentrates the neurogenic factor FGF2 [Kerever et al., 2007]. The neural progenitor cells within the SVZ niche also make cell-to-cell contacts with vascular endothelial cells and astrocytes [Kokovay et al., 2010]. Hair follicle stem cells in close proximity to sensory nerves receive an Shh signal from the nerves that is important for maintenance of a multipotential cell population capable of becoming epidermal stem cells [Brownell et al., 2011]. Thus, cell-cell interactions within niche environments are critical for maintaining progenitor cells capable of contributing to tissue homeostasis and repair.
The adventitia of large elastic arteries is a mechanically active environment. The primary physiological function of these vessels is to absorb ventricular pulse pressures and dampen the propagation of the pulse pressure gradient [Wagenseil and Mecham, 2009]. This is accomplished by expansion of the elastic fiber-containing artery wall and its relaxation again with each heartbeat. The largest changes in wall diameter will occur in the outer layers, e.g. the adventitia. Adventitial progenitor cells will therefore find themselves in a tissue environment that expands outward and relaxes inward with each cardiac cycle. It is becoming well established that stem and progenitor cells respond to biomechanical forces that influence their survival, proliferation, and differentiation [Discher et al., 2009; Guilak et al., 2009; Castillo and Jacobs, 2010; Fu et al., 2010]. It can be anticipated, therefore, that adventitial progenitor cells will be responsive to a mechanically active environment through matrix receptor-mediated signaling [Hahn and Schwartz, 2009; Hynes, 2009; Shattil et al., 2010]. Moreover, this mechanical activity will likely be found to influence adventitial progenitor cell survival, proliferation, and differentiation in conjunction with soluble factors and cell-cell interactions found in the adventitial niche environment.
The stereotypical organization of endothelial cells and SMCs in a radial pattern in blood vessel walls suggests that these cells possess a planar orientation in vivo. If so, progenitor cells in the adventitia may also have an intrinsic cell polarity that is reflected in their flattened appearance and clustered organization in the artery wall (fig. 2). This would have important implications for the adventitial niche since intrinsic polarity in the progenitor cells suggests that asymmetric cell division might be an important mechanism for generation of more differentiated progeny in the adventitia [Williams et al., 2011]. Whether or not this is the case for adventitial progenitor cells will be important to examine in developing and injured arteries in future studies.
Fig. 2.
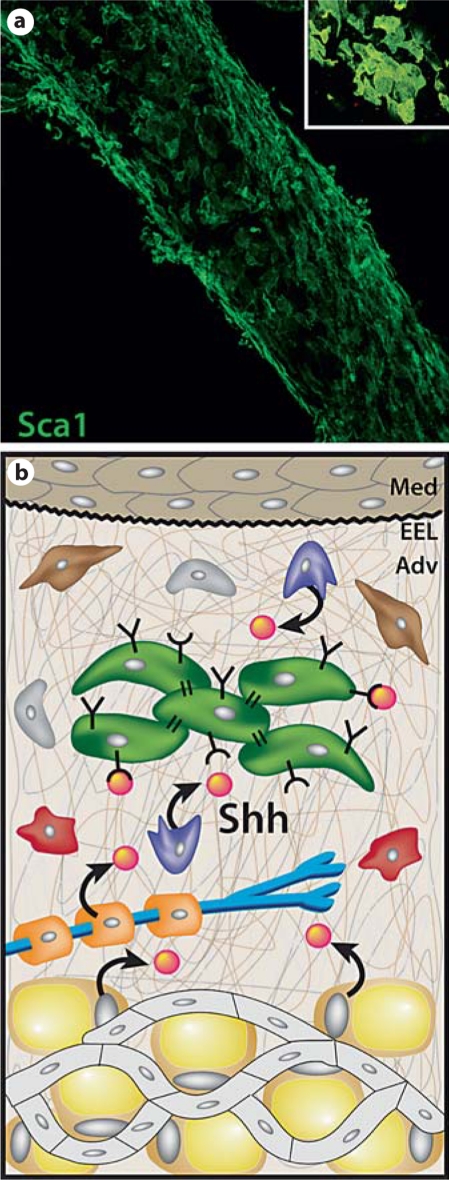
The adventitia as a progenitor cell niche in the artery wall. a The left common carotid artery from a postnatal day 9 mouse was fixed and immunostained for Sca1 expression and examined by confocal microscopy. Shown is a reconstructed low power image of stacked Z-plane confocal images of adventitial Sca1+ progenitor cells. Inset A high power view of the characteristic cluster arrangement of Sca1+ progenitor cells in the mouse carotid artery adventitia. b Schematic representation of a proposed progenitor cell niche in the arterial adventitia. We propose that this niche environment contains soluble factors (orange spheres) including Shh [Passman et al., 2008] and other secreted factors not yet described, and ECM proteins (thin brown, grey and white lines) including various collagens, proteoglycans, and hyaluronic acid. Adventitial progenitor cells will possess matrix adhesion receptors (Y) and cell surface receptors for soluble factors (U) that together signal maintenance of a progenitor phenotype, promote survival of progenitor cells, and prevent their premature differentiation. Upon injury to the artery wall, an influx of inflammatory cells into the adventitia likely directs downregulation of niche signaling leading to differentiation of progenitor cells to form SMCs, pericytes, and possibly other vascular cell types.
