Abstract
Objective
Current evidence supports the efficacy of hypnosis for reducing the pain associated with experimental stimulation and various acute and chronic conditions; however, the mechanisms explaining how hypnosis exerts its effects remain less clear. The hypothalamic-pituitary-adrenal (HPA) axis and pro-inflammatory cytokines represent potential targets for investigation given their purported roles in the perpetuation of painful conditions; yet, no clinical trials have thus far examined the influence of hypnosis on these mechanisms.
Design
Healthy participants, highly susceptible to the effects of hypnosis, were randomized to either a hypnosis intervention or a no-intervention control. Using a cold pressor task, assessments of pain intensity and pain unpleasantness were collected prior to the intervention (Pre) and following the intervention (Post) along with pain-provoked changes in salivary cortisol and the soluble receptor of tumor necrosis factor-α (sTNFαRII).
Results
Compared to the no-intervention control, data analyses revealed that hypnosis significantly reduced pain intensity and pain unpleasantness. Hypnosis was not significantly associated with suppression of cortisol or sTNFαRII reactivity to acute pain from Pre to Post; however, the effect sizes for these associations were medium-sized.
Conclusions
Overall, the findings from this randomized controlled pilot study support the importance of a future large-scale study on the effects of hypnosis for modulating pain-related changes of the HPA axis and pro-inflammatory cytokines.
Keywords: Hypnosis, Hypnotic analgesia, Pain, Cortisol, Tumor necrosis factor-α, HPA axis, Inflammation
INTRODUCTION
Clinical hypnosis has a long history in pain medicine and the effectiveness of hypnosis as an analgesic and sedative technique has been confirmed in recent meta-analyses [1,2]. In particular, current evidence attests to the efficacy of hypnosis in reducing pain in healthy subjects during exposure to laboratory pain stimuli [2,3], acute pain in clinical patients during medical/surgical procedures [4,5,6], and in patients with persistent pain [7,8,9,10]. Following the establishment of hypnosis as an effective intervention for pain relief and management, several lines of research began to focus on whether hypnosis has a greater effect on sensory (i.e., intensity) or affective (i.e., unpleasantness) components of pain [11,12,13,14,15]. The sensory component is thought to reflect the overall magnitude and intensity of the felt pain, whereas the affective component reflects what the pain feels like (e.g., “dull” and “aching”) and its general unpleasantness [16]. Such differentiation has led some to hypothesize that hypnosis may be more effective at reducing the affective and unpleasantness components of pain than its intensity component [9 for review]. In a series of earlier studies conducted by Price and colleagues [11,14], it was initially reported that affective components of pain showed a greater reduction with hypnosis than did sensory ones [11], but subsequently it was suggested that both components could show a reduction, and that the amount of change depended on the nature of the hypnotic suggestion [14]. Indeed, it has since been demonstrated, through functional magnetic resonance imaging (fMRI), that different areas of the brain are differentially impacted by hypnosis as a function of the specific post-hypnotic suggestion used rather than hypnosis in general [13]. For example, post-hypnotic suggestions of reductions in pain affect are associated with altered activity in the anterior cingulate cortex, whereas suggestions for reductions in pain intensity alter activity in both the anterior cingulate cortex and sensory cortices [15,17]. Additional research specifically addressing the sensory and affective components of pain could further assist with teasing apart the effects of hypnosis on the multidimensional experience of pain.
Despite the evidence supporting the effectiveness of hypnosis for treating pain, the mechanisms explaining how hypnosis exerts its effects remain less clear. Results from previous laboratory studies that used functional magnetic resonance imaging (fMRI) to examine the effects of clinical hypnosis suggest that hypnotic suggestions for relief of pain may prevent nociceptive inputs from reaching higher cortical structures responsible for pain perception [3,18]. In addition, a trial of self-hypnosis was shown to not only reduce pain and anxiety, but also reduce oxygen desaturation, and hemodynamic instability in patients undergoing a variety of invasive medical procedures [19]; thereby implicating cardiovascular factors in hypnosis effects. Noticeably lacking from the research addressing the neurological and physiological correlates of hypnosis has been an evaluation of the efficacy of hypnosis for modulating pain-related neuroendocrine (e.g., hypothalamic-pituitary-adrenal (HPA) axis) and immunological (e.g., inflammation) changes. Such research seems warranted because, in general, the experience of pain is often associated with enhanced HPA axis activity (e.g., cortisol production) and release of pro-inflammatory cytokines, which in turn sensitize the nervous system and amplify the transmission of pain signals [20,21,22,23,24]. Indeed, cortisol and pro-inflammatory cytokines such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) show positive associations with pain severity from quantitative sensory testing [25,26,27], burn wounds [28,29,30], and discogenic low back pain [31,32,33]. Further, it has been suggested that prolonged activation of the HPA axis and pro-inflammatory response to pain may constitute important mechanisms whereby acutely painful conditions develop into the phenomenon of chronic pain and chronic pain is perpetuated [33,34,35]. Importantly, previous clinical trials have tentatively demonstrated the ability of self-hypnosis and other mind-body treatments such as cognitive-behavioral therapy and mindfulness meditation to not only improve pain experiences but also affect immune functioning [36,37,38,39]. Additional research demonstrating the potential of hypnosis to improve pain outcomes, and influence the HPA axis and pro-inflammatory cytokine responses to acute invasive medical procedures, laboratory pain tasks, and persistent pain, would represent a significant advancement in mind-body medicine. If it is confirmed that hypnosis reliably induces a decrease in HPA axis and pro-inflammatory cytokine activity, this intervention modality may potentially be used as an adjuvant in clinical therapy involving medical conditions where the over expression of the HPA axis and inflammation occurs (e.g., burn injuries, rheumatoid arthritis). The purpose of the current pilot study was to examine whether hypnosis not only produced reductions in the sensory and affective components of pain, but also to examine whether hypnosis directly influenced HPA axis and pro-inflammatory reactivity to acute pain in the laboratory.
