Abstract
Widely divergent vertebrates share a common central temporal mechanism for representing periodicities of acoustic waveform events. In the auditory nerve, periodicities corresponding to frequencies or rates from about 10 Hz to over 1,000 Hz are extracted from pure tones, from low-frequency complex sounds (e.g., 1st harmonic in bullfrog calls), from mid-frequency sounds with low-frequency modulations (e.g., amplitude modulation rates in cat vocalizations), and from time intervals between high-frequency transients (e.g., pulse-echo delay in bat sonar). Time locking of neuronal responses to periodicities from about 50 ms down to 4 ms or less (about 20–300 Hz) is preserved in the auditory midbrain, where responses are dispersed across many neurons with different onset latencies from 4–5 to 20–50 ms. Midbrain latency distributions are wide enough to encompass two or more repetitions of successive acoustic events, so that responses to multiple, successive periods are ongoing simultaneously in different midbrain neurons. These latencies have a previously unnoticed periodic temporal pattern that determines the specific times for the dispersed on-responses.
Keywords: Periodic sounds, Fundamental pitch, Auditory midbrain, Pulse rate, Response synchrony
Introduction
Important advances are being made in auditory science from an invigorated comparative approach based on the different uses that individual species have evolved from the same basic auditory components. Many of these advances can be traced to the theoretical and empirical foundations laid by Gerhard Neuweiler in the collaborative research center “Nachrichtenaufnahme und Verarbeitung im Hörsystem von Vertebraten (Munich) 1983–1997” led by Neuweiler, Eberhard Zwicker, and Geoffrey Manley (see Manley et al. 2000). Using both Weld and laboratory methods, Neuweiler and his group pioneered the neuroethological analysis of the perceptual and physiological foundations of hearing in a wide variety of animal species. Here, we show how this kind of cross-species comparative approach can produce additional insights into the understanding of audition, using as an example the neural coding of the principal perceptual dimension of human hearing—pitch. We focus on how different species (frogs, cats, bats, and humans) represent common, low-frequency periodicities in their natural communication sounds. The shared underlying auditory mechanism involves synchronization of single spikes in individual auditory nerve and auditory brainstem neurons to successive periodic acoustic “events” (e.g., cycles, envelope peaks, or pulses), and transmission of these timed events to the auditory midbrain where they evoke synchronized spikes distributed over a large pool of neurons having different characteristic latencies. The key features of this system are that robust synchrony still occurs in neural responses above the level of the auditory nerve or brainstem, and that the latencies of these responses are widely dispersed in time.
Pitch and its neural representation
Because of its critical role in perception of speech and music, pitch and its auditory bases have dominated both the history and the substance of auditory theory (Licklider 1951; Lyon and Shamma 1996; Moore 2003). For human listeners, a well-defined sensation of pitch is evoked by many different kinds of stimuli, including pure tones, multiple harmonic sounds (such as vowels and musical notes), amplitude-modulated (AM) tones or noise bursts, and pulse trains with particular pulse repetition rates (PRRs). The frequency range over which clear pitch percepts can be perceived extends up to about 5,000 Hz for musical notes (Semal and Demany 1990), up to about 1,400 Hz for multiple harmonic stimuli with missing fundamental frequencies (Moore 2003), up to about 800 Hz for AM sounds (Ritsma 1962), and between 50 and 2,000 Hz for iterated ripple noise (Yost and Hill 1978). Pitch strength or salience is typically greater for lower compared to higher frequencies within these ranges. The psychophysical prominence of low periodicities for evoking strong pitch sensations is consistent with the low periodicity range of human vowel sounds (Stevens 1998).
For most of the past century, models of pitch perception have concentrated on identifying how spectral and temporal features of complex sounds related to the percept of pitch are encoded in the auditory system. These models have focused on the relative roles of the tuning of auditory neurons to different sound frequencies (spectral or place theories) and the synchronization of neural discharges to salient events in the waveform or envelope of the stimulus (temporal or periodicity theories). The essentially duplex nature of pitch perception has been appreciated for a long time (Wever 1949; Licklider 1951), and numerous, fairly complete models that account for pitch at different levels of the auditory pathway have been proposed using purely spectral domain codes, purely temporal domain codes, and combined spectral–temporal (duplex) codes converging onto a common pitch dimension (see recent reviews by Lyon and Shamma 1996; Shamma 2001; Moore 2003; Rees and Langner 2005; Walker et al. 2010). Spectral and temporal codes each have constraints related to how particular features of complex sounds are coded in the auditory nerve (nVIII) and then transmitted to and transformed in the central auditory system.
The principal limitation on spectral processing is the capacity of nVIII fibers to separate, or resolve, closely spaced frequencies in complex sounds. If a complex sound contains individual harmonics that are far enough apart to fall within the tuning curves of different neurons, they would be resolvable by “place”, i.e., by intrinsically spectral cues. The sound’s harmonic spectral pattern (i.e., fundamental frequency or frequency separation of adjacent harmonics) can be extracted from the corresponding tono-topic spacing of the excited neurons in conjunction with the intervening neurons that are less excited, all without reference to temporal cues about periodicity. The sharpness of tuning of individual mammalian nVIII fibers suggests that, at frequencies up to about 5,000 Hz, depending on the specific tuning characteristics in different species, a place or spectral code could be used to determine the pitch of pure tones. On the other hand, spectral codes are not as useful in extracting the low (<500 Hz) periodicities of complex sounds because the broader tuning in these low-frequency regions often fails to resolve closely spaced harmonic components (Young and Sachs 1979; Joris et al. 2004; Cedolin and Delgutte 2005).
The principal limitation on temporal processing is the capacity of nVIII fibers to synchronize their responses to individual cycles of the sound or to salient envelope events at higher repetition rates. In many species, synchrony is very robust for periodicities up to about 300–500 Hz. Then, as frequency increases, synchrony declines until it is discernable only statistically, with the upper limit around 5,000 Hz for pure tones (squirrel monkeys: Rose et al. 1967) and around 1,300 Hz for multiple harmonic stimuli (domestic cats: Cedolin and Delgutte 2005). The problem for determining the operating range of the temporal mechanism is thus specifying, for a given species, the point at which synchrony declines sufficiently that it becomes less effective as a neuronal code for pitch. Overall, though, temporal models of periodicity extraction based on autocorrelation or pooled interval distributions are effective in explaining how low periodicity pitches within the range of human vowel or animal communication sounds can be extracted from series of high-frequency harmonics (Cariani and Delgutte 1996; Simmons et al. 1996a, b; Suga et al. 1990).
