SUMMARY
In periodontitis, an effective host-response is primarily related to neutrophils loaded with serine proteases, including elastase (NE) and protease 3 (PR3), which extracellular activity is tightly controlled by endogenous inhibitors. In vitro these inhibitors are degraded by gingipains, cysteine proteases produced by Porphyromonas gingivalis. The purpose of this study was to determine the level of selected protease inhibitors in gingival-crevicular fluid (GCF) in relation to periodontal infection. GCF collected from 31 subjects (9 healthy controls, 7 with gingivitis, 5 with aggressive periodontitis and 10 with chronic periodontitis was analyzed for the levels of elafin and secretory leukocyte protease inhibitor (SLPI), two main tissue-derived inhibitors of neutrophil serine proteases. In parallel, activity of NE, PR3, and arginine-specific gingipains (Rgps) in GCF was measured. Finally loads of P. gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia, and Treponema denticola were determined. The highest values of elafin were found in aggressive periodontitis and the lowest in controls. The quantity of elafin correlated positively with the load of P. gingivalis, T. forsythia, and T. denticola, as well as with Rgps activity. In addition, NE-activity was positively associated with the counts of those bacterial species, but not with amount of elafin. In contrast, the highest concentrations of SLPI were found in periodontally healthy subjects whereas amounts of this inhibitor were significantly decreased in patients infected with P. gingivalis. Periodontopathogenic bacteria stimulate the release of NE and PR3 which activities escape the control due to degradation of locally produced inhibitors (SLPI and elafin) by host- and bacteria-derived proteases.
Keywords: Porphyromonas gingivalis, periodontal disease, elafin, SLPI, neutrophil elastase, Proteinase 3
INTRODUCTION
In many respects pathogenesis of periodontal diseases results from an interaction of certain periodontal pathogens with host immune responses. Among the bacterial species being strongly associated with periodontitis, Aggregatibacter actinomycetemcomitans and bacteria of the „red complex“ (Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola) seem to play a major role in disease initiation and progression (1996; Borrell & Papapanou, 2005; Holt & Ebersole, 2005; Bamford et al., 2010). P. gingivalis is one of the major pathogens of severe chronic periodontitis (Lopez, 2000) but it can also be found in large numbers in patients with aggressive periodontitis (Takeuchi et al., 2001; Miura et al., 2005). Although a variety of virulence factors, including lipopolysaccharides, capsular material, and fimbriae are implicated in the pathogenicity of P. gingivalis (Sundqvist, 1993{Holt, 2005 #3; Holt & Ebersole, 2005), proteases are central to deterrence of host antimicrobial defenses by this bacterium (Potempa & Pike, 2009). Among several different types of proteolytic enzymes secreted by P. gingivalis (Potempa et al., 2003), cysteine proteases referred to as gingipains are of the main importance. While arginine-specific gingipains (RgpA and RgpB) are encoded by two genes (rgpA and rgpB), a lysine-specific enzyme is a product of a single gene (kgp) (Potempa et al., 2000). Acting alone or in concert gingipains are able to impair neutrophil function, manipulate the complement pathway, interfere with coagulation and kallikrein/kinin cascades, cleave immunoglobulins, inactivate endogenous protease inhibitors, as well as degrade the extracellular matrix proteins and bioactive peptides (Potempa & Pike, 2009; Guo et al., 2010).
A primary host-response to bacteria colonizing the subgingival tooth surface is infiltration of the gingival tissue and sulcus by large numbers of neutrophils (PMNs) (Garant, 2003), which constitute the main source of proteolytic activity and antimicrobial peptides, including α-defensins 1–4 and hCAP18/LL-37 (Gallo et al., 2002; RL Gallo; Murakami, 2002). The serine proteases, protease 3 (PR3), neutrophil elastase (NE) and cathepsin G are stored in primary granules and together with antimicrobial peptides are involved in non-oxidative killing of microorganisms (Korkmaz et al., 2008; Pham, 2008). Moreover, they participate in inflammation and destruction of periodontal tissues. E.g., both NE and PR3 are capable to increase production of interleukin-8 and monocyte chemoattractant protein 1 in gingival fibroblasts (Uehara et al., 2003), NE degrades periodontal ligament (Ujiie et al., 2007).
To maintain homeostasis in tissues activity of neutrophil proteases has to be tightly regulated by blood plasma- and tissue-derived protease inhibitors. In gingiva the main serine protease inhibitors produced locally include SLPI (secretory leukocyte protease inhibitor) and elafin (Williams et al., 2006). While SLPI inhibits mainly NE (Fritz et al., 1978; Bergenfeldt et al., 1992), elafin targets both NE and PR 3 (Zani et al., 2004). Recently, it has been shown that P. gingivalis induces the expression of protease inhibitors, including SPLI and elafin but in the same time it was noticed that these inhibitors can be degraded by proteolytic enzymes produced by this bacterium (Yin et al., 2010). Indeed, in more detailed study it was clearly shown that both RgpA and RgpB can efficiently abolish SLPI ability to inhibit NE (Into et al., 2006). Furthermore, all three gingipains (RgpA, RgpB, Kgp) were found to cleave elafin and inactivate its inhibitory activity, with RgpB being far more effective than other gingipains (Kantyka et al., 2009). Taken together these in vitro findings suggest that infections with P. gingivalis can exert a mutually opposite effect on the level of protease inhibitors in the inflamed gingival tissue. Therefore, the aim of this pilot study was to investigate a correlation between P. gingivalis counts in subgingival plaque as well as this bacterium-derived Rgps activity and the level of elafin and SLPI in GCF collected from periodontitis and gingivitis patients. Based in vitro degradation of elafin and SLPI by Rgps (Into et al., 2006; Kantyka et al., 2009) we hypothesize that the level of the inhibitors in GCF will inversely correlate with the presence of P. gingivalis and Rgp activity. Furthermore, since the inhibitor presence should have a bearing on neutrophil protease activity, we have also determined the level of NE and PR3 activity in GCF.
METHODS
Subject recruitment
Thirty one subjects were recruited from patients of the Department of Conservative Dentistry, University Hospital of Jena. The definition of aggressive and chronic periodontitis was based on the classification system of the “International Workshop for a Classification System of Periodontal diseases and Conditions” from 1999 (Armitage, 1999). Subjects suffering from systemic disease (e.g. diabetes mellitus, cancer or coronary heart disease), or on antibiotic therapy within the last 6 months and pregnant, or lactating females were excluded. A further exclusion criterion was a periodontitis treatment within the last two years. Study was made in agreement with the guidelines of the Helsinki Declaration, revised in 2008. Ethical approval was obtained from local ethics committee of the University of Jena. A written informed consent was obtained from each subject prior to participation.