Development of the Adventitia
What is the embryonic origin of adventitial progenitor cells and when do they first appear in developing vessel wall? Evidence to date suggests that Sca1+ progenitor cells in the mouse first appear in the aortic root region around E16.5 [Passman et al., 2008]. This is around the same time that the final layer of smooth muscle and elastic fibers in the tunica media of large elastic arteries is formed and development of the tunica adventitia is thought to begin [Kelleher et al., 2004; Wagenseil et al., 2010]. The origin of progenitor cells found in the adventitia is not known. Hu et al. [2004] showed that adventitial progenitors expressing the Sca1 marker in adult ApoE-deficient mice do not arise from bone marrow, and Passman et al. [2008] showed that in aortic segments composed of neural crest-derived SMCs the adjacent Sca1-expressing progenitor cells in the adventitia were not derived from the neural crest. Wasteson et al. [2008] traced the origin of descending aorta SMCs to progenitors in the somite. In those studies the aortic SMCs could clearly be identified as having a somite origin, while few if any cells in the adventitia were labeled by the somite lineage markers used [Wasteson et al., 2008]. Thus the developmental origins of AdvSca1 cells differ from those of their neighboring SMCs [Majesky, 2007; Passman et al., 2008] and are as yet unknown.
In principle, the adventitial niche environment could either be formed before progenitor cells occupy the niche or, alternatively, be produced at the same time that progenitor cells appear in the adventitia. In the first case, progenitor cells would find and occupy an existing signaling environment implying they had no direct role in producing the niche. In the second case, the progenitor cells themselves may actively participate in constructing a niche environment that they will reside in. The early evidence favors the former possibility as Shh signaling in the mouse aortic root adventitia is detectable with PtclacZ/+ reporter mice at least 1 day before Sca1-expressing progenitor cells are detected there [Passman et al., 2008]. It also remains possible that adventitial progenitors move from a previous niche into the arterial adventitia during late stages of vascular development. If true, they would resemble HSCs that move from the yolk sac to the periaortic zone to fetal liver and then to bone marrow as development proceeds. Since adventitial progenitors can be recovered from the adventitia of adult mouse and human arteries [Hu et al., 2004; Zengin et al., 2006; Pasquinelli et al., 2007; Passman et al., 2008; Campagnolo et al., 2010], their residence time in the adventitial niche environment may be quite long. Much additional work is required to characterize the origins and regulation of both the adventitial progenitor cells and the proposed adventitial niche-signaling environment.