This pilot study used a 2 group (hypnosis group vs. a no-intervention control group) randomized controlled design to examine the effects of hypnosis on the following variables: participant ratings of pain intensity (PI) and pain unpleasantness (PU), salivary cortisol and the soluble receptor for TNF-α (sTNFαRII). Ratings of PI and PU represented individuals' sensory and affective experience of pain, respectively; whereas salivary cortisol and sTNFαRII represented HPA axis and pro-inflammatory activity, respectively. The following specific hypotheses were tested: 1) Relative to the no-intervention control group (Control), the hypnosis group (Hypnosis) will demonstrate greater reductions in PI and PU following the intervention, with the greatest reductions occurring for PU..2) Hypnosis will demonstrate suppressed salivary cortisol and sTNFαRII reactivity to the pain stimulus when compared with Control following the intervention.
MATERIAL AND METHODS
Overview of Study Plan
Hypnotic susceptibility
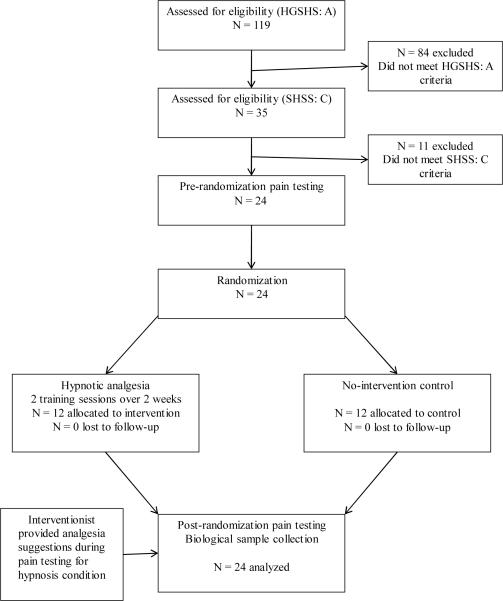
The flow of the participants through the study is shown in Figure 1. Participation began with a two stage hypnotic suceptibility screening process that incorporated the Harvard Group Scale of Hypnotic Susceptibility (HGSHS), Form A [40] and the Stanford Hypnotic Susceptibility Scale (SHSS), Form C [41]. These screeners are widely considered the gold standards for ensuring consistency in hypnotic abilities among subjects participating in research [42]. Both scales have scores that range from 0 to 12, and both include hypnosis induction and suggestions for 12 characteristic hypnotic experiences (e.g., arm levitation, age regression, a dream, and a post-hypnotic suggestion of amnesia until cued to remember among others) [43]. Participants classified as highly susceptible to hypnosis (i.e., score of 7 or greater on both the HGSHS and SHSS) were selected for study enrollment. A cut score of 7 (for each scale) has been validated in previous studies and has been shown to reliably differentiate highly hypnotizable individuals from those who are not [44]. Only individuals with high hypnotic susceptibility were included in the current study to maximize the potential for hypnosis to reduce pain and alter physiological reactivity [45]. Of the 119 potential participants who were screened for study appropriateness, a total of 24 highly hypnotizable individuals (50% women) were identified and scheduled for study inclusion. The most common reasons for study exclusion were use of oral contraceptives and sub-threshold hypnotic suceptibility.
Figure 1.
Study flow chart.