Sampling of gingival crevicular fluid (GCF)
Samples were collected in the morning, 2–3 h after breakfast from the deepest site per quadrant. The sites to be sampled were isolated with cotton rolls and gently air-dried. Crevicular washes were obtained using a previously described method (Sigusch et al., 1992; Guentsch et al., 2011). A capillary tip was carefully inserted into the crevice at a level of approximately 1 mm below the gingival margin. At each site, three sequential washes with 10 µL of 0.9% sodium chloride were performed using a micropipette. The washes of one patient were pooled and transferred into a microcentrifuge tube, immediately frozen and kept at – 20°C until analysis.
Enzyme activities of neutrophil elastase and proteinase 3
Enzyme activity of NE was determined by measuring the rate of release of p-nitroanilide (p-NA) from N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide (MeSuc-AAPV-pNA) used as substrate (Sigma, Munich, Germany). The assay was performed in total volume of 150 µL with 10 µL of the GCF sample and 0.75 mM final substrate concentration in 50 mM Tris-HCl, pH 7.5. The rate of pNA released was recorded at 405 nm using a Spectromax 250 (Molecular Devices Corp., Sunnyvale, USA) for 30 min.
PR3 activity was determined using Abz-GVADnVADYQ-Y(NO2)-D as a substrate at final concentration of 50 µM in 0.1 M Tris-HCl, 5 mM EDTA, 0.15 M NaCl, 0.05% Tween-20, 5% dimethylforamide, pH 7.5, added to 10 µL GCF. Substrate hydrolysis was measured as an increase of fluorescence at λex = 320nm and λem = 420nm for 3 h at 37°C using a Spectramax GEMINI XS (Molecular Devices Corp., Sunnyvale, USA).
The activities of NE and PR3 in GCF are expressed as increase in absorbance per minute, or increase in relative fluorescence per minute, respectively.
Microbiology of periodontal pathogens
The DNA was extracted by using a DNA extraction system (A&A Biotechnology, Gdynia, Poland) from 5 µL of the GCF wash according to recommendations of the manufacturer. Periodontopathogens were determined by using micro-IDent® (Hain Lifescience, Nehren, Germany) according to the manufacturer’s instruction. In short, PCR amplification was carried out in a reaction volume of 25 µL consisting of 2.5 µL of template DNA and 22.5 µL of reaction mixture containing 17.5 µL of primer–nucleotide mix (micro-IDent®), 2.5 µL of 10 × PCR buffer, 2.5 µL of 25 mm MgCl2 and 1 U Taq polymerase (Fermentas Life Science, St. Leon-Rot, Germany). PCR cycling was carried out in a Mastercycler (Eppendorf, Hamburg, Germany). The cycling conditions comprised an initial denaturation step at 95°C for 5 min, 10 cycles at 95°C for 30 s and at 60°C for 2 min, 20 cycles at 95°C for 10 s, at 55°C for 30 s and at 72°C for 30 s, and a final extension step at 72°C for 10 min. In the subsequent reverse hybridization, the biotinylated amplicons were denatured and incubated at 45°C with hybridization buffer and strips coated with two control lines and five species-specific probes. After PCR products was bound to their respective complementary probe, a highly specific washing step removed any none specifically bound DNA. Streptavidin conjugated alkaline phosphatase was added, the samples were washed and hybridization products were visualized by adding a substrate for alkaline phosphatase. Then, the intensity of the band was measured as described recently (Eick et al., 2011). In addition, real-time PCR was used to quantify the level of P. gingivalis by addition of primers described by Ashimoto et al. (Ashimoto et al., 1996) and the GoTaq(R) qPCR Master Mix (Promega AG, Dübendorf, Switzerland) and using 7500 Real time PCR System (Applied Biosystem-Life Technologies, Carlsbad, CA, USA). The positive control was P. gingivalis ATCC 33277 in the range of 102 – 107 cells/sample.
Activity of arginine specific gingipains (Rgps)
The activities of Rgps in the GCF were determined using the chromogenic substrate NBenzoyl-l-Arginine-p-Nitroanilide (BApNA) (Sigma, St. Louis, MO, USA). Ten µL of GCF samples were pre-incubated in 200 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2, pH 7.6, supplemented with 10 mM cysteine, for 5 min at 37°C and assayed for amidase activity with 0.5 mM substrate in the total volume of 200 µL. The release of p-nitroanilide was monitored spectrophotometrically at 405 nm, as described above.
Determination of protease inhibitors
The amount of SLPI within the GCF was determined using ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instruction, GCF samples were diluted 1 : 100 before being applied to the microplate. The detection level of the kit was 100 pg/ml.
For determination of elafin, GCF samples were diluted 4 times with sample buffer (0.125 mM Tris-HCl, 20% glycerol, 4% SDS), and resolved by SDS-PAGE (15% acrylamide) using the Tris-Tricine discontinuous buffer system (Schagger et al., 1988). As the reference 20 pg elafin (kind gift of Proteo Biotech AG Kiel, Germany) was always run on a gel. Western blot was performed by electrotransfer of SDS-PAGE resolved proteins onto a PVDF membrane, followed by overnight blocking with 2% 0.22 µm-filtered bovine serum albumin in Tris-buffered saline with 0.05% Tween 20 (TTBS). Blocked membrane was incubated with primary antibody (1:500, biotinylated goat anti-human Trappin-2; BAF-1747; R&D, Wiesbaden-Nordenstadt, Germany), then washed 3 times with TTBS and streptavidin-HRP was applied for 1 h (1:20,000, RPN-1231; GE Healthcare, Munich, Germany). ECL + (GE Healthcare) was used as the chemoluminescence substrate according to the manufacturer`s instruction and membranes were exposed to x-ray films (Kodak).
Data analysis
The clinical data were expressed as means ± standard deviation (SD). Laboratory variables are presented as median including quartiles. Groups were compared with Kruskal-Wallis and Mann-Whitney tests. The correlation between tested variables was made using Spearman-test. Statistical software (PASW 18.0, SPSS, Chicago, IL, USA) was used for all statistical analyses.