Existing models of pitch extraction in the central auditory system (Langner 1981; Langner and Schreiner 1988; Shamma 2001; Joris et al. 2004; Rees and Langner 2005) place differing emphases on the roles of spectral, temporal or duplex codes in explaining pitch perception. One difficulty for the use of temporal or duplex codes is the more restricted range of synchrony to pure tones (up to about 1,000 Hz in guinea pigs; Liu et al. 2006) and to AM stimuli (typically less than 300 Hz in rodents; Joris et al. 2004) in the auditory midbrain compared to nVIII. We show below that this restricted range of synchrony passed from nVIII to midbrain encompasses the frequency region of periodicities in the natural vocalizations of many animal species. Moreover, we identify the existence of a common temporal code in the auditory midbrain for extracting low periodicities across several different vertebrate groups.
Periodicity in animal vocalizations
Many of the sounds used by different vertebrate species for communication and orientation contain the same physical features that affect pitch sensations in humans. Even though we have no direct evidence of an animal’s internal, psychological reaction to these features, we can nonetheless use their external, behavioral responses as an index for understanding behavioral and neural coding of pitch-like percepts. In different behavioral contexts, animals perceive frequencies and periodicities to distinguish among sounds and to control their behavior in a manner that suggests they perceive a dimension analogous to pitch in humans. This congruence of behaviors evoked by various types of sounds in humans and in other vertebrates encourages a comparative approach to seek out common mechanisms. Figure 1 shows examples of natural vocal signals used by the three species we will discuss in this paper as contributing to the understanding of pitch: the bullfrog (Rana catesbeiana), the domestic cat (Felis catus), and the big brown bat (Eptesicus fuscus). All three species use complex, multiple harmonic signals for communication or orientation, and they represent evolutionary diverse vertebrate groups. These species were chosen for comparison because their signals and their hearing sensitivities (Fig. 2) cover a wide frequency range, but nevertheless aspects of their auditory behavior appear to involve pitch-like perceptions for low-frequency periodicities. Moreover, the spectrograms of animal calls in Fig. 1 contain time–frequency patterns that are recognizable in human speech, such as onset transients and vowel-like formant frequencies that sweep up or down while dropping in and out over the course of the sound (Stevens 1998).
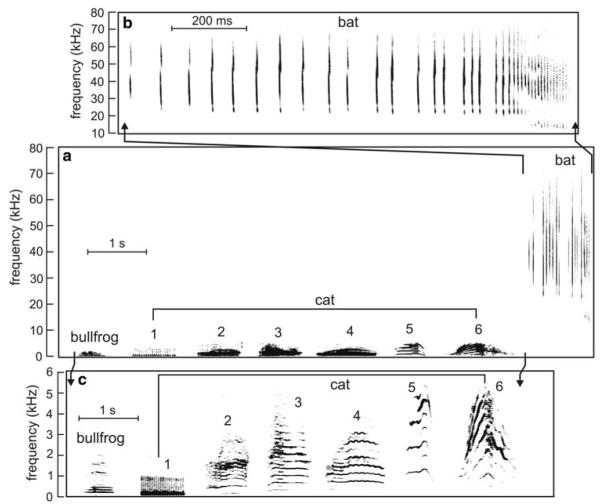
Fig. 1.
a Spectrograms of complex natural sounds from the bullfrog, domestic cat and the big brown bat to illustrate the complexity of these vocalizations and their different frequency ranges. Bat sounds expanded in (b) bullfrog and cat sounds expanded in (c) (note different frequency and time scales). Spectrograms were prepared using Adobe Audition v. 1.5. The bullfrog advertisement notes (one note is shown) and domestic cat vocalizations (1, “purr;” 2, “growl;” 3, 4, “meow;” 5, “kitten distress call;” 6, “angry cat call”) overlap in the low-frequency range below 5 kHz. The pursuit sequence of the big brown bat extends from 20 to 80 kHz and does not overlap in frequency with either bullfrog or cat calls. The bullfrog note is from Suggs and Simmons (2005); cat vocalizations are from on-line sources (http://www.kessels.nl/CatSounds/index.html; http://www.pawsonline.info/feline_sounds.htm); and the big brown bat pursuit sequence is from Simmons et al. (1995)
Fig. 2.
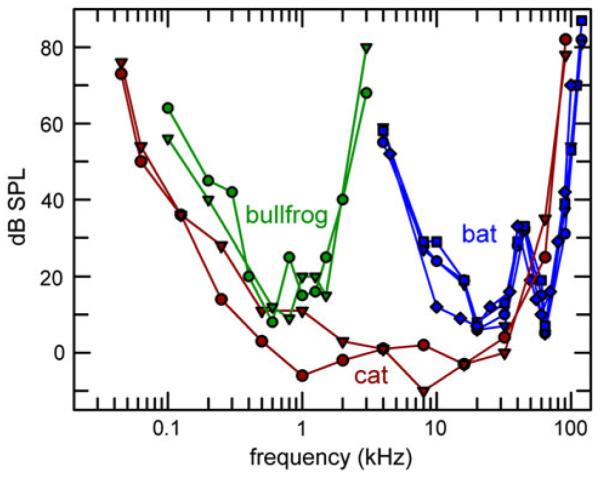
Behavioral audiograms for two bullfrogs (green circles and triangles, Megela-Simmons et al. 1985), two domestic cats (red circles and triangles, Heffner and Heffner 1985), and two big brown bats (blue diamonds, Dalland 1965; blue circles, squares, Koay et al. 1997). Big brown bats may also detect lower frequencies down to about 1 kHz, but with higher thresholds than those in the ultrasonic range (Poussin and Simmons 1982) and those in the audiogram of the domestic cat or the bullfrog. Within the most sensitive frequency range of hearing, the audiogram of the domestic cat includes frequencies common to the bullfrog and to the big brown bat, which otherwise illustrate two extreme ends of the frequency scale
The bullfrog note (Fig. 1) was recorded from a natural chorus of vocalizing males (Suggs and Simmons 2005). Male advertisement calls consist of three to ten such notes, each consisting of a harmonic series with individual frequency components spread over 200–500 Hz in the lower range and between 1,000 and 1,600 Hz in the higher range. The fundamental frequency around 100 Hz is missing, but overall waveform periodicity of individual notes ranges from about 80 to 125 Hz for different individual males, and the envelopes of these notes can also contain superimposed, prominent AM around 10–20 Hz (Suggs and Simmons 2005). This waveform periodicity is important in mediating the calling behavior of male bullfrogs (Hainfeld et al. 1996; Bee and Bowling 2002).