Pre-intervention pain testing
On a separate day following the screening of hypnotic susceptibility, all participants were scheduled to return to the laboratory to complete pre-intervention pain testing that included exposure to a cold pressor task (CPT). The CPT is a reliable and valid method to assess pain without tissue damage [46], and has demonstrated clinical pain relevance [47]. The CPT was used in the current study because recent evidence suggests that the CPT may be the experimental pain modality best suited for examining cortisol and sTNFαRII reactivity to acute pain [48]. The CPT procedure involved a NESLAB RTE-10 liter refrigerated water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) filled with circulating cold water maintained at approximately 4°C (± 0.2°C). Standardized, audiotaped instructions were used to direct participants during all laboratory pain testing procedures. Participants were instructed to place their dominant hand into the cold water up to their wrist and to keep their hands immersed “for the entire duration of the procedure or until the pain becomes intolerable”. Unbeknownst to participants, the maximum duration of CPT exposure was 300 seconds, at which time subjects were instructed to remove their hand from the cold water. Before, during, and after completion of the pre-intervention pain testing, all participants provided oral fluid samples for the assessment of cortisol and sTNFαRII via the procedures described below. For consistency and because of the substantial circadian variation in salivary cortisol levels, all pre- and post-intervention pain testing sessions occurred between 4:00 p.m. and 7:00 p.m. [49]. These laboratory pain and physiological reactivity data constituted the pre-intervention (Pre) assessment portfolio. Following the completion of Pre pain testing, participants were then randomized to Control (N = 12) or to Hypnosis (N = 12). Sex was balanced across the two groups and all participants completed the study.
Post-Intervention pain testing
The post-intervention (Post) pain testing sessions were scheduled approximately four weeks following completion of the Pre sessions. All pain testing sessions were facilitated by trained research assistants familiar with the study protocol and blinded to randomization. All study participants completed the Post pain testing session exactly as described above for Pre. However, during the Post pain testing session, participants randomized to Hypnosis were engaged by a research assistant in the hypnotic induction with analgesia suggestions during the 15 minutes prior to initiation of the CPT. Control participants were given a world atlas and allowed to look at neutral images during the 15 minutes prior to initiation of the CPT. Before, during, and after completion of the Post pain testing, all participants again provided oral fluid samples for additional assessment of cortisol and sTNFαRII.
Participants
This study was carried out in accordance with the 1975 Declaration of Helsinki (and its subsequent revisions) guidelines for ethical conduct of research and was approved by the University's Institutional Review Board. Individuals interested in study participation completed an on-line web-based survey as part of an initial health screening. Healthy, pain-free adults (18 – 45 years old) of both sexes and all races were eligible for study enrollment. Potential participants were excluded if they self-reported any of the following criteria: (a) age less than 18 or over 45 years; (b) ongoing chronic pain problems; (c) diagnosed with hypertension or taking medication for blood pressure; (d) circulatory disorders; (e) history of cardiac events; (f) history of metabolic disease or neuropathy; (g) pregnant; (h) currently using prescription analgesics, tranquilizers, antidepressants, or other centrally acting agents; (i) use of nicotine, (j) use of prescription medication (e.g., corticosteriods, oral contraceptives), (k) psychiatric disorders (e.g., depression), or (l) chronic or acute health problems that affect the neuroendocrine or immune system. Individuals who met health criteria for inclusion into the study were then contacted by telephone and invited to present to our laboratory to complete an additional screener assessing hypontic suceptibility. During their first visit to our laboratory, participants gave their consent to participate in the trial after having been informed about its nature and purpose, participation and termination conditions, risk, and benefits. On appointment days, participants were asked to not exercise, consume alcohol, or use nonprescription medication on the day of any study appointment. All participants were compensated for their participation.
Intervention Summary
Participants randomized to Control received no intervention or education on behalf of our study. Participants who were randomly assigned to Hypnosis were scheduled for two appointments, approximately one week apart, to receive individual training in hypnosis. All of the hypnosis sessions were facilitated by doctoral-level research assistants extensively trained and continuously supervised by a licensed clinical psychologist. The first intervention training session occurred within one week of the Pre pain testing session. During these training sessions, participants met with a research assistant to practice hypnotic induction and post-hypnotic suggestions of analgesia.
A semi-standardized hypnotic protocol was followed which consisted of the following components. A modified version of the hypnotic induction developed by Dr. D. Spiegel [50] and used by Lang and colleagues [19,50], that incorporated eye fixation, kinesthetic arm levitation on the dominant arm (the arm that was exposed to laboratory pain testing), dissociation, imaginative absorption, and distraction. Following induction, participants were given the post-hypnotic suggestion that after their initial hypnotic training session they would be able to easily enter hypnosis through the Spiegel 1 2 3 eye fixation method [19]. We developed a comprehensive script of positively framed analgesia suggestions [19,50] that targeted PI and PU relevant to the laboratory pain model [12,51] using both direct and indirect suggestions and imagery [11]. This script consisted of five post-hypnotic suggestions that included: 1) a glove anesthesia suggestion of the dominant hand/arm, 2) a dissociative suggestion to a place of comfort where nothing was bothersome, 3) replacement of any negative bodily sensation with numbness, 4) mental imagery (e.g., putting any negative bodily sensation into a box, and that box inside another box, and placing it at the end of a long hallway, so that one might be aware of it but not bothered by it), and 5) suggestions for relaxation and comfort. During the first hypnotic practice session, each participant experienced all five of the post-hypnotic suggestions and was then asked to pick the two suggestions he/she liked the most. During the second hypnosis practice session, participants again underwent hypnotic induction and were provided with the two post-hypnotic suggestions of their choosing. Following completion of the second session, participants were asked to choose their most preferred post-hypnotic suggestion. This suggestion was incorporated into the Post pain testing session. The hypnotic induction and analgesia suggestion was designed so that it could be effectively completed in 15 minutes, the amount of time allotted in the Post pain testing session. The hypnotic induction and analgesia suggestions comprising the intervention for this study were consistent with the hypnosis interventions shown to be successful in reducing pain in previous studies [12,13,19,51].