RESULTS
Clinical data
Nine periodontally healthy subjects, 7 gingivitis patients, 10 patients with chronic periodontitis (CP) and 5 with aggressive periodontitis (AP) were recruited and participated in the study. The study group consisted of 15 women and 16 men; the mean age of all participants was 39.4±10.2 years. Demographic and clinical data characterizing the patients’ groups are summarized in Table 1. The patients in the aggressive periodontitis group were with a mean age of 39.4±8.6 years, which is higher than normal from AP. All included patients had the generalized form of AP and the beginning of the disease was registered first before the age of 35 years. Periodontal destruction characterized by the mean pocket depths of 4.40 – 4.67 mm was found in the periodontitis groups. A high bleeding on probing (BoP) incidence as a sign of inflammation was also detected in both periodontitis and gingivitis groups.
Table 1.
Demographic and clinical data
| Control Group n=9 |
Gingivitis n=7 |
Chronic Periodontitis n=10 |
Aggressive Periodontitis n=5 |
|
|---|---|---|---|---|
| Age (mean ± SD) | ||||
| (years) | 33.2 ± 9.8 | 38.6 ± 7.8 | 45.6 ± 12.9 | 39.4 ± 8.6 |
| Gender (m:f) | 5:4 | 3:4 | 5:5 | 3:2 |
| PD (mean ± SD) (mm) | 1.65 ± 0.37 | 2.23 ± 0.78 | 4.40 ± 0.75 | 4.67 ± 0.88 |
| BoP (mean ± SD) (%) | 7.20 ± 10.65 | 80.34 ± 9.88 | 90.56 ± 18.05 | 89.67 ± 10.54 |
| Teeth (mean ± SD) | 27.80 ± 2.23 | 28.33 ± 1.63 | 27.02 ± 2.36 | 27.31 ± 2.43 |
Bacterial species associated with periodontitis
Selected bacterial species, which are associated with periodontal inflammation (P. gingivalis, T. forsythia, T. denticola, A. actinomycetemcommitans, and P. intermedia), were not found in periodontally healthy controls (Table 2). High counts of A. actinomycetemcomitans were detectable in two cases (40%) of AP and in two cases (20%) of CP. In one case of AP the very high counts of A. actinomycetemcomitans were associated with none of the other four investigated pathogens. Whereas none of the pathogens was detectable in the control group, all pathogens had been detected in the gingivitis, CP and AP groups. In the groups showing signs of periodontal inflammation (AP, CP, gingivitis), T. denticola was prevalent (about 60% cases). T. forsythia was detected in at least 80% of the cases. P. intermedia was only rarely present (Table 2).
Table 2.
Detection of periodontopathogens within groups by using semi-quantitative strip technology
| Controls n=9 |
Gingivitis n=7 |
Chronic Periodontitis n=10 |
Aggressive Periodontitis n=5 |
|
|---|---|---|---|---|
| P.gingivalis | ||||
| Positive | 0 (0%) | 4 (57%) | 9 (90%) | 4 (80%) |
| High load* | 0 (0%) | 2 (29%) | 3 (30%) | 2 (40%) |
| T. forsythia | ||||
| Positive | 0 (0%) | 6 (86%) | 8 (80%) | 4 (80%) |
| High load* | 0 (0%) | 3 (43%) | 0 (0%) | 1 (20%) |
| T. denticola | ||||
| Positive | 0 (0%) | 4 (57%) | 6 (60%) | 3 (60%) |
| High load* | 0 (0%) | 2 (29%) | 0 (0%) | 1 (20%) |
| A. actinomycetemcomitans | ||||
| Positive | 0 (0%) | 1 (14%) | 2 (20%) | 2 (40%) |
| High load* | 0 (0%) | 0 (0%) | 2 (20%) | 2 (40%) |
| P. intermedia | ||||
| Positive | 0 (0%) | 1 (14%) | 1 (10%) | 1 (20%) |
| High load* | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
high load was defined as an intensity of the band of ≥50% in relation to the hybridization control
P. gingivalis was detected in the half of gingivitis patients and in more than 80% of the periodontitis patients-derived samples. Qualitatively, all samples found positive by using the hybridization-based strip-technology were shown containing a given pathogen using the real-time PCR technique. Similarly, negative samples with the first technique were confirmed negative with the other. Quantitatively, the correlation coefficient R for detection of pathogens using both methods was 0.952 (p<0.001). However, because only real-time PCR allows counting bacterial cells within GCF, quantitative results obtained by this technique are discussed in the follow up text. Accordingly, the highest numbers of P. gingivalis were determined in GCF samples from the AP group (median 1.07×106), followed by the CP group (median 2.27 × 105) and the gingivitis group (median 1.40×103; Fig 1).
Figure 1.
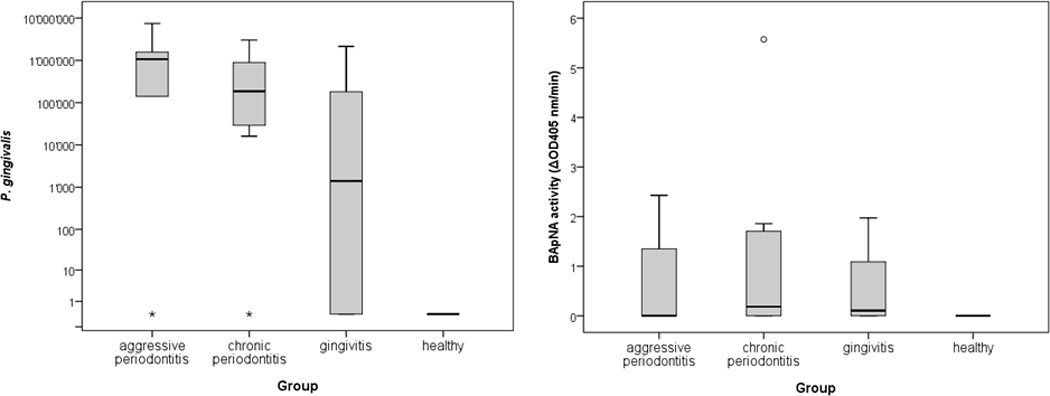
Counts of P. gingivalis (median and 25 and 75 percentiles) and Rgps activity (median and 25 and 75 percentiles) determined by the release of p-nitroanalide from BApNA in GCF obtained from patients with AP (aggressive periodontitis), (CP) chronic periodontitis, and gingivitis as well as from periodontally healthy subjects
The arginine specific amidolytic activity was the highest in the CP group with a median of 0.18 U, the value significantly higher in comparison to the Rgps activity in controls (p=0.024; Fig 1).