Many domestic cat vocalizations (Fig. 1), such as the purr, growl, meow, and kitten calls, are multiple harmonic sounds that evoke different sensations of pitch in humans (Nicastro 2004; Nicastro and Owren 2003). These vocalizations not only overlap the bullfrog advertisement call in the low-frequency range, but also contain higher frequencies, very prominently up to about 5,000 Hz. The harmonically structured periodicities of frog and many cat vocalizations are broadly similar, with 1st-harmonic frequencies up to several hundred Hertz. Indeed, psychophysical tests reveal that domestic cats can perceive the missing fundamental frequency of complex sounds (Heffner and WhitWeld 1976).
The sequence of big brown bat echolocation sounds (Fig. 1) was recorded from an aerial pursuit that culminated in a successful capture of an insect (Simmons et al. 1995). Each individual sonar pulse is very brief and consists of several harmonic frequency-modulated (FM) sweeps extending from 20 kHz up to 80–100 kHz. Initially, the periodicity of the pulses is 100–150 ms, but, as the bat approaches its prey, the successive sonar pulses are emitted at progressively shorter intervals of 20–50 ms, culminating in a terminal capture stage featuring pulse intervals of 6–8 ms. Echoes of these pulses reach the bat’s ear at delays from about 30 ms down to 2–3 ms. These pulse-to-pulse periods and echo delays are crucial acoustic features for orientation and prey capture by sonar. Being entirely ultrasonic, the bat calls are inaudible to humans, but when they are transformed into pulses at the same repetition rates by a “bat detector” (Neuweiler 1990), they evoke in humans a gliding, buzzing pitch corresponding to the gradually increasing PRR. These comparisons show that the common feature of the auditory behavior of bullfrogs, cats, and big brown bats is reliance on sounds containing the same general range of waveform periodicities from about 10 to 1,000 Hz, in spite of large differences in the frequencies and acoustic structure of the sounds that convey these periodicities.
Hearing ranges in different species
Although vertebrates of many taxa may hear the same variety of acoustic stimuli that evoke sensations of pitch in humans, their inner-ear organ(s) often have radically different structures and generate different auditory codes for the same stimuli. Due to diversity in the morphology of auditory sense organs across different taxa, the same type of sound might come to be represented differently in different animals. Some fishes hear only in the frequency range from a few tens of Hertz up to a few hundred Hertz and exhibit little or no frequency tuning of auditory nerve fibers beyond that supplied by the simple resonance of the entire end-organ itself (Fay 1988). For these animals, distinguishing among sounds having acoustic features related to pitch depends primarily on representing the temporal structure of waveforms that evoke cycle-by-cycle synchronized responses in nVIII fibers. Conservatively stated, the relevant range of time intervals is roughly from 1ms (1,000 Hz) to 50 ms (20 Hz). In birds and mammals, the frequency range of hearing is broader than in most fishes and amphibians, reaching frequencies from about 5–10 kHz to as high as 100–200 kHz in echolocating species (Fay 1988). Although relatively sharp neuronal frequency tuning is present at these higher frequencies, tuning remains broad in the same low-frequency range, from about 20 to 1,000 Hz, where temporal representation prevails in species with broadly tuned auditory nerve fibers. The ubiquity of low-frequency hearing in tandem with varying degrees of high-frequency hearing (none in turtles, some in fishes and frogs, more in birds, very much in some owls and in small mammals; Fay 1988) suggests that vertebrate auditory systems contain a common temporal mechanism for extracting periodicities in the range of 1–50 ms, whether the sounds themselves are at low, middle, or high frequencies, and despite differences in the shape and extent of behavioral audiograms.
Figure 2 shows how the behavioral audiograms for bullfrogs (Megela-Simmons et al. 1985), domestic cats (Heffner and Heffner 1985), and big brown bats (Dalland 1965; Koay et al. 1997) extend over wide, partially overlapping frequency ranges. The bullfrog auditory range extends from about 100 to 2,000 Hz. Within this range, nVIII fibers innervating the frog’s two peripheral auditory organs (amphibian and basilar papilla) are generally broadly tuned, and thus cannot precisely resolve individual harmonics in complex advertisement call notes (horizontal bands in spectrogram of bullfrog note in Fig. 1). On the other hand, all of the bullfrog’s nVIII fibers, regardless of their spectral tuning or site of innervation in the inner ear, synchronize robustly to the missing fundamental frequency around 100 Hz in these notes (Schwartz and Simmons 1990). The bullfrog is thus an example of an animal where temporal coding of complex signals appears unambiguously to pre-dominate over spectral coding.
The domestic cat has a broad frequency range of hearing, extending from 100 Hz up to 60 kHz (Fig. 2). Across the 200–5,000 Hz range of frequencies that are well-represented in the cat’s vocal signals (Fig. 1), the physiological substrate for a spectral, place representation of sounds in nVIII exists (Kiang et al. 1965). In addition, the potential for temporal mechanisms to represent pitch is evident in the robust synchrony of cat nVIII responses to cycles of tones up to 1 kHz, with significant but declining synchrony extending to around 5,000 Hz, and to lower frequency periodicities of salient envelope features in most cat vocalizations (Rose et al. 1967; Brugge et al. 1969; Young and Sachs 1979; Cariani and Delgutte 1996). Thus, for sounds in the mid-frequency range so important for cats, the emergence of pitch-like percepts could plausibly be due to either spectral or temporal cues.