The Adventitia in Arterial Wound Repair
Like most tissues, blood vessels activate intrinsic mechanisms for tissue repair when injured or diseased. This capacity for repair of the artery wall is substantial. For example, when subjected to balloon dilation injury replication rates in all layers of rat carotid or porcine coronary artery wall can increase over 100-fold [Clowes et al., 1983; Reidy et al., 1983; Scott et al., 1996]. In commonly used animal models of arterial wound repair where preexisting intimal cells that are characteristically found in human arteries [Schwartz et al., 1995] are not present, migration of medial SMCs to the intima is thought to be essential for neointimal formation. This conventional view has been challenged by evidence suggesting that adventitial cells can respond to arterial injury, migrate into the injured wall, and contribute to neointimal formation [Sartore et al., 2001]. For example, Scott et al. [1996] employed single injections of 5-bromo-2-deoxyuridine (BrdU) between days 2 and 3 after balloon catheter injury to porcine coronary arteries [Scott et al., 1996]. One day later, most of the labeled cells were found in the adventitia. When examined at 14 days after injury, the presence of significant numbers of BrdU-positive cells in the neointima suggested that cells in the adventitia could migrate within the injured artery wall and reach the neointima in this model [Scott et al., 1996]. Similarly, Holifield et al. [1996] used controlled enzyme digestion from the adventitial side of uninjured canine carotid arteries and isolated cells that did not express smooth muscle marker proteins. They referred to these nonmuscle cells as ‘type 2’ cells in contrast to the controlled digestion from the luminal side that produced smooth muscle marker-positive cells [Holifield et al., 1996]. Type 2 cells proliferated when placed in serum-containing culture medium and expressed SMα-actin but not SM-MHC. By contrast, vascular SMCs from adult carotid media did not spread or proliferate in vitro but remained viable and expressed SMC marker proteins including SM-MHC at undiminished levels. Of particular interest was the finding that the neointima produced by balloon injury was composed of cells that were morphologically and immunologically identical to type 2 cells [Holifield et al., 1996]. Transplantation of adventitial cells that were labeled in various ways to the adventitial side of an artery in rodents and then monitoring the movement of these cells within the vessel wall following arterial injury has also provided evidence that adventitial cells can move into the media and that some can continue migration and reach the intima [Mason et al., 1999; Li et al., 2000; Hu et al., 2004].
It is intriguing to consider that at least part of the migrating population of adventitia-derived cells originates within the progenitor cell pools in the adventitia. For example, when obtained from genetically marked donor mice expressing β-galactosidase from the Rosa locus (Rosa26) and transferred to the outside of an experimental vein graft placed into the arterial circulation, adventitial Sca1+ cells were found in the media of the vein graft at 2 weeks and in the graft neointima at 4 weeks after surgery [Hu et al., 2004]. These findings are consistent with the migration of progenitor cells from the adventitia through the wall of the vein graft and into the neointima where they differentiated to SMCs. By contrast, if Sca1-negative adventitial cells (mostly fibroblasts) were grafted instead of Sca1+ cells, and then a great majority of Sca1-negative cells remained in the graft adventitia after 4 weeks and were only rarely found in the neointima. Therefore, adventitial Sca1+ cells with a potential to form SMC-like cells can migrate from the adventitia to the vein graft neointima and promote intimal lesion growth [Hu et al., 2004; Torsney and Xu, 2011].
Dysregulation of the Adventitial Niche: Aging and Arterial Disease
Recent studies suggest that loss of function of niche-dependent signaling may be an important contributing factor or even a proximal cause of various pathologies associated with aging including alopecia, kyphosis, osteoporosis, hair graying, fibrosis, and thymic involution [Conboy et al., 2005; Janzen et al., 2006; Brack et al., 2007; Capell et al., 2007; Olive et al., 2010]. Depletion of endogenous progenitor cell pools has been associated with congenital defects [Hardouin et al., 2011], a reduced capacity to repair injuries, atrophy of functional tissue mass, and degenerative changes in different tissues [Ruzankina et al., 2007; Gopinath and Rando, 2008; Day et al., 2010]. There are even age-related changes in stem/progenitor cell niches that are proposed to direct young progenitor cells to adopt an aged phenotype [Janzen et al., 2006; Krishnamurthy et al., 2006; Brack et al., 2007; Carlson and Conboy, 2007]. Reductions or loss of progenitor cell pools in the artery wall may predispose to microvascular (vasa vasorum) rarefaction such as that seen in rapid aging syndromes [Olive et al., 2010], medial dissection and aneurysm formation in the thoracic aorta [Wilens et al., 1965; Heistad et al., 1981; Guo et al., 2007; Zhang et al., 2011], and adventitial fibrosis [Mahoney et al., 2011]. As we acquire more information about the natural fate of adventitial progenitor cells in injured and aging arteries, we will be better able to refine our hypotheses about the consequences of depleted progenitor pools for the structure and function of large elastic arteries. Similarly, as further progress is made in the identification of critical soluble signals and cell-cell interactions that comprise the adventitial niche environment, we will have additional tools, targets, and pathways for intervention in degenerative changes in artery walls that are commonly seen in aging blood vessels.