Activities of serine-proteases
There was low variation in the level of the NE activity within individual groups of patients. Whereas the highest activity was detected in the gingivitis group, slightly lesser activities have been determined in samples obtained from CP and AP subjects. Again, the activity in periodontally healthy subjects was significantly (p=0.039) lower than in gingivitis patients (Fig 2).
Figure 2.
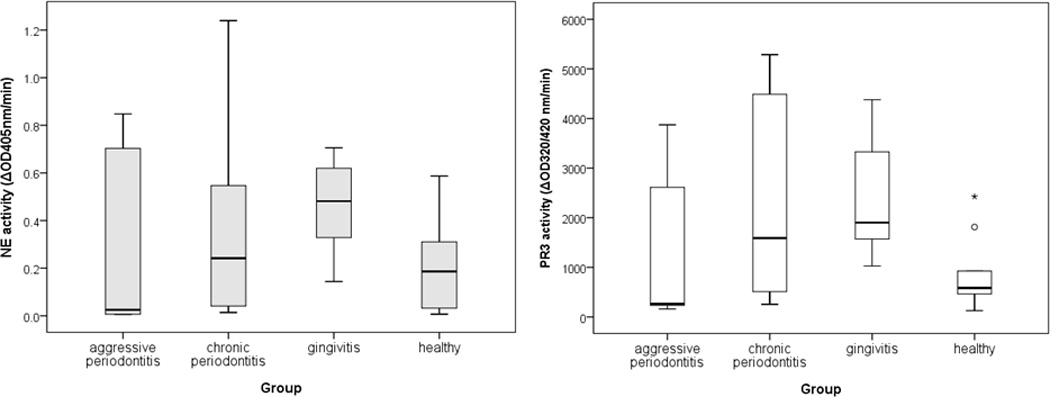
Activities of the neutrophil serine proteases, PR3 determined by using fluorogenic substrate (Abz-GVADnVADYQ-Y(NO2)-D) and NE by using chromogenic substrate (N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide) in GCF obtained from patients with AP (aggressive periodontitis), CP (chronic periodontitis), and gingivitis as well as from periodontally healthy subjects
The highest activity of PR3 was detected in gingivitis patients, followed by CP and AP patients. Periodontally healthy subjects showed a generally low activity, significantly lower (p=0.007) than in gingivitis patients.
Levels of protease inhibitors
SLPI was found to be present in very high concentrations in periodontally healthy subjects. The detectable amount of SLPI was lower in all patients’ groups characterized by gingival inflammation. The difference between periodontally healthy subjects and CP patients was statistically significant (p=0.043) (Fig 3).
Figure 3.
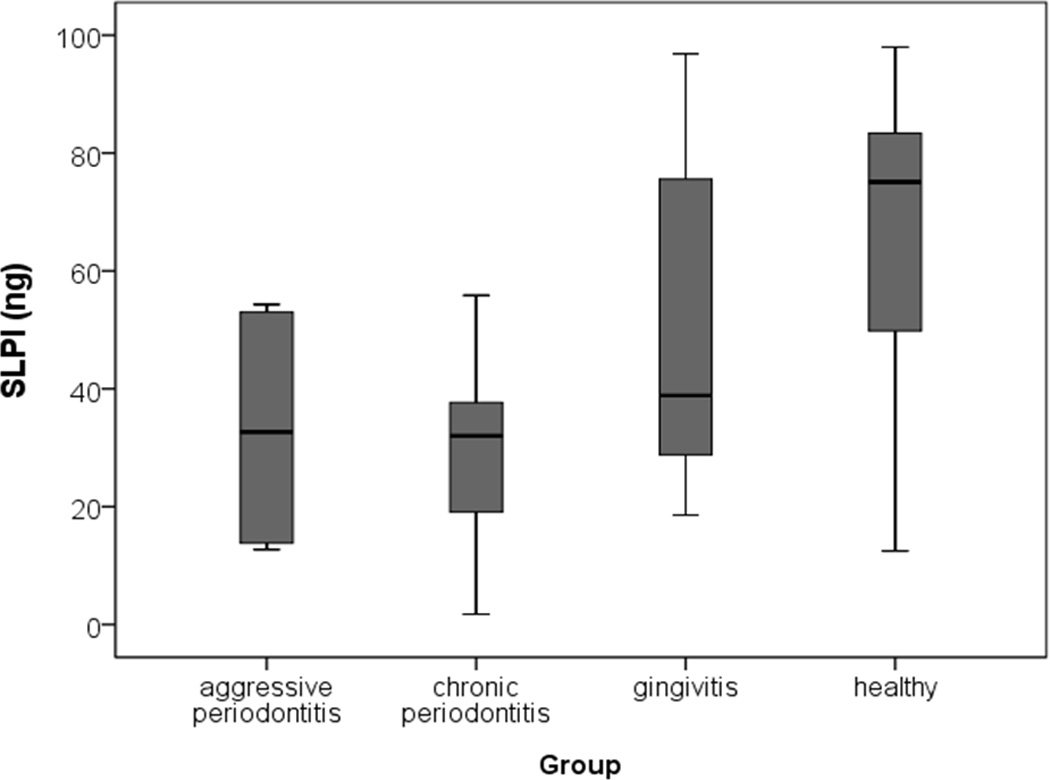
Level of SLPI determined by using ELISA in GCF obtained from patients with AP (aggressive periodontitis), CP (chronic periodontitis), and gingivitis as well as from periodontally healthy subjects.
The levels of elafin have been determined by using specific antibodies and Western blot technique. As expected from the fact that in vivo elafin is secreted in the higher molecular form referred to as trappin and is usually covalently linked to connective tissue proteins (Guyot et al., 2005), the molecular weight of the detected immunoreactive bands in GCF was higher than that of free elafin. This result confirms that also in the gingival tissue elafin occurs in the form linked to extracellular matrix components.
For quantification of elafin content in GCF all immunoreactive bands were scanned and their total intensity was used for calculation. The amount of elafin differed between the groups (p=0.001). Elafin was found in significantly highest quantities in AP patients than in patients diagnosed with CP (p=0.040) and gingivitis (p=0.042). The most profound difference was observed between AP patients and healthy controls (p=0.001). The level of elafin did not differ significantly between CP and gingivitis patients. In both these groups quantities of the detected inhibitor were higher in comparison to control subjects (p=0.008 and p=0.016 respectively) (Fig 4).