The hearing sensitivity of the big brown bat offers a sharp contrast to that of most other vertebrates, and highlights auditory mechanisms at the opposite end of the frequency spectrum from bullfrogs (Fig. 2). Big brown bats hear best in the frequency range of 10–100 kHz (Dalland 1965; Koay et al. 1997), which encompasses the frequency range of their largely ultrasonic sonar and communication sounds and also covers the full range of sharp frequency tuning for neurons at all stages of the bat’s auditory system (Ferragamo et al. 1998; Haplea et al. 1994; Ma and Suga 2008; Wu and Jen 2008). In big brown bats, synchronization of neural responses to cycles in the waveforms of pure tones at these ultrasonic frequencies is not observed; instead, synchronization occurs to such envelope events as tone-burst onsets, AM, and the transient occurrence of an individual neuron’s tuned frequency in FM sweeps. In particular, neural responses evoked by FM sounds that mimic echolocation signals are time-locked to the occurrence of their tuned frequency in the FM sweep, so that each sonar broadcast and each echo is represented by the timing of responses across a wide range of frequencies. In spite of the ultrasonic frequency range used for echolocation, temporal cues in the range of 1–50 ms are used for processing of the PPRs and echo delays crucial for perceiving objects by sonar (Simmons et al. 1996a, b; Neuweiler 1990). In contrast to the overlap of potential temporal and spectral auditory representations across the mid-frequency range in cats (and humans), the scale of spectral representations associated with frequency content in bats is segregated from the scale of temporal representations associated with representation of echo delay and pulse intervals.
Temporal coding of periodicity in the auditory midbrain
To illustrate common features of temporal processing schemes beyond nVIII in the bullfrog, domestic cat, and big brown bat, we present data on responses to AM stimuli in the auditory midbrain (torus semicircularis, TS, in bullfrogs; inferior colliculus, IC, in domestic cats and big brown bats). The auditory midbrain was chosen because data are available for all three species (there are no data on neural processing in the big brown bat’s nVIII; bullfrogs do not have an auditory cortex).
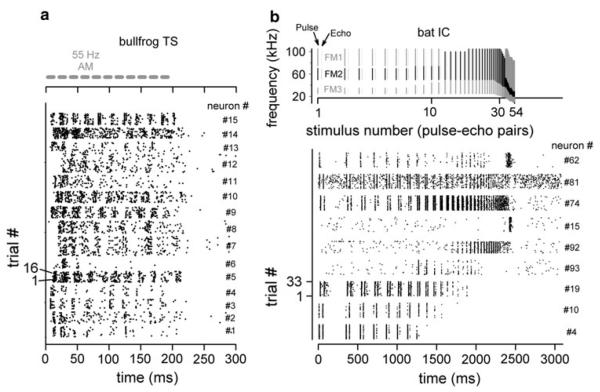
Figure 3a shows dot raster plots of responses from a sample of single neurons in the bullfrog’s TS to AM noise (data from Simmons et al. 2000). Neural responses to AM rates of 55 Hz cluster at intervals around 18 ms. But there is considerable variability in initial or on-response latency between different neurons and in the persistence of synchrony throughout the duration of the sound. For this group of neurons, the degree of synchrony to this AM rate was similar to that observed in responses of nVIII fibers. Figure 3b shows dot raster plots for responses of neurons in the big brown bat’s IC to a train of FM pulses and “echoes” that simulate an entire aerial interception maneuver (data from Sanderson and Simmons 2005). Here, too, individual neurons differ in their onset latencies and in the persistence of synchronized responding across the sequence as a whole. Some neurons follow pulses and echoes across the entire sequence, while others respond synchronously only to segments of the sequence depending on the momentary pulse rate. These response preferences are a reflection in big brown bats of selective responding to PRR that is manifested as tuning to particular AM rates in other animals (Rees and Langner 2005).
Fig. 3.
Dot raster plots showing responses of a 15 individual neurons from the bullfrog TS to repetitive 55 Hz AM noise bursts (from Simmons et al. 2000), and b responses of 9 individual neurons from the bat IC to repetitive pulse-echo patterns simulating an aerial pursuit sequence (from Ferragamo et al. 1998). Stimulus structure and timing are shown at the top of the corresponding dot raster displays. In these displays, time (response latency) is shown on the horizontal axis, and sequential stimulus presentations (trial numbers) are plotted vertically, going up from the bottom. Neuron number is shown on the right vertical axis of each plot. Responses recorded from individual neurons thus appear as vertically stacked horizontal bands stretching from left to right across time. Dots within each band register individual responses from successive stimulus presentations to that neuron. Bullfrog data are based on responses to 15–18 stimulus presentations and big brown bat data are based on responses to 32 stimulus presentations
Figure 4 summarizes the degree of neural synchrony to different AM (PRR) rates in the auditory midbrain of our three model species, plotted as temporal modulation transfer functions for the bullfrog and the bat, or the proportion of neurons with different best AM rates for the cat. In addition, data on synchronization of responses are plotted from auditory cortical recordings made from humans (Brugge et al. 2009). Synchrony is quantified variously by vector strength of the response (VS, ranging from 0 to 1.0; Joris et al. 2004) or from the relative contribution of synchrony to overall response power (Brugge et al. 2009). As previously described in several mammalian species (Joris et al. 2004), VS declines with increasing AM rate, with a dramatically sharp cut-off frequency of about 150–200 Hz for unambiguous synchrony in the IC (Fig. 4), in contrast to the higher frequency cut-off (between 1,000 and 5,000 Hz, depending on species) in the nVIII. What is most striking about Fig. 4 is that the AM response data for the bullfrog and the big brown bat are similar to the data from the domestic cat. The curves showing the proportion of neurons with high VS or with different best AM rates all have the same low-pass shape and about the same cut-off frequency—excellent synchrony up to about 100–150 Hz, even though for these three species the most sensitive range of hearing (Fig. 2) and the nature of typical acoustic stimuli (Fig. 1) differ so dramatically. This suggests that temporal codes continue to operate in the auditory midbrain even though the range of AM rates coded by synchronization is more limited than that in nVIII, and even though IC neurons also respond to changes in AM rate by changes in spike probability. Similarly, in horseshoe bats, IC neurons exhibit measurable synchrony of responses to AM rates as high as 300–400 Hz on an ultrasonic carrier of about 80 kHz (Reimer 1987), although the degree of synchrony has declined substantially relative to that observed at 50–200 Hz. Comparable midbrain auditory recordings are not available from humans, but, intriguingly, auditory cortical recordings from humans reveal the same robust synchronization to PRRs up to about 100–200 Hz, which is surprisingly similar to the other species in Fig. 4 (Brugge et al. 2009).