Concluding Remarks
The studies described above suggest new functions for the adventitial layer of blood vessels. They provide evidence that progenitor cells with the potential to produce cell types commonly found in blood vessel walls normally reside within the adventitia of large arteries and veins. At this point, many questions remain to be answered before the picture becomes clear. For example, we still know very little about how the adventitia forms in embryonic blood vessels. Despite the intense interest in molecular pathways controlling early vascular development, factors required for the latter stages including formation of the medial and adventitial layers remain largely unknown. Molecular markers that specifically label the adventitia are lacking. In their absence we can only reliably identify this tissue after the EEL is laid down. Does that mean adventitia formation begins only after the EEL is completed, or are developmental pathways at work in preadventitial mesenchyme that dictate when the last layer of media is formed and thus pattern the future adventitia? Does the onset of Shh signaling in mouse aortic adventitia around E15.5 to E16.5 [Passman et al., 2008] reflect formation of a media-adventitia border and, if so, what genes act upstream of Shh to control the timing and location of Shh pathway activation? What is the origin of adventitial cells in the embryo and what roles do individual adventitial cell types play in the morphogenesis of this tissue layer? In addition to Shh, what other growth factors, morphogens, ECM proteins, and cell-cell signaling molecules are important constituents of the adventitial niche? Does signaling within the adventitial niche maintain vascular progenitor cell pool sizes and does depletion of this progenitor cell pool predispose the artery wall to degenerative changes commonly seen in aging and vascular disease? This exciting new view of the adventitia as a progenitor cell niche in the vessel wall must now be pursued by careful investigation across different species and experimental models to provide for a more complete understanding of the integrated roles of the adventitia in vascular development, physiology, and disease.
Acknowledgements
We acknowledge helpful discussions with members of the Majesky, Daum, and Mahoney laboratories and with Drs. M. Bothwell, R. Nicosia, M. Reyes, M. Rosenfeld, and S. Schwartz of the Adventitia Group at the University of Washington. The authors’ work was supported by NIH grants HL-93594 (M.W.M.), HL-88374 (G.D.), and HL-87513 (W.M.M. Jr.), American Heart Association grant 09PRE2060165 (V.H.), the Curriculum for Genetics and Molecular Biology, University of North Carolina at Chapel Hill, and the Seattle Children's Research Institute.
List of abbreviations used in this paper
- AdvSca1
adventitial Sca1+ progenitor cell
- BMP2
bone morphogenetic protein-2
- ECM
extracellular matrix
- EEL
external elastic lamina
- Hh
hedgehog
- HSCs
hematopoietic stem cells
- Ptc
hedgehog receptor patched
- Sca1
stem cell antigen-1, also called Ly6A/E
- Shh
sonic hedgehog
- SMC
smooth muscle cell
- SVZ
subventricular zone
- VEGF
vascular endothelial growth factor
References
- Aguirre A., Rubio M.E., Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cell in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Biressi S., Rando T.A. Heterogeneity in the muscle satellite cell population. Semin Cell Dev Biol. 2010;21:845–854. doi: 10.1016/j.semcdb.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A.S., Conboy M.J., Roy S., Lee M., Kuo C.J., Keller C., Rando T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brownell I., Guevara E., Bai C.B., Loomis C.A., Joyner A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo P., Cesselli D., Al Haj Zen A., Beltrami A.P., Krankel N., Katare R., Angelini G., Emanueli C., Madeddu P. Human adult vena saphena contain perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell B.C., Collins F.S., Nabel E.G. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- Carlson M.E., Conboy I.M. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo A.B., Jacobs C.R. Mesenchymal stem cell mechanobiology. Curr Osteoporos Rep. 2010;8:98–104. doi: 10.1007/s11914-010-0015-2. [DOI] [PubMed] [Google Scholar]
- Chen X.D., Dusevich V., Feng J.Q., Manolagas S.C., Jilka R.L. Extracellular matrix made by bone marrow cells facilitates expansion to marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- Clowes A.W., Reidy M.A., Clowes M.M. Kinetics of cellular proliferation after arterial injury. 1. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy I.M., Rando T.A. The regulation of notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Shugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Day K., Shefer G., Shearer A., Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D.E., Mooney D.J., Zandestra P.W. Growth factors, matrices and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinane M., Mollmark J., Zagorchev L., Moodie K., Sun B., Hall A., Shipman S., Morganelli P., Simons M., Mulligan-Kehoe M.J. The antiangiogenic activity of rPAI-1(23) inhibits vasa vasorum and growth of atherosclerotic plaque. Circ Res. 2009;104:337–345. doi: 10.1161/CIRCRESAHA.108.184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley B., Palumbo C., Nalepka J., Molyneaux K. BMP signaling controls formation of a primordial germ cell niche within the early genital ridges. Dev Biol. 2010;343:84–93. doi: 10.1016/j.ydbio.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Wang Y.K., Yang M.T., Desai R.A., Yu X., Liu Z., Chen C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E., Kadl A., Sanders J., Varughese D., Sarembock I.J., Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U., DasGupta R., Degenstein L., Fuchs E. De novo hair follicle morphogenesis and hair tumore in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gopinath D.S., Rando T.A. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., Chen C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.C., Pannu H., Tran-Fadulu V., Papke C.L., Yu R.K., et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- Gutterman D.D. Adventitia-dependent influences on vascular function. Am J Physiol Heart Circ Physiol. 1999;277:1265–1272. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- Hahn C., Schwartz M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardouin S.N., Guo R., Romeo P.H., Nagy A., Aubin J.E. Impaired mesenchymal stem cell differentiation and osteoclastogenesis in mice deficient for Igf2-P2 transcripts. Development. 2011;138:203–213. doi: 10.1242/dev.054916. [DOI] [PubMed] [Google Scholar]
- Haurani M.J., Pagano P.J. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Heistad D.D., Marcus M.L., Larsen G.E., Armstrong M.L. Role of vasa vasorum in nourishment of the aortic wall. Am J Physiol Heart Circ Physiol. 1981;240:H781–H787. doi: 10.1152/ajpheart.1981.240.5.H781. [DOI] [PubMed] [Google Scholar]
- Holifield B., Helgason T., Jemelka S., Taylor A., Navran S., Allen J., Seidel C. Differentiated vascular myocytes: are they involved in neointimal formation. J Clin Invest. 1996;97:814–825. doi: 10.1172/JCI118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Chiba H., Nagai K., Ishii G., Ochiai A. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368:305–310. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- Howson K.M., Aplin A.C., Gelati M., Alessandri G., Parati E.A., Nicosia R.F. The postnatal rat aorta contains pericyte progenitor cells that for spheriodal colonies in suspension culture. Am J Physiol Heart Circ Physiol. 2005;289:C1396–C1407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- Hsu Y., Pasolli H.A., Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhang Z., Torsney E., Afzal A.R., Davison F., Metzler B., Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C., DasGupta R., Kocieniewski P., Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V., Forkert R., Fleming H.E., Saito Y., Waring M.T., Dombkowski D.M., Cheng T., DePinho R.A., Sharpless N.E., Scadden D.T. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jones D.L., Wagers A.J. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kelleher C.M., McLean S.E., Mecham R.P. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- Kerever A., Schnack J., Vellinga D., Ichikawa N., Moon C., Arikawa-Hirasawa E., Efird J.T., Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milleu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kimble J.E., White J.G. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kobielak K., Pasolli H.A., Alonso L., Polak L., Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E., Goderie S., Wang Y., Lotz L., Lin G., Sun Y., Roysam B., Shen Q., Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–273. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov V.A., Schwartz S.M., Berk B.C. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J., Ramsey M.R., Ligon K.L., Torrice C., Koh A., Bonner-Weir S., Sharpless N.E. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- Kuang S., Gillespie M.A., Rudnicki M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Le Grand F., Jones A.E., Seale V., Scime A., Rudnicki M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Chen S.J., Oparil S., Chen Y.F., Thompson J.A. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- Lie D.C., Colamarino S.A., Song H.J., Desire L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Mahoney W.M., Jr, Fleming J.N., Schwartz S.M. A unifying hypothesis for scleroderma: identifying a target cell for scleroderma. Curr Rheumatol Rep. 2011;13:28–36. doi: 10.1007/s11926-010-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M.W. Developmental basis for vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Majesky M.W., Dong X.R., Regan J.N., Hoglund V.J. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D.P., Kenagy R.D., Hasenstab D., Bowen-Pope D.F., Seifert R.A., Coats S., Hawkins S.M., Clowes A.W. Matrix metalloproteinase-9-overexpression enhances vascular smooth muscle cells migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayranpaa M.I., Trosien J.A., Fontaine V., Folkesson M., Kazi M., Eriksson P., Swedenborg J., Heldin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. [DOI] [PubMed] [Google Scholar]
- Moore K.A., Lemischka I.R. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Moulton K.S., Vakii K., Zurakowski D., Soliman M., Butterfield C., Sylvin E., Lo K.M., Gillies S., Javaherin K., Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atheroslclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M., Harten I., Mitchell R., Beers J.K., Djabali K., Cao K., Erdos M.R., Blair C., Funke B., Smoot L., Gerhard-Herman M., Machan J.T., Kutys R., Virmani R., Collins F.S., Wight T.N., Nabel E.G., Gordon L.B. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with vascular aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pasquinelli G., Tazzari P.L., Vaselli C., Foroni L., Buzzi M., Storci G., Alviano F., Ricci F., Bonafe M., Orrico C., Bagnara G.P., Stella A., Conte R. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- Passman J.N., Dong X.R., Wu S.P., Maguire C.T., Hogan K.A., Bautch V.L., Majesky M.W. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan, J.N. (2010) Regulation of Adventitia-Resident Progenitor Cells. Ph.D; dissertation, University of North Carolina at Chapel Hill, Chapel Hill.