Figure 4.
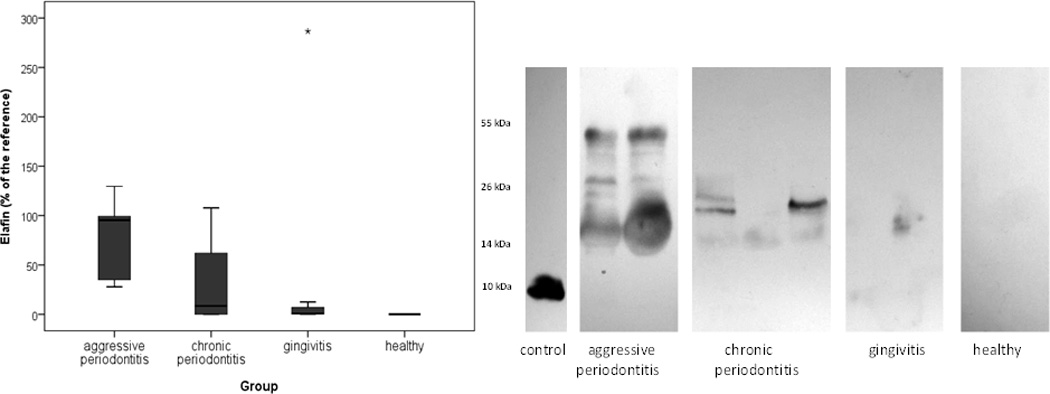
Level of elafin determined using densitometry after Western blot technique according to the reference (20 pg elafin) in GCF obtained from patients with AP (aggressive periodontitis), CP (chronic periodontitis), and gingivitis as well as from periodontally healthy subjects and examples of Western blots
Associations between serine proteases, inhibitors of serine proteases and P. gingivalis
Out of total 31 analyzed GCF samples 17 (54.8%) were tested positive for P. gingivalis. The neutrophil protease activities were higher in the P. gingivalis-positive group than in P. gingivalis-negative group but the difference was statistically significant only for the PR3 activity (p=0.011). Interestingly, the elafin level was significantly higher in P. gingivalis infected patients in comparison to non-infected ones (p=0.002) while SLPI levels were inversely correlated (p=0.026) with the P. gingivalis presence (Fig 5).
Figure 5.
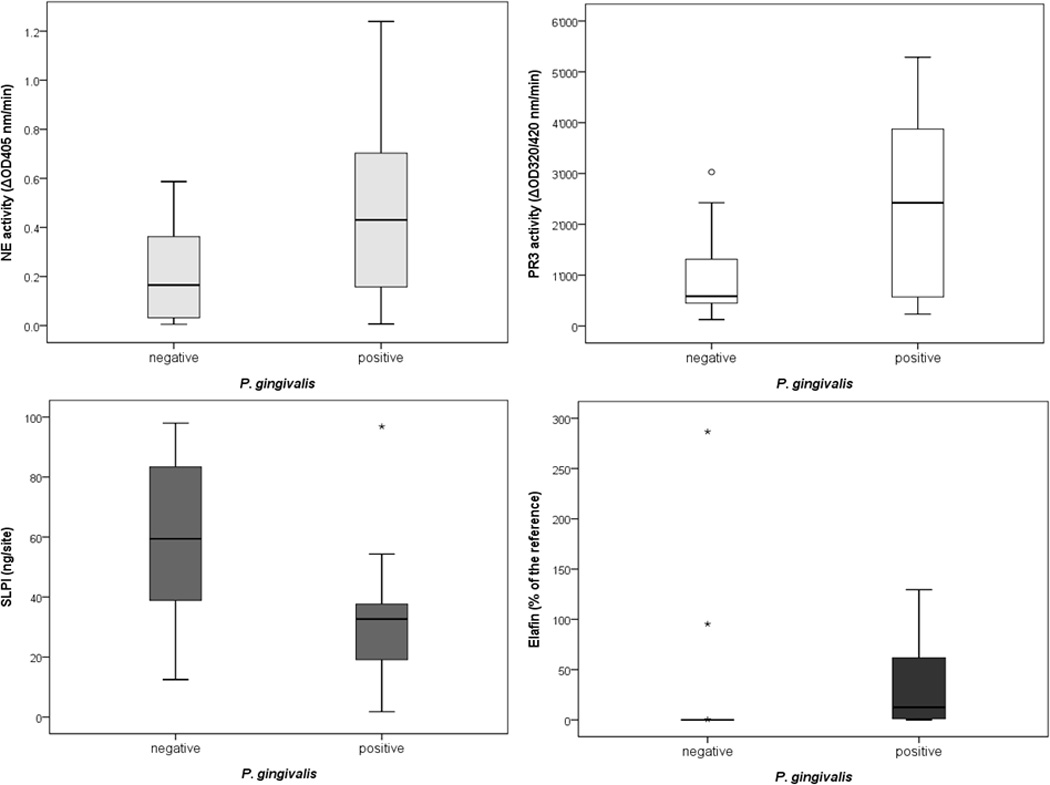
Activities of the neutrophil serine proteases, PR3 and NE, as well as the levels of the protease inhibitors SLPI and elafin in GCF obtained from patients being tested negatively and positively for P. gingivalis.
These findings are further supported by the strong correlation between the counts of P. gingivalis and the Rgps activity in GCF. Furthermore, P. gingivalis counts and the arginine-specific amidolytic activity showed a positive correlation with activities of neutrophil serine proteases and, remarkably, also with elafin. The concentration of elafin was inversely correlated with the level of SLPI. Also, the association between the load of P. gingivalis and SLPI has a tendency to be inverse but without reaching the significance. Finally, it should be noted that the activities of PR3 and NE strongly correlated one with another. In contrast, no association between the protease activities with the levels of SLPI and elafin was registered (Table 3).
Table 3.
Significant Correlations (Spearman) between different variables within GCF
| Coefficient R | P | |
|---|---|---|
| Elafin | ||
| SLPI | −0.378 | 0.036 |
| P. gingivalis | 0.618 | <0.001 |
| BApNA | 0.643 | <0.001 |
| Neutrophil elastase activity | ||
| Proteinase 3 activity | 0.790 | <0.001 |
| P. gingivalis | 0.493 | 0.013 |
| BApNA | 0.678 | <0.001 |
| Proteinase 3 activity | ||
| P. gingivalis | 0.496 | 0.005 |
| BApNA | 0.825 | <0.001 |
| P. gingivalis | ||
| BApNA | 0.726 | <0.001 |
The activities of NE and PR3, as well as the level of elafin positively correlated with loads of T. forsythia and T. denticola (correlation coefficient between 0.406 and 0.714; p<0.05 each). Conversely, A. actinomycetemcomitans was negatively correlated with SLPI (R=−0.497; p=0.004).