Primary Outcome Measures
Pain intensity (PI) and pain unpleasantness (PU)
At Pre and Post pain testing sessions, a description of the difference between PI (“How strong the pain feels”) and PU (“How unpleasant or disturbing the pain is for you”) was read to all participants. Following this, PI and PU were assessed during the CPT by asking participants for verbal self-reports on 0–100 scales, with 0 = “No pain” (or “Not at all unpleasant”) and 100 = “Pain as intense as I can imagine” (or “Pain as unpleasant as I can imagine). Numeric rating scales of PI and PU have demonstrated validity through their ability to detect treatment effects, as well as their strong association to other measures of sensory and affective components of pain [52]. PI and PU ratings were obtained every 30 seconds throughout the 300 second painful procedure (maximum 10 ratings for each pain descriptor), and at the exact time the task was discontinued. PI and PU ratings were averaged over the course of the CPT trial and included in statistical analyses. Whether participants completed the entire CPT, or terminated the task prior to the allotted maximum time of exposure, the duration of exposure was recorded and classified as cold pressor pain tolerance (CPTo).
Salivary cortisol and sTNFαRII
Consistent with the procedures incorporated by Dickerson and colleagues [53], the cortisol and sTNFαRII levels in this study were obtained from oral fluids, which provide an established method for assessing cortisol [49] and has been validated for assessing certain pro-inflammatory products [54]. We chose to assess sTNFαRII because it is more stable and reliably measured than TNF-α [55], and it has been demonstrated that levels of sTNFαRII in oral fluids are significantly and highly correlated with those obtained from plasma [54]. Therefore, oral fluid collection seems to be a less reactive and invasive means for reliably measuring neuroendocrine and immune activity relative to a needle stick with blood draws.
To minimize potential error associated with the collection of oral fluid samples, participants were asked to not eat foods that may cause bleeding of the gums (e.g, potato chips) or brush their teeth for at least 2 hours prior to the testing session. This is because blood leakage from microinjuries of the oral mucosa may confound the measurement of salivary cortisol and OMT sTNFαRII [56]. An OraSure collection device (Epitope, Beaverton, OR) was placed into the mouth, between the lower cheek and gum, for 2.5 minutes per sampling time-point; this placement collects samples mainly containing oral mucosal transudate (OMT). Along with the OraSure device, salivettes (Sarsted, Leicester, UK) were concurrently placed into the mouth, on top of the tongue, for saliva collection. OMT and saliva are both oral fluids; OMT is a filtrate of blood plasma while saliva contains enzymes and other particles from the parotid and salivary glands. Cortisol in saliva is in its unbound, biologically active form, and its concentration is independent of saliva flow rate. OMT was collected for sTNFαRII rather than saliva because sTNFαRII is more readily available in OMT.
After obtaining the OMT and saliva samples, the samples were immediately refrigerated before being transferred and stored at −80°C until batch assayed. Cortisol was measured using high sensitivity salivary cortisol immunoassay kits (Salimetrics, State College, PA). Intra- and interassay variability was less than 8%. sTNFαRII was measured using Quantikine Human sTNFαRII enzyme immunoassay kits (R&D Systems, Minneapolis, MN). The Bradford method [57] was used to quantify protein in the oral fluids, using the Bio-Rad protein assay kit with bovine plasma albumin as the standard. The sTNFαRII results are reported using the ratio of the experimental value for the analyte to the protein concentration in the test sample. This ratio controls for changes in salivary flow rate, which can be altered by experimental procedures or vary between individuals. The ratio values are more reliable than the analyte values alone [54]. The intra- and interassay variability was less than 5%.
Saliva and OMT samples were collected at baseline (prior to completion of the 15 min hypnotic induction or 15 min rest period for controls), immediately following termination of the CPT (i.e., pain offset), and at pre-determined intervals during recovery from the pain testing procedure (15, 20, 25, 30 and 40 minutes post-CPT initiation). These sampling time-points were chosen based on a meta-analysis of prior research showing that peak changes in cortisol occur at approximately 30 minutes following stressor onset [58]. Additionally, a recent study has reported that sTNFαRII reactivity to acute stress have been found to occur at that same timepoint [53].