Fig. 4.

Temporal modulation transfer functions based on response synchronization to AM stimuli or PRR of pulse trains (bullfrog TS: AM noise, green circles; PRR, green squares) (big brown bat IC: PRR, blue diamonds) or on the distribution of best AM rates for AM synchronization and tuning (cat IC: AM, red triangles). For bullfrogs, the AM curve shows mean VS for a sample of 56 neurons in the TS tested at each AM rate (data from Simmons et al. 2000). These neurons differed in their AM tuning as defined by changes in spike rate with AM rate, but showed little difference in VS at any particular rate. For big brown bats, the curve shows mean VS at different pulse rates for eight representative AM low-pass neurons (data from Lu et al. 1998; see also Pinheiro et al. 1991). For domestic cats, the curve shows the normalized proportion of neurons at each best AM rate (data from Langner and Schreiner 1988). For comparison, PRR synchronization is shown for the human AC (posteromedial Heschyl’s gyrus, gray hexagons), expressed as the mean power difference between synchronized and unsynchronized responses obtained from nine human subjects (data from Fig. 5 in Brugge et al. 2009). By these measures, the auditory midbrain of frogs, cats, and bats and part of the AC of humans contain neurons that respond robustly and synchronously to AM stimuli up to 100 Hz, with progressively declining synchronization from 100 to 200–250 Hz. The temporal code for these stimuli thus is preserved from the auditory brainstem to the midbrain or forebrain, indicating that each acoustic event (AM cycle or pulse) triggers its own time-locked response for evaluation of its particular time separation from the previous event
Periodic distributions of latency
Some models of periodicity extraction in the central auditory system include the operation of a cross-correlation or autocorrelation mechanism for detection of periodicity. Licklider’s (1951) duplex model requires synchronization of neural responses to the period or AM rate of the stimulus, followed by the use of delay lines that allow responses to successive periods to catch up with each other so they can occur simultaneously in neurons with different latencies. Coincidence-detecting neurons can then separate different periodicities into higher level neurons tuned to different periodicities by the relative latencies of the inputs to the coincidence detectors. This hypothesis implies that the delay lines will vary systematically in their latencies of response so that an entire spectrum of periodicities can be accommodated by the latency differences among various combinations of neurons. In essence, the latencies of neurons responding to the periodic stimuli would have to be spread over a range wide enough to encompass the longest periods likely to be received and processed. For example, to capture and display time intervals for successive periods occurring at a rate of 30 Hz, the latency spread would have to extend at least out to about 35 ms. Shorter periods, such as 5–10 ms for rates up to 100–200 Hz, would fit into this 35-ms latency spread and be available for subsequent, still temporal, processing. This next stage of processing involves coincidence detection to separate different periodicities into higher level neurons “tuned” to different periodicities as a consequence of the neuronal connections they receive from delay line or dispersed-latency neurons having different latencies. This hypothesis implies that the delay lines will vary systematically in their latencies of response from one cell to another so that an entire spectrum of periodicities up to about 35 ms can be accommodated by the latency differences among various combinations of neurons (Licklider 1951; Suga et al. 1990). Delay lines are not the only way to obtain dispersed latencies, however. Langner’s (1981; Langner and Schreiner 1988) model relies on the presence of intrinsic oscillations in neuronal responses in combination with a spectrum of on-response latencies to serve as a time basis for periodicity extraction. Both of these models predict that neuronal latency spread is a significant stage of temporal processing for using temporal coding.
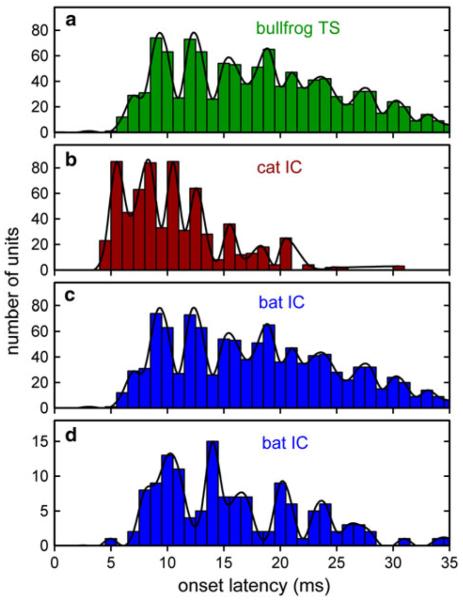
We examined whether systematic dispersions in neuronal latencies would correlate with the degree of synchronization to AM stimuli. Histograms for latencies of on-responses in the TS and IC to AM and PRR across three species show considerable similarity in structure (Fig. 5). In the bullfrog TS (Fig. 5a; data from Simmons et al. 2000), the mean on-response latency across 175 neurons is 21.0 ms (±5.6 ms SD), but different units have systematically distributed onset latencies that are poorly represented by the mean of the population. The shortest on-response latencies pooled across neurons and effective AM rates are about 5 ms, with a wide dispersion of longer latencies extending densely to values of about 25 ms and more sparsely to values of 30 ms or more. The distribution of onset latencies over the latency range in the TS has a finer temporal structure than is represented by the overall width of the distribution. Instead of a shape that tapers uniformly down from an initial peak at about 5 ms to progressively lower peaks from 5 to 35 ms, the histogram is broken into a succession of peaks and valleys that reveals a periodicity intrinsic to the on-responses themselves. Over this time span, there are approximately nine successive cycles of this latency periodicity, for a cycle length of about 3 ms. This corresponds to a repetition frequency of ~300 Hz.
Fig. 5.