Discussion
In this pilot study four different groups of patients were included. Clinical parameters did not differ between the two periodontitis groups. In addition BoP values, an accepted measure of gingival inflammation, were similar in gingivitis and periodontitis patients. Among analyzed groups the neutrophil serine proteases activity was the highest in gingivitis. The finding of the higher NE activity in gingivitis than in periodontitis patients contests the data presented in other report (Figueredo et al., 2005). The discrepancy may result partially from different methods used to collect GCF, which were shown to affect NE recovery from sampling device (Guentsch et al., 2011). Alternatively, a design of this study as a pilot one with relatively low number of subjects may weaken differences between the groups. Results of several studies suggested that the NE presence and/or activity in GCF from discrete periodontitis sites can be used to identify differences in disease severity within patients and to determine success of periodontal treatment (Binder et al., 1987; Lamster et al., 1988; Yin et al., 2010). The PR3 activity has not yet been determined in gingival fluid. Therefore the finding that the PR3 activity correlates strongly with the NE activity suggests this enzyme as a suitable biomarker for disease severity and progression. The enzymes release due to neutrophil lysis during GCF freezing is rather unlikely to contribute to this correlation since cathepsin G, NE and PR3 are stored in azurophilic granules in tight association with proteoglycans and their release requires high ionic strength (Reeves et al., 2002).
In vivo activity of NE and PR3 escaped from neutrophils during these cells degranulation and NET formation or due to frustrated phagocytosis and necrosis (Fox et al., 2010) are instantly quenched by endogenous inhibitors. SLPI and elafin are two inhibitors produced locally in tissues. Although SLPI is expressed in macrophages (Mihaila & Tremblay, 2001) this protein is predominantly made in epithelial cells (van Wetering et al., 2000), including gingival epithelial cells (Yin et al., 2010). In epithelial cells, SLPI is constitutively expressed (Sallenave et al., 1994), with the level of expression stimulated by lipopolysaccharide, lipoteichonic acid, interleukin (IL)-6, IL-10 (Jin et al., 1998), IL-1β, tumor necrosis factor (TNF)α (Sallenave et al., 1994). Also, epithelia cell interaction with P. gingivalis enhances SLPI synthesis through yet unknown mechanism (Yin et al., 2010). It the same time it is known that Arg-gingipains are able to cleave SLPI (Into et al., 2006; Yin et al., 2010). In vitro at concentrations of 100 nM Rgps totally degraded SLPI whereas at 10 nM already eliminated SLPI ability to inhibit NE (Into et al., 2006). In the light of recent findings that the GCF concentration of Arg-gingipains is up to 1500 nM, with the median about 58 nM at P. gingivalis positive sites (Guentsch et al., 2011), it is expected that SLPI can also be degraded or at least inactivated in vivo. In keeping, here we found a low level of SLPI in GCF from patients being positive for P. gingivalis in agreement with the previous finding (Into et al., 2006). Unexpectedly this inverse correlation between SLPI and P. gingivalis was not as strong as that between A. actinomycetemcomitans counts and the SLPI level. It may be assumed that leukotoxin and cytolethal distenting toxin produce by A. actinomycetemcomitans (Mayer et al., 1999; Kachlany, 2010; Fong et al., 2011) inhibit expression of SLPI. Finally, SLPI sensitivity to degradation by host-derived proteases such as cathepsins B, L and S (Taggart et al., 2001) may compound the correlation. Indeed, it is known that active cathepsin B occurs at the high level in GCF of periodontitis patients (Ichimaru et al., 1996). Taken together it is very likely that host and pathogen derived proteases, together with yet unknown factors that may inhibit SLPI expression, contribute to significant depletion of this inhibitor in the infected periodontitis sites.
Similar to SLPI, elafin is also mainly expressed in epithelial cells (van Wetering et al., 2000; Yokota et al., 2007; Lee et al., 2009) in a manner significantly stimulated by chronic inflammatory conditions, e.g. chronic sinusitis (Lee et al., 2009). Consistently, we detected high levels of elafin in patients with periodontitis and gingivitis. Interestingly, we found a strong positive correlation between elafin levels and P. gingivalis counts, as well as elafin and the Arg-gingipain activity. Especially the latter correlation seems to be at odds with Rgps ability to cleave and efficiently inactivate elafin in vitro (Kantyka et al., 2009).
Elafin is the extremely stable protein resistant to proteolytic degradation (Guyot et al., 2010). Nevertheless, Rgps exert limited proteolysis of a single peptide bond within the active site loop of elafin. The cleavage efficiently inactivates protein inhibitory activity but does not abolish its recognition by antibodies in western blot analysis in non-reducing conditions (Kantyka et al., 2009). While proteolytic inactivation of elafin occurs at subnanomolar Rgps concentrations, visible degradation of the inhibitor requires 100 nM enzyme concentrations (Kantyka et al., 2009). This inefficiency of elafin degradation correlates with the absence of elafin degradation products in analyzed GCF samples. On the other hand, taking into account Rgps concentration in GCF (Guentsch et al., 2011) at least 100-fold higher than required to cleave the elafin’s reactive loop, it is highly implausible that the inhibitor detected in GCF is active.
Our western blot analysis has clearly shown that elafin in GCF occurs in high molecular mass forms apparently representing elafin in the form of trappin-2 conjugated to fragments of extracellular matrix proteins (Guyot et al., 2005; Baranger et al., 2011). In this context it is important to reiterate that in vivo elafin occurs predominantly as trappin-2 immobilized in a meshwork of the extracellular matrix (ECM) by the action of a type 2 transglutaminase (Guyot et al., 2005). Immobilization of elafin prevents diffusion of the inhibitor from the location it is most needed to protect fragile matrix proteins from proteolytic degradation by the neutrophil-derived serine proteases.