Hypnotic Experiences Questionnaire (HEQ)
To assess for experiential differences between Hypnosis and Control, we administered a modified version of the hypnotic experience questionnaire short form (HEQ-S) [59] originally developed by Kelly [60]. The HEQ-S is a 16 item questionnaire that assesses three domains: altered state/dissociation, relaxation, and rapport. In the current study, the modified HEQ was comprised of nine items that asked individuals about their experiences with hypnotic absorption/altered state/ dissociation (e.g., to what degree did you experience your body as being different from normal?), physical and mental relaxation (e.g., how mentally and emotionally relaxed did you feel in today's session?), and pain relief (e.g., to what degree did you experience any pain relief as occurring automatically without trying to make it happen?). Respondents rated the items on an 11-point Likert scale from 0 (not at all) to 10 (extremely, or the greatest degree possible). Higher scores on this modified HEQ indicate a greater (i.e., deeper) hypnotic experience (range = 0 to 90). The modified HEQ was administered following the Post pain testing session, and was given to every participant (i.e., Hypnosis and Control) to assess whether the hypnotic induction with analgesia suggestions did indeed produce a qualitatively different experience for Hypnosis. The internal consistency of the modified HEQ in the current study was excellent (α = 0.88).
Data Reduction and Analysis
A significant amount of skew in the distributions of cortisol and sTNFαRII data was discovered. Accordingly, these data were subjected to logarithmic transformation using a log10 function, which was effective for reducing skew according to Shapiro-Wilk's tests (p's > 0.05). Subsequent statistical analysis of cortisol and sTNFαRII reactivity was completed using transformed values; however, study figures show anti-log mean values for easier interpretation of results. Pearson correlations were used to examine the bivariate relationships among all study variables separately for Hypnosis and Control. An analysis of variance (ANOVA) was completed to examine the difference between Hypnosis and Control on the modified HEQ (i.e., hypnotic experience). All primary outcomes were analyzed according to the intention-to-treat principle [61]; however, data imputation was not necessary given the lack of participant attrition. To determine the effects of hypnosis on Pre to Post ratings of PI and PU, a series of two repeated-measures analysis of variance (RM-ANOVA) with Greenhouse-Geisser corrections for violations of spherecity were carried out. Significant interaction and main effects were further analyzed by Bonferroni adjusted post-hoc tests. Results include the partial η2 as a measure of effect size where appropriate. Following the conventions of Cohen [62], partial η2 = 0.01 is considered a small effect, partial η2 = 0.06 a medium-sized effect and partial η2 =0.14 a large effect. Per the specifications of Pruessner and colleagues [63], a measure of area under the curve with respect to increase (AUCI) was calculated to summarize the reactivity of cortisol and sTNFαRII to the CPT. With endocrine and immune data, AUCI is a parameter that emphasizes the changes of a physiological marker over time and is more related to sensitivity of the system. An additional series of six repeated measures RM-ANOVAs was carried out to separately examine Pre and Post cortisol and sTNFαRII reactivity, and Pre-Post changes in the AUCI summary indicators of cortisol and sTNFαRII. SPSS for windows (version 19.0) was used for all analyses.
RESULTS
Participant characteristics
The two groups of study participants were homogenous. No significant differences were found for age, distribution by sex, distribution by ethnicity, or body mass index (BMI) between the two experimental groups (all p's = ns; Table 1). As can be seen in Table 2, participants' sex was not significantly associated with either Pre or Post CPTo, pain reports, or any of the AUCI summary indicators for cortisol and sTNFαRII reactivity (all p's = ns). Accordingly, participants' sex was not included in any subsequent analyses. As expected, ANOVA revealed that participants in Hypnosis (M = 63.7, SD = 9.9) reported significantly greater hypnotic experiences on the modified HEQ compared to Control participants (M = 36.7, SD = 17.5) (F(1,22) = 21.67, p < 0.001). This finding supports that the hypnotic induction with analgesia suggestions did produce a qualitatively different experience for Hypnosis compared to Control. It is worth noting that participants in Control had an average modified HEQ score that was significantly different from zero. The reason for this result is not apparent at this time, nor is it clear whether this response represents a normal score for persons highly susceptible to the effects of hypnosis but not subjected to hypnotic suggestions.
Table 1.
Means and standard deviations for demographic and anthropometric measures
| Hypnosis | Control | |||
|---|---|---|---|---|
| Measures | M | SD | M | SD |
| Age | 20.2 | 3.6 | 19.4 | 2.4 |
| Body mass index | 23.0 | 3.3 | 23.7 | 3.5 |
|
|
||||
| Sex | Men = 6 | Men = 6 | ||
| Women = 6 | Women = 6 | |||
|
|
||||
| Ethnicity | Caucasian American = 7 | Caucasian American = 8 | ||
| Asian American = 3 | Asian American = 3 | |||
| African American = 2 | African American = 1 | |||
*= p < 0.05
Table 2.