Histograms of on-response latencies in the auditory midbrain of three vertebrate species. Distribution of onset latencies in the bullfrog TS (a, 175 neurons; from Simmons et al. 2000). Data are on-responses to AM noise bursts, pooled across responses to AM rates with VS > 0.1 (lower VS values were not included in the plot). Because these are onset latencies, each neuron is recorded as having only one latency value at each AM rate. Distributions of onset latencies in the IC of the domestic cat (b, 762 neurons; from Langner and Schreiner 1988) and the big brown bat (c, 351 neurons; from Ferragamo et al. 1998; d, 133 neurons from Sanderson and Simmons 2000, 2005). Data are on-responses to tone bursts at each neuron’s best frequency. In all three species, latencies for on-responses range from an initial 4–5 ms to at 25–35 ms. Solid curves trace “preferred latencies” to highlight common temporal organization across these four latency distributions. In spite of considerable differences in experimental protocols in different experiments (e.g., species, anesthetic state, nature of stimuli), robust “preferred latencies” appear as oscillations in all four latency distributions. Oscillations are at intervals of about 2.5–3.5 ms in bullfrogs, domestic cats, and big brown bats
Similar periodicities in the latency histogram are shown in the data from the domestic cat (Fig. 5b; data from Langner and Schreiner 1988) and the big brown bat (Fig. 5c, data from Ferragamo et al. 1998; Fig. 5d, data from Sanderson and Simmons 2000, 2005). In both of these species, on-responses in the IC also start at about 4–5 ms and then continue densely until about 15–20 ms in the cat and 25–30 ms in the bat. The 4–5 ms starting latency can be related to axonal conduction delays from nVIII up through medullary and lemniscal pathways. The problem is accounting for the longer latencies of 20–30 ms exhibited by some of these neurons, which are much too long just for axonal conduction and synaptic delays. Instead, the underlying mechanism is thought to be arrival of an initial inhibitory input to each neuron, followed by a prolonged interval of inexcitability until the inhibition has waned (Cassedy and Covey 1996).
Besides this broad latency distribution for on-responses, these curves in Fig. 5 also exhibit an internal periodicity in the latency distributions that is common to all three species. This intrinsic periodicity is about 3 ms in the bullfrog, 2.5 ms in the cat, and 3–3.5 ms in the big brown bat. This overall periodic temporal organization imposed on the entire population of latencies may be different than the latency organization built into the Langner model. Latencies for individual neurons in the domestic cat’s IC (Langner and Schreiner 1988) exhibit oscillations in integer multiples of about 0.4 ms (the presumed minimal step size for an oscillatory process that generates neuronal response delays). The data in Fig. 5a show that the population onset latencies of bullfrog TS neurons exhibit an internal periodicity with a cycle length of about 3 ms, approximately 7 times this proposed 0.4 ms oscillation interval for reverberating circuits. The overall cat data in Fig 5b contain an internal periodicity about 6 times the 0.4 ms minimum, and the bat data in Fig. 5c and d contain an internal periodicity about 7–9 times the 0.4 ms minimum. Interestingly, the shortest delay for which delay-tuned neurons have been found in bats is 0.4 ms (Edamatsu and Suga 1993). The periodic peaks in the latency distributions of Fig. 5 are only a few milliseconds wide at most. These peaks could not manifest themselves unless an appreciable proportion of the on-responses has stimulus-to-stimulus variability that is smaller than the widths of the peaks. The presence of relatively stable on-response latencies for individual neurons combined with the wide distribution of latencies across all neurons suggests that the occurrence of the on-responses coincides not only with the end of the initial period of inhibition (different in different neurons) but also with the arrival of an explicitly excitatory input to each neuron that drives the neurons at the intervals shown by the peaks in the latency distributions.
The presence of the same periodic pattern in the latency distributions in the auditory midbrain of all three species seems to be an important feature of the process whereby responses to the timing of acoustic events are dispersed across a wide enough span of time to allow for comparisons from one event to the next—to determine the length of time between events. These distributions are an intrinsic property of the population of neurons; they are not visible in an individual neuron’s successive responses to an ongoing stimulus but just in the first response evoked by the beginning of the stimulus. They reveal the presence of a pervasive, robust oscillatory phenomenon that applies to the first response across all neurons—an ensemble or “gang” effect, in contrast to the periodic responses to an ongoing stimulus seen in individual chopper cells (Joris et al. 2004).
The delay line/oscillatory hypothesis has been supported by research on the extraction of a specific type of periodicity between successive sounds—the delay of biosonar echoes after biosonar broadcasts in echolocating bats. Big brown bats and other species of bats use the dispersed latencies of on-responses in the IC to retain a registration of the time-of-occurrence of each frequency in the broadcasts for comparison with those same frequencies in echoes. The comparison takes the form of coincidence detections between long-latency responses to the broadcast and shorter latency responses to echoes. Coincidence-detecting neurons are delay-tuned to the broadcast-to-echo time difference implied by the difference in latency between their long-latency and short-latency inputs from the IC. Echoes can be detected from flying insects at a maximum range of about 5 m, which is about 30 ms of delay, about the length of the delay lines created in the IC (Fig. 5c, d). Crucially, the neuronal delay lines need to be long enough to encompass one or more full cycles of the periods to be discriminated. The lengths of the delay lines should be able to accommodate repetition rates as low as 30–50 Hz.
Concluding remarks
Bullfrogs, domestic cats, and big brown bats—all model species for auditory neuroethology—possess neurons in the auditory midbrain that robustly synchronize their responses to individual cycles of AM or individual pulses in pulse trains at rates up to roughly 100 Hz. In each case, the actual frequencies of the sounds differ, but the temporal pattern nevertheless is faithfully registered in the temporal pattern of responses in the auditory midbrain. Several conclusions are warranted: Neuronal mechanisms for processing periodicities as short as 10 ms are ubiquitous across species, and they involve the combination of latency dispersion and temporal synchrony of responses to accommodate successive responses driven by the periodicity of the stimulus. These mechanisms can reasonably be regarded as having evolved early in vertebrate evolution and subsequently persisting along widely divergent lineages. The presence of similar patterns of temporal coding in these three species raises the possibility that the presumably highly specialized processing involved in bat echolocation may have taken advantage of temporal mechanisms for processing complex sounds that already were present in the ancestral stem for bats.