Finally, the striking correlation between elafin and P. gingivalis/Rgps activity points at gingipains as a main sheddases of ECM-linked inhibitor. In keeping, gingipains can easily degrade fibronectin, the protein abundant in gingiva (Talonpoika, 1991; Figueredo & Gustafsson, 2000), which is the main anchorage ECM protein for trappin (Guyot et al., 2005). It is also likely that elafin is released by fibronectin degradation by proteases of T. forsythia and T. denticola (Bamford et al., 2010) since loads of these highly proteolytic species correlates with elafin levels in GCF. The contention that periodontal pathogen-derived proteases, including gingipains, can work as elafin sheddeses is corroborated by the presence of fibronectin fragments of 40 kDa, 68 kDa and 120 kDa in GCF which quantity increased with disease severity (Huynh et al., 2002).
Taken together based on our analysis of GCF content the following scenario can be suggested. Results of ex vivo studies (Yin et al., 2010) strongly suggest that in inflamed infected periodontal/gingival tissues expression of both SPLI and elafin is increased. While soluble SPLI is degraded in situ by bacteria and host-derived proteases, the ECM conjugated elafin, although resists proteolytic degradation, it can be inactivated by limited proteolysis at the inhibitory reactive site loop (Kantyka et al., 2009). The inhibitory capacity of any intact elafin and any intact SPLI is then saturated and neutrophil serine proteases are free to exert their broad range of biological activities. In addition this will destroy other functions of inhibitors, such as mediation of wound healing by SLPI (Ashcroft et al., 2000), chemoattractant and opsonin activity of elafin (Huynh et al., 2002), and both inhibitors antibacterial activity as well as ability to suppresses host response to LPS (Talonpoika et al., 1991; Samsom et al., 2007); (Hiemstra et al., 1996; Baranger et al., 2008). Apart from releasing neutrophil proteases from control, potential abrogation of the immunomodulatory functions of these inhibitors by bacterial proteases may profoundly contribute to severity and progression of the periodontal disease.
Acknowledgement
Most the study was founded by the participating departments. The authors acknowledge the support of Oliver Laugisch by the German Academic Exchange Service (grant No: 314-D/08/48763) and a grant for a foreign training by the German Society of Periodontology. In addition, this study was partially supported by grants from European Community (FP7-HEALTH-2010-261460 “Gums&Joints” and Marie Curie ITN-290246 “RAPID”), Foundation for Polish Science (TEAM project DPS/424-329/10), and the National Institutes of Health, USA (Grant DE 09761). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union (grant No: POIG.02.01.00-12-064/08 – “Molecular biotechnology for health”).
Footnotes
The authors declare that there are no conflicts of interest in this study.
References
- Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Lei K, Jin W, et al. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Bamford CV, Francescutti T, Cameron CE, Jenkinson HF, Dymock D. Characterization of a novel family of fibronectin-binding proteins with M23 peptidase domains from Treponema denticola. Mol Oral Microbiol. 2010;25:369–383. doi: 10.1111/j.2041-1014.2010.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranger K, Zani ML, Chandenier J, Dallet-Choisy S, Moreau T. The antibacterial and antifungal properties of trappin-2 (pre-elafin) do not depend on its protease inhibitory function. FEBS J. 2008;275:2008–2020. doi: 10.1111/j.1742-4658.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- Baranger K, Zani ML, Labas V, Dallet-Choisy S, Moreau T. Secretory leukocyte protease inhibitor (SLPI) is, like its homologue trappin-2 (pre-elafin), a transglutaminase substrate. PLoS One. 2011;6:e20976. doi: 10.1371/journal.pone.0020976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenfeldt M, Axelsson L, Ohlsson K. Release of neutrophil proteinase 4(3) and leukocyte elastase during phagocytosis and their interaction with proteinase inhibitors. Scand J Clin Lab Invest. 1992;52:823–829. doi: 10.3109/00365519209088387. [DOI] [PubMed] [Google Scholar]
- Binder TA, Goodson JM, Socransky SS. Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res. 1987;22:14–19. doi: 10.1111/j.1600-0765.1987.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32 Suppl 6:132–158. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Eick S, Straube A, Guentsch A, Pfister W, Jentsch H. Comparison of real-time polymerase chain reaction and DNA-strip technology in microbiological evaluation of periodontitis treatment. Diagn Microbiol Infect Dis. 2011;69:12–20. doi: 10.1016/j.diagmicrobio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Figueredo CM, Fischer RG, Gustafsson A. Aberrant neutrophil reactions in periodontitis. J Periodontol. 2005;76:951–955. doi: 10.1902/jop.2005.76.6.951. [DOI] [PubMed] [Google Scholar]
- Figueredo CM, Gustafsson A. Increased amounts of laminin in GCF from untreated patients with periodontitis. J Clin Periodontol. 2000;27:313–318. doi: 10.1034/j.1600-051x.2000.027005313.x. [DOI] [PubMed] [Google Scholar]
- Fong KP, Tang HY, Brown AC, et al. Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Mol Oral Microbiol. 2011;26:262–276. doi: 10.1111/j.2041-1014.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S, Leitch AE, Duffin R, Haslett C, Rossi AG. Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun. 2010;2:216–227. doi: 10.1159/000284367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H, Schiessler H, Gieger R, Ohlsson K, Hochstrasser K. Naturally occurring low molecular weight inhibitors of neutral proteinases from PMN-granulocytes and of kallikreins. Agents Actions. 1978;8:57–64. doi: 10.1007/BF01972403. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- Garant P. Oral cells and tissues. Carol Stream, IL: Quintessence Publishing Co Inc.; 2003. [Google Scholar]
- Guentsch A, Kramesberger M, Sroka A, et al. Comparison of Gingival Crevicular Fluid Sampling Methods in Patients With Severe Chronic Periodontitis. J Periodontol. 2011 doi: 10.1902/jop.2011.100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot N, Bergsson G, Butler MW, et al. Functional study of elafin cleaved by Pseudomonas aeruginosa metalloproteinases. Biol Chem. 2010;391:705–716. doi: 10.1515/BC.2010.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot N, Zani ML, Maurel MC, Dallet-Choisy S, Moreau T. Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase. Biochemistry. 2005;44:15610–15618. doi: 10.1021/bi051418i. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS, Maassen RJ, Stolk J, et al. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Huynh QN, Wang S, Tafolla E, et al. Specific fibronectin fragments as markers of periodontal disease status. J Periodontol. 2002;73:1101–1110. doi: 10.1902/jop.2002.73.10.1101. [DOI] [PubMed] [Google Scholar]