- Reidy M.A., Clowes A.W., Schwartz S.M. Endothelial regeneration. 5. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983;49:569–575. [PubMed] [Google Scholar]
- Rey F.E., Pagano P.J. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol. 2002;22:1962–1971. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- Ritman E.L., Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Zediak V.P., Velez M., Bhandona A., Brown E.J. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;7:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S., Chiavegato A., Faggin E., Franch R., Puato M., Ausoni S., Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;81:46–53. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- Scadden D.T. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schwartz S.M., deBlois D., O'Brien E.R. The intima: soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- Scott N.A., Cipolla G.D., Ross C.E., Dunn B., Martin F.H., Simonet L., Wilcox J.N. Identification of a potential role for the adventitia in vascular lesion formation after overstretch balloon injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- Shattil S.J., Kim C., Ginsberg M.H. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Wang Y., Kolovay E., Lin G., Chuang S.M., Goderie S.K., Roysam B., Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.D., Bryant S.R., Couper L.L., Vary C.P., Gotwals P.J., Koteliansky V.E., Lindner V. Soluble transforming growth factor-β type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res. 1999;84:1212–2222. doi: 10.1161/01.res.84.10.1212. [DOI] [PubMed] [Google Scholar]
- Song X., Call G.B., Kirilly D., Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Spiegelman B.M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980;255:8811–8818. [PubMed] [Google Scholar]
- Spradling A., Drummond-Barbosa D., Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Swedenborg J., Maryanpaa M.I., Kovanen P.T. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:734–740. doi: 10.1161/ATVBAHA.110.213157. [DOI] [PubMed] [Google Scholar]
- Tang P.C., Qin L., Zielonka J., Zhou J., Matte-Martone C., Bergaya S., van Rooijen N., Shlomchik W.D., Min W., Sessa W.C., Pober J.S., Tellides G. MyD88-dependent superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med. 2008;205:3159–3171. doi: 10.1084/jem.20081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M., Van der Veken L., Silva-Vargas V., Loussaint M., Colonna L., Zaidi B., Garcia-Verdugo J.M., Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu B.C., Lee C., Sun H., LeJeune W., Recinos A., 3rd, Ju X., Spratt H., Guo D.C., Milewicz D., Tilton R.G., Brasier A.R. An adventitial IL6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney E., Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Wagenseil J.E., Ciliberto C.H., Knutsen R.H., Levy M.A., Kovacs A., Mecham R.P. The importance of elastin to aortic development in mice. Am J Physiol Heart Circ Physiol. 2010;299:H257–H264. doi: 10.1152/ajpheart.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil J.E., Mecham R.P. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P., Johansson B.R., Jukkola T., Breuer S., Akyurek L.M., Partanen J., Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- Watt F.M., Hogan B.L. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Wilens S.L., Malcolm J.A., Vasquez J.M. Experimental infarction (medial necrosis) of the dog's aorta. Am J Pathol. 1965;47:695–711. [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Beronja S., Pasolli H.A., Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin E., Chalajour F., Gehling U.M., Ito W.D., Treede H., Lauke H., Weil J., Reichenspurner H., Killic N., Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- Zhang J., Sun J., Lindholt J.S., Sukhova G.K., Sinnamon M., Stevens R.L., Adachi R., Libby P., Thompson R.W., Shi G.P. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Tang P.C., Qin L., Gayed P.M., Li W., Skokos E.A., Kyriakides T.R., Pober J.S., Tellides G. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med. 2010;207:1951–1966. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi P., Alpin A.C., Smith K.D., Nicosia R.F. The rat aorta contains resident mononuclear phagocytes with proliferative capacity and proangiogenic properties. J Leukoc Biol. 2010;88:1051–1059. doi: 10.1189/jlb.0310178. [DOI] [PMC free article] [PubMed] [Google Scholar]