Panel A: Zero-order correlations for control condition
| Panel A: Zero-order correlations for control condition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 1. Sex | — | |||||||||||
| 2. HEQ | .00 | — | ||||||||||
| 3. Pre-CPTo | .32 | −.06 | — | |||||||||
| 4. Post-CPTo | .35 | −.44 | .27 | — | ||||||||
| 5. Pre-PI | −.14 | .20 | −.74** | −.04 | — | |||||||
| 6. Post-PI | −.53 | .18 | −.28 | −.86** | .12 | — | ||||||
| 7. Pre-PU | −.48 | .45 | −.55 | −.18 | .72** | .30 | — | |||||
| 8. Post-PU | −.50 | .31 | −.22 | −.85** | .04 | .97** | .35 | — | ||||
| 9. Pre-cortisol AUCI | .34 | .28 | .53 | .00 | −.11 | −.14 | −.09 | −.11 | — | |||
| 10. Post-cortisol AUCI | .28 | .17 | .23 | .02 | −.11 | −.25 | −.20 | −.22 | .66* | — | ||
| 11. Pre-sTNFαRII AUCI | −.05 | .05 | −.13 | −.18 | .16 | .44 | .42 | .42 | .08 | .08 | — | |
| 12. Post-sTNFαRII AUCI | −.37 | −.23 | .16 | −.16 | −.45 | .33 | −.19 | .43 | −.09 | −.12 | .01 | — |
| Panel B: Zero-order correlations for hypnosis condition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 1. Sex | — | |||||||||||
| 2. HEQ | .30 | — | ||||||||||
| 3. Pre-CPTo | .19 | .04 | — | |||||||||
| 4. Post-CPTo | .10 | .39 | .13 | — | ||||||||
| 5. Pre-PI | .14 | .29 | −.76** | −.07 | — | |||||||
| 6. Post-PI | .06 | −.14 | −.22 | −.85** | .23 | — | ||||||
| 7. Pre-PU | .20 | .20 | −.82** | −.13 | .88** | .28 | — | |||||
| 8. Post-PU | .02 | −.15 | −.38 | −.81** | .30 | .96** | .40 | — | ||||
| 9. Pre-cortisol AUCI | .27 | .09 | −.13 | −.05 | .38 | .16 | .12 | .21 | — | |||
| 10. Post-cortisol AUCI | .50 | .44 | −.35 | .47 | .33 | −.22 | .38 | −.13 | .34 | — | ||
| 11. Pre-sTNFαRII AUCI | .35 | .05 | .02 | −.20 | .26 | .19 | −.05 | .16 | .77** | .15 | — | |
| 12. Post-sTNFαRII AUCI | .30 | .08 | −.41 | .36 | .44 | −.02 | .41 | .02 | .10 | .59* | −.06 | — |
p < .05
p < .01
Note: pre-, pre-randomization; post-, post-randomization, HEQ, hypnotic experiences questionnaire; CPTo, cold pressor pain tolerance; PI, pain intensity rating; PU, pain unpleasantness rating; AUCI, area under the curve with respect to increase.
Pain ratings of intensity and unpleasantness
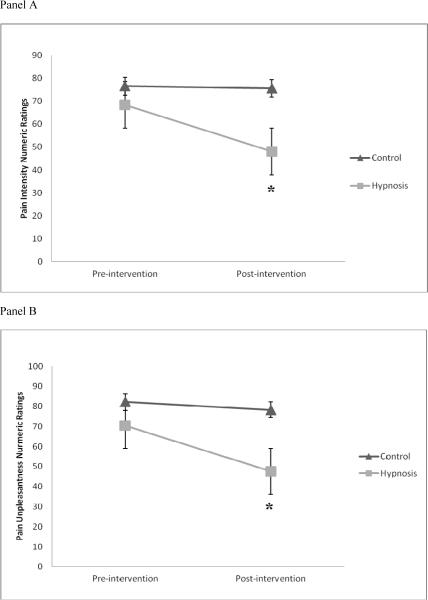
Pre and Post CPTo was significantly and inversely correlated with Pre and Post ratings of PI and PU, respectively (rrange = −0.74 to −0.86, all p's < 0.05; Table 2). These findings suggests that individuals who produced greater PI and PU ratings were less willing to tolerate the painful cold water, whereas individuals with diminished pain ratings had longer CPTo. Examination of the CPTo means in Table 3 demonstrates that Hypnosis and Control were not sigificantly different for CPTo assessed at Pre (p = ns) and Post (p = ns). Accordingly, CPTo was not included as a covariate in subsequent analyses of PI and PU pain ratings.There were no significant differences between Hypnosis (PI = 68.5 ± 26.7; PU = 70.4 ± 25.7) and Control (PI = 76.5 ± 18.7; PU = 82.2 ± 18.1) for mean ratings of PI (F(1, 22) = 0.74, p = ns, η2 = 0.033) or PU (F(1, 22) = 1.68, p = ns, η2 = 0.071) at Pre. This suggests Hypnosis and Control had roughly equivalent PI and PU ratings prior to initiation of the intervention phase of the study. RMANOVAs revealed significant study group (Hypnosis and Control) by time (Pre and Post) interactions for PI (F(1, 22) = 5.06, p = 0.04, η2 = 0.187) and PU (F(1, 22) = 8.27, p = 0.01, η2 = 0.273); see Figure 2, panels A and B, respectively. The effect sizes for these interactions were large. Post-hoc tests of these significant interactions revealed that, when compared to Pre ratings, the Post ratings of PI and PU were significantly decreased for Hypnosis (Bonferroni adjusted post-hoc tests: p's < 0.01) However, Pre-Post PI and PU ratings were virtually unchanged for Control (p's = ns). Mean ratings of PI (F(1, 22) = 7.39, p = 0.01, η2 = 0.251) and PU (F(1, 22) = 8.36, p = 0.01, η2 = 0.275) were found to be significantly lower for Hypnosis (PI = 48.1 ± 29.1; PU = 47.6 ± 31.7) compared to Control (PI = 75.6 ± 19.4; PU = 78.3 ± 18.7) at Post.