- Ichimaru E, Tanoue M, Tani M, et al. Cathepsin B in gingival crevicular fluid of adult periodontitis patients: identification by immunological and enzymological methods. Inflamm Res. 1996;45:277–282. doi: 10.1007/BF02280991. [DOI] [PubMed] [Google Scholar]
- Into T, Inomata M, Kanno Y, et al. Arginine-specific gingipains from Porphyromonas gingivalis deprive protective functions of secretory leucocyte protease inhibitor in periodontal tissue. Clin Exp Immunol. 2006;145:545–554. doi: 10.1111/j.1365-2249.2006.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F, Nathan CF, Radzioch D, Ding A. Lipopolysaccharide-related stimuli induce expression of the secretory leukocyte protease inhibitor, a macrophage-derived lipopolysaccharide inhibitor. Infect Immun. 1998;66:2447–2452. doi: 10.1128/iai.66.6.2447-2452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res. 2010;89:561–570. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantyka T, Latendorf T, Wiedow O, et al. Elafin is specifically inactivated by RgpB from Porphyromonas gingivalis by distinct proteolytic cleavage. Biol Chem. 2009;390:1313–1320. doi: 10.1515/BC.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Lamster IB, Oshrain RL, Fiorello LA, Celenti RS, Gordon JM. A comparison of 4 methods of data presentation for lysosomal enzyme activity in gingival crevicular fluid. J Clin Periodontol. 1988;15:347–352. doi: 10.1111/j.1600-051x.1988.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Lee CW, Kim TH, Lee HM, et al. Upregulation of elafin and cystatin C in the ethmoid sinus mucosa of patients with chronic sinusitis. Arch Otolaryngol Head Neck Surg. 2009;135:771–775. doi: 10.1001/archoto.2009.97. [DOI] [PubMed] [Google Scholar]
- Lopez NJ. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J Periodontol. 2000;71:948–954. doi: 10.1902/jop.2000.71.6.948. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bueno LC, Hansen EJ, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaila A, Tremblay GM. Human alveolar macrophages express elafin and secretory leukocyte protease inhibitor. Z Naturforsch C. 2001;56:291–297. doi: 10.1515/znc-2001-3-420. [DOI] [PubMed] [Google Scholar]
- Miura M, Hamachi T, Fujise O, Maeda K. The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitis. J Periodontal Res. 2005;40:147–152. doi: 10.1111/j.1600-0765.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Murakami MO T, Zaiou M. Biology and clinical relevance of naturally occuring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 1994;11:733–741. doi: 10.1165/ajrcmb.11.6.7946401. [DOI] [PubMed] [Google Scholar]
- Samsom JN, van der Marel AP, van Berkel LA, et al. Secretory leukoprotease inhibitor in mucosal lymph node dendritic cells regulates the threshold for mucosal tolerance. J Immunol. 2007;179:6588–6595. doi: 10.4049/jimmunol.179.10.6588. [DOI] [PubMed] [Google Scholar]
- Schagger H, Aquila H, Von Jagow G. Coomassie blue-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for direct visualization of polypeptides during electrophoresis. Anal Biochem. 1988;173:201–205. doi: 10.1016/0003-2697(88)90179-0. [DOI] [PubMed] [Google Scholar]
- Sigusch B, Klinger G, Holtz H, Suss J. In vitro phagocytosis by crevicular phagocytes in various forms of periodontitis. J Periodontol. 1992;63:496–501. doi: 10.1902/jop.1992.63.6.496. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. Pathogenicity and virulence of black-pigmented gram-negative anaerobes. FEMS Immunol Med Microbiol. 1993;6:125–137. doi: 10.1111/j.1574-695X.1993.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Taggart CC, Lowe GJ, Greene CM, et al. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Umeda M, Sakamoto M, et al. Treponema socranskii, Treponema denticola, and Porphyromonas gingivalis are associated with severity of periodontal tissue destruction. J Periodontol. 2001;72:1354–1363. doi: 10.1902/jop.2001.72.10.1354. [DOI] [PubMed] [Google Scholar]
- Talonpoika J. Characterization of fibrin(ogen) fragments in gingival crevicular fluid. Scand J Dent Res. 1991;99:40–43. doi: 10.1111/j.1600-0722.1991.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Talonpoika J, Soderling E, Tiekso J, Paunio K. Gingival crevicular fluid plasmin activity in different clinical conditions and after periodontal treatment. Proc Finn Dent Soc. 1991;87:329–337. [PubMed] [Google Scholar]
- Uehara A, Muramoto K, Takada H, Sugawara S. Neutrophil serine proteinases activate human nonepithelial cells to produce inflammatory cytokines through protease-activated receptor 2. J Immunol. 2003;170:5690–5696. doi: 10.4049/jimmunol.170.11.5690. [DOI] [PubMed] [Google Scholar]
- Ujiie Y, Oida S, Gomi K, Arai T, Fukae M. Neutrophil elastase is involved in the initial destruction of human periodontal ligament. J Periodontal Res. 2007;42:325–330. doi: 10.1111/j.1600-0765.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- van Wetering S, van der Linden AC, van Sterkenburg MA, et al. Regulation of SLPI and elafin release from bronchial epithelial cells by neutrophil defensins. Am J Physiol Lung Cell Mol Physiol. 2000;278:L51–L58. doi: 10.1152/ajplung.2000.278.1.L51. [DOI] [PubMed] [Google Scholar]
- van Wetering S, van der Linden AC, van Sterkenburg MA, et al. Regulation of secretory leukocyte proteinase inhibitor (SLPI) production by human bronchial epithelial cells: increase of cell-associated SLPI by neutrophil elastase. J Investig Med. 2000;48:359–366. [PubMed] [Google Scholar]
- Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- Yin L, Swanson B, An J, et al. Differential effects of periopathogens on host protease inhibitors SLPI, elafin, SCCA1, and SCCA2. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v2i0.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Bui T, Liu Y, et al. Differential regulation of elafin in normal and tumor-derived mammary epithelial cells is mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2007;67:11272–11283. doi: 10.1158/0008-5472.CAN-07-2322. [DOI] [PubMed] [Google Scholar]
- Zani ML, Nobar SM, Lacour SA, et al. Kinetics of the inhibition of neutrophil proteinases by recombinant elafin and pre-elafin (trappin-2) expressed in Pichia pastoris. Eur J Biochem. 2004;271:2370–2378. doi: 10.1111/j.1432-1033.2004.04156.x. [DOI] [PubMed] [Google Scholar]