Acknowledgments
This work was supported by ONR Grant # N00014-04-l-0415, by NIH Grant # R01-MH069633, and by NSF Grant # IOS-0843522 (JAS), and by NIH grant DC05257 (AMS). We thank the members of the Brown bat and frog labs for discussion of this work.
Abbreviations
- AM
Amplitude modulation
- FM
Frequency modulation
- PRR
Pulse repetition rate
- nVIII
Auditory nerve
- TS
Torus semicircularis
- IC
Inferior colliculus
- VS
Vector strength
Contributor Information
James A. Simmons, Department of Neuroscience, Brown University, Box GLN, Providence, RI 02912, USA; Department of Cognitive, Linguistic and Psychological Sciences, Brown University, Providence, RI 02912, USA
Andrea Megela Simmons, Department of Neuroscience, Brown University, Box GLN, Providence, RI 02912, USA.
References
- Bee MA, Bowling AC. Socially-mediated pitch alteration by territorial male bullfrogs, Rana catesbeiana. J Herpetol. 2002;36:140–143. [Google Scholar]
- Brugge JF, Anderson DJ, Hind JE, Rose JE. Time structure of discharges in single auditory nerve fibers of the squirrel monkey in response to complex periodic sounds. J Neurophysiol. 1969;32:386–401. doi: 10.1152/jn.1969.32.3.386. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Nourski KV, Oya H, Reale RA, Kawasaki H, Steinschneider M, Howard MA., III Coding of repetitive transients by auditory cortex on Heschyl’s gyrus. J Neurophysiol. 2009;102:2358–2374. doi: 10.1152/jn.91346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariani PA, Delgutte B. Neural correlates of the pitch of complex tones. I. Pitch and pitch salience. J Neurophysiol. 1996;76:1698–1716. doi: 10.1152/jn.1996.76.3.1698. [DOI] [PubMed] [Google Scholar]
- Cassedy JH, Covey E. A neuroethological theory of the operation of the inferior colliculus. Brain Behav Evol. 1996;47:311–336. doi: 10.1159/000113249. [DOI] [PubMed] [Google Scholar]
- Cedolin L, Delgutte B. Pitch of complex tones: rate-place and interspike interval representations in the auditory nerve. J Neurophysiol. 2005;94:347–362. doi: 10.1152/jn.01114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalland J. Hearing sensitivity in bats. Science. 1965;150:1185–1186. doi: 10.1126/science.150.3700.1185. [DOI] [PubMed] [Google Scholar]
- Edamatsu H, Suga N. Differences in response properties of neurons between two delay-tuned areas in the auditory cortex of the mustached bat. J Neurophysiol. 1993;69:1700–1712. doi: 10.1152/jn.1993.69.5.1700. [DOI] [PubMed] [Google Scholar]
- Fay RR. Hearing in vertebrates: a psychophysics databook. Hill-Fay; Winnetka, IL: 1988. [Google Scholar]
- Ferragamo MJ, Haresign T, Simmons JA. Frequency tuning, latencies, and responses to FM sweeps in the inferior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol A. 1998;182:65–79. doi: 10.1007/s003590050159. [DOI] [PubMed] [Google Scholar]
- Hainfeld C, Boatright-Horowitz SL, Boatright-Horowitz SS, Simmons AM. Discrimination of phase spectra in complex sounds by the bullfrog, Rana catesbeiana. J Comp Physiol A. 1996;178:75–87. doi: 10.1007/BF00193436. [DOI] [PubMed] [Google Scholar]
- Haplea S, Covey E, Casseday JH. Frequency tuning and response latencies at three levels in the brainstem of the echolocating bat, Eptesicus fuscus. J Comp Physiol A. 1994;174:671–683. doi: 10.1007/BF00192716. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Hearing range of the domestic cat. Hear Res. 1985;19:85–88. doi: 10.1016/0378-5955(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Heffner H, WhitWeld IC. Perception of the missing fundamental by cats. J Acoust Soc Am. 1976;59:915–919. doi: 10.1121/1.380951. [DOI] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neuronal processing of amplitude-modulated sounds. Physiol Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Watanabe T, Thomas T, Clark LF. Discharge patterns of single fibers in the cat’s auditory nerve. MIT Press; Cambridge, MA: 1965. [Google Scholar]
- Koay G, Heffner HE, Heffner RS. Audiogram of the big brown bat (Eptesicus fuscus) Hear Res. 1997;105:202–210. doi: 10.1016/s0378-5955(96)00208-0. [DOI] [PubMed] [Google Scholar]
- Langner G. Evidence for neuronal periodicity detection in the auditory system of the guinea fowl: implications for pitch analysis in the time domain. Exp Brain Res. 1981;52:333–355. doi: 10.1007/BF00238028. [DOI] [PubMed] [Google Scholar]
- Langner G, Schreiner C. Periodicity coding in the inferior colliculus of the cat. I. Neuronal mechanisms. J Neurophysiol. 1988;60:1799–1822. doi: 10.1152/jn.1988.60.6.1799. [DOI] [PubMed] [Google Scholar]
- Licklider JCR. A duplex theory of pitch perception. Experientia. 1951;7:128–134. doi: 10.1007/BF02156143. [DOI] [PubMed] [Google Scholar]
- Liu L-F, Palmer AR, Wallace MN. Phase-locked responses to pure tones in the inferior colliculus. J Neurophysiol. 2006;95:1926–1935. doi: 10.1152/jn.00497.2005. [DOI] [PubMed] [Google Scholar]
- Lu Y, Jen PH-S, Wu M. GABAergic disinhibition affects responses of bat inferior collicular neurons to temporally patterned sound pulses. J Neurophysiol. 1998;79:2303–2315. doi: 10.1152/jn.1998.79.5.2303. [DOI] [PubMed] [Google Scholar]
- Lyon R, Shamma S. Auditory representation of timbre and pitch. In: Hawkins HL, McMullen TA, Popper AN, Fay RR, editors. Auditory computation. Springer; New York: 1996. pp. 221–270. [Google Scholar]