Table 3.
Cold pressor tolerance (i.e., CPTo) times measured in seconds.
| Pre-Intervention | Post-Intervention | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | % to 300s | Mean ± SD | Range | % to 300s | |
| Hypnosis | 205.7 ± 111.3 | 52 – 300 | 50% | 243.3 ± 105.5 | 19 – 300 | 75% |
| Control | 214.6 ± 126.4 | 15 – 300 | 58.3% | 209.5 ± 128.7 | 15 – 300 | 58.3% |
Note: Mean and SD measured in seconds. % to 300s = the percentages of participants who tolerated the CPT for the maximum duration of time allowed (i.e., 300 seconds).
Figure 2.
Panel A: Pre- to post-intervention change in pain intensity. *= significant change for hypnosis.
Panel B: Pre- to post-intervention change in pain unpleasantness. *= significant change for hypnosis.
Pre cortisol and sTNFαRII reactivity to the CPT
First, the baseline measures for Pre cortisol and sTNFαRII were compared between Hypnosis and Control. Significant differences were not found for mean baseline (i.e., prior to the CPT) values of cortisol (F(1, 22) = 0.35, p = ns, η2 = 0.016) or sTNFαRII (F(1, 22) = 0.00, p = ns, η2 = 0.00). The absence of statistical differences for baseline cortisol and sTNFαRII between the two study groups suggests that participants had equivalent cortisol and sTNFαRII levels prior to initiation of CPT. Accordingly, neither baseline cortisol nor sTNFαRII required statistical control in subsquent analyses. CPTo was statistically controlled in the analyses of Pre cortisol and sTNFαRII given the potential for individual differences in CPTo to confound physiological reactivity to the CPT.
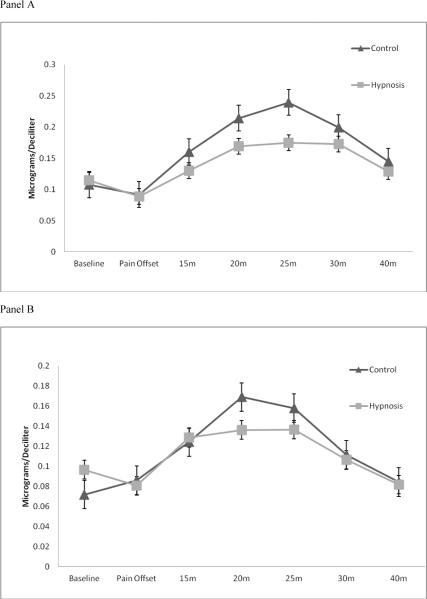
Pre cortisol reactivity is shown in Figure 3, panel A. Analysis of Pre physiological reactivity included a between-subjects factor for study group (Hypnosis, Control) and a within-subjects factor for time (baseline, pain offset, 15, 20, 25, 30, and 40 minutes post-CPT initiation). RM-ANOVA with Greenhouse-Geisser correction revealed a marginally significant main effect of time for cortisol reactivity (F(6, 126) = 2.43, p = 0.08, η2 = 0.104); however, the main effect of study group difference was non-significant (F(1, 21) = 0.02, p = ns, η2 = 0.001) and the interaction effect between study group and time was also non-significant (F(6, 126) = 0.41, p = ns, η2 = 0.019). The effect size for the main effect of time was medium-sized while the other effect sizes were small. No post-hoc tests for probing between- and within-group differences were completed due to the lack of statistical significance.
Figure 3.
Panel A: Pre-intervention cortisol reactivity; error bars represent SEM
Panel B: Post-intervention cortisol reactivity; error bars represent SEM
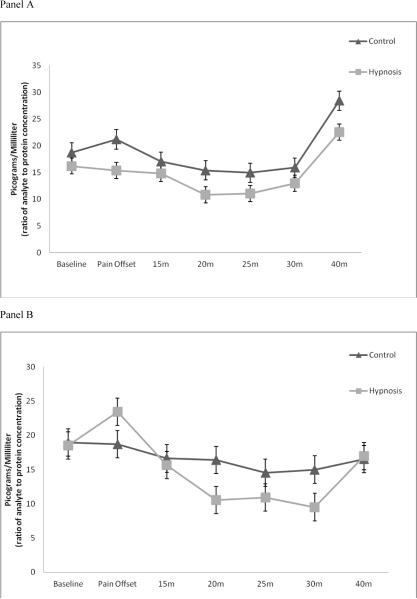
Pre sTNFαRII reactivity is shown in Figure 4, panel A. Results of an additional RM-ANOVA with Greenhouse-Geisser correction demonstrated non-significant main effects for time (F(6, 126) = 0.82, p = ns, η2), study group (F(1, 21) = 0.48, p = ns, η2 = 0.022), and their interaction (F(6, 126) = 0.91, p = ns, η2 = 0.041). All effects sizes were small. Again, no post-hoc tests were completed due to the lack of statistical significance.
Figure 4.
Panel A: Pre-intervention sTNFαRII reactivity; error bars represent SEM
Panel B: Post-intervention sTNFαRII reactivity; error bars represent SEM
Post cortisol and sTNF αRII reactivity to the CPT
No significant differences were found between Hypnosis and Control for mean baseline values of post-intervention cortisol (F(1, 22) = 2.19, p = ns, η2= 0.09) or sTNFαRII (F(1, 22) = 0.14, p = ns, η2 = 0.016). This suggests that the two study groups had equivalent cortisol and sTNFαRII levels prior to initiation of CPT; neither baseline cortisol nor sTNFαRII required statistical control. Similar to the pre-intervention physiological analyses, CPTo was again statistically controlled in the analyses of post-intervention cortisol and sTNFαRII.
Post-intervention cortisol reactivity is shown in Figure 3, panel B. Analysis of Post physiological reactivity also included a between-subjects factor for study group (Hypnosis, Control) and a within-subjects factor for time (baseline, pain offset, 15, 20, 25, 30, and 40 minutes post-CPT initiation). Results of RM-ANOVA revealed a significant main effect of time for cortisol reactivity (F(6, 126) = 13.33, p < 0.001, η2 = 0.388). Neither the main effect of study group (F(1, 21) = 0.23, p = ns, η2 = 0.011) nor the interaction effect between study group and time (F(6, 126) = 1.00, p = ns, η2 0.051) were statistically significant. The effect sizes for the main effect of time and the interaction effect of study group by time on cortisol were large and medium, respectively. Post-hoc analyses of the main effect of time on Post cortisol reactivity indicated that for all participants (irrespective of Hypnosis or Control) cortisol was significantly elevated from baseline at 15, 20, and 25 minutes following initiation of the CPT (Bonferroni adjusted post-hoc tests: p's < 0.01).
Post-intervention sTNFαRII reactivity is shown in Figure 4, panel B. Findings from RMANOVA again demonstrated a significant main effect of time for sTNFαRII reactivity (F(6, 126) = 7.92, p < 0.001, η2 = 0.274). The main effect of study group on sTNFαRII reactivity was non-significant (F(1, 21) = 0.21, p = ns, η2 = 0.010); however, the study group by time interaction effect was marginally significant (F(6, 126) = 2.14, p = 0.08, η2 = 0.092). Similar to cortisol, the effect sizes for the main effect of time and the interaction effect of study group by time on sTNFαRII were large and medium. Post-hoc tests of the main effect of time on sTNFαRII reactivity suggested that for all participants (irrespective of Hypnosis or Control) sTNFαRII was significantly decreased from baseline at 20, 25, and 30 minutes following initiation of the CPT (Bonferroni adjusted post-hoc tests: p's < 0.01).
Pre-Post changes in cortisol and sTNFαRII reactivity
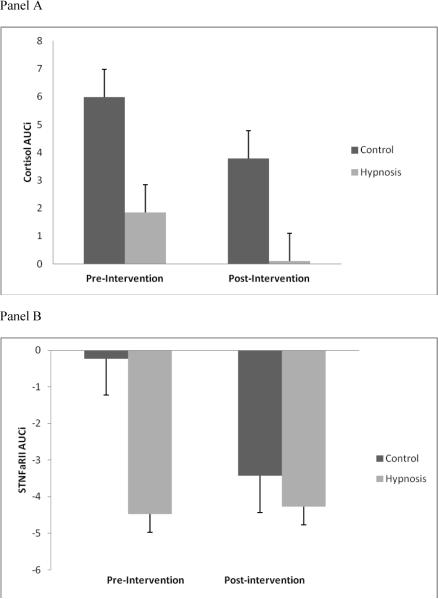
To assess change in cortisol and sTNFαRII reactivity across the study, their respective summary indicators (AUCI) were examined from Pre-Post controlling for Pre-Post change in CPTo. Analysis of Pre-Post change in physiological reactivity included a between-subjects factor for study group (Hypnosis, Control) and a within-subjects factor for time (Pre AUCI, Post AUCI). RM-ANOVA revealed a non-significant main effect of time (F(1, 21) = 1.43, p = ns, η2 = 0.064), a non-significant main effect of study group (F(1, 21) = 1.39, p = ns, η2 = 0.062), and a non-significant interaction effect of study group by time (F(1, 21) = 0.01, p = ns, η2 = 0.002) for cortisol reactivity (Figure 5, panel A). The main effects of study group and time on Pre-