- Ma X, Suga N. Corticofugal modulation of the paradoxical latency shifts of inferior collicular neurons. J Neurophysiol. 2008;100:1127–1134. doi: 10.1152/jn.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Fastl H, Kössl M, Oeckinhhaus H, Klump G, editors. Auditory worlds: sensory analysis and perception in animals and man. Wiley-VCH, Weinheim; Germany: 2000. [Google Scholar]
- Megela-Simmons A, Moss CF, Daniel KM. Behavioral audiograms of the bullfrog (Rana catesbeiana) and the green tree frog (Hyla cinerea) J Acoust Soc Am. 1985;78:1236–1244. doi: 10.1121/1.392892. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. An introduction to the psychology of hearing. 5th edn Academic Press; San Diego: 2003. [Google Scholar]
- Neuweiler G. Auditory adaptations for prey capture in echolocating bats. Physiol Rev. 1990;70:615–641. doi: 10.1152/physrev.1990.70.3.615. [DOI] [PubMed] [Google Scholar]
- Nicastro N. Perceptual and acoustic evidence for species-level differences in meow vocalizations by domestic cats (Felis catus) and African wild cats (Felis silvestris lybica) J Comp Psych. 2004;118:287–296. doi: 10.1037/0735-7036.118.3.287. [DOI] [PubMed] [Google Scholar]
- Nicastro N, Owren MJ. Classification of domestic cat (Felis catus) vocalizations by native and experienced human listeners. J Comp Psych. 2003;117:44–52. doi: 10.1037/0735-7036.117.1.44. [DOI] [PubMed] [Google Scholar]
- Pinheiro AD, Wu M, Jen PH. Encoding repetition rate and duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Comp Physiol A. 1991;169:69–85. doi: 10.1007/BF00198174. [DOI] [PubMed] [Google Scholar]
- Poussin C, Simmons JA. Low-frequency hearing sensitivity in the echolocating bat, Eptesicus fuscus. J Acoust Soc Am. 1982;72:340–342. [Google Scholar]
- Rees A, Langner G. Temporal coding in the auditory midbrain. In: Winer JA, Schreiner CE, editors. The inferior colliculus. Springer; New York: 2005. pp. 346–376. [Google Scholar]
- Reimer K. Coding of sinusoidally amplitude modulated acoustic stimuli in the inferior colliculus of the rufous horseshoe bat, Rhinolophus rouxi. J Comp Physiol A. 1987;161:305–313. doi: 10.1007/BF00615250. [DOI] [PubMed] [Google Scholar]
- Ritsma RJ. Existence region of the tonal residue. I. J Acoust Soc Am. 1962;34:1224–1229. [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Sanderson MI, Simmons JA. Neural responses to overlapping FM sounds in the inferior colliculus of echolocating bats. J Neurophysiol. 2000;83:1840–1855. doi: 10.1152/jn.2000.83.4.1840. [DOI] [PubMed] [Google Scholar]
- Sanderson MI, Simmons JA. Target representation of naturalistic echolocation sequences in single unit responses from the inferior colliculus of big brown bats. J Acoust Soc Am. 2005;118:3352–3361. doi: 10.1121/1.2041227. [DOI] [PubMed] [Google Scholar]
- Schwartz JJ, Simmons AM. Encoding of a spectrally-complex communication sound in the bullfrog’s auditory nerve. J Comp Physiol A. 1990;166:489–500. doi: 10.1007/BF00192019. [DOI] [PubMed] [Google Scholar]
- Semal C, Demany L. The upper limit of musical pitch. Music Percept. 1990;8:165–175. [Google Scholar]
- Shamma S. On the role of space and time in auditory processing. Trends Cogn Sci. 2001;5:340–348. doi: 10.1016/s1364-6613(00)01704-6. [DOI] [PubMed] [Google Scholar]
- Simmons JA, Ferragamo MJ, Saliant PA, Haresign T, Wotton JM, Dear SP, Lee DN. Auditory dimensions of acoustic images in echolocation. In: Popper AN, Fay RR, editors. Hearing by bats. Springer; New York: 1995. pp. 146–190. [Google Scholar]
- Simmons AM, Shen Y, Sanderson MI. Neural and computational basis for periodicity extraction in frog peripheral auditory system. Audit Neurosci. 1996a;2:109–133. [Google Scholar]
- Simmons JA, Saillant PA, Ferragamo MJ, Haresign T, Dear SP, Fritz JB, McMullen TA. Auditory computations for acoustic imaging in bat sonar. In: Hawkins HL, McMullen TA, Popper AN, Fay RR, editors. Auditory computation. Springer; New York: 1996b. pp. 401–468. [Google Scholar]
- Simmons AM, Sanderson MI, Garabedian CE. Representation of waveform periodicity in the auditory midbrain of the bullfrog, Rana catesbeiana. J Assoc Res Otolaryngol. 2000;1:2–24. doi: 10.1007/s101620010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KN. Acoustic phonetics. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Suga N, Olsen JF, Butman JA. Specialized subsystems for processing biologically important complex sounds: cross-correlation analysis for ranging in the bat’s brain. Cold Spring Harbor Symp Quant Biol. 1990;55:585–597. doi: 10.1101/sqb.1990.055.01.056. [DOI] [PubMed] [Google Scholar]
- Suggs DN, Simmons AM. Information theory analysis of patterns of modulation in the advertisement call of the male bullfrog, Rana catesbeiana. J Acoust Soc Am. 2005;117:2330–2337. doi: 10.1121/1.1863693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KMM, Bizley JK, King AJ, Schnupp JWH. Cortical encoding of pitch: recent results and open questions. Hear Res. 2010 doi: 10.1016/j.heares.2010.04.015. doi:10.1016/j.heares.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever EG. Theory of hearing. Wiley; New York: 1949. [Google Scholar]
- Wu CH, Jen PH. Echo frequency selectivity of duration-tuned inferior collicular neurons of the big brown bat, Eptesicus fuscus, determined with pulse-echo pairs. Neuroscience. 2008;156:1028–1038. doi: 10.1016/j.neuroscience.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Yost WA, Hill R. Strength of the pitches associated with ripple noise. J Acoust Soc Am. 1978;64:485–492. doi: 10.1121/1.382021. [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. J Acoust Soc Am. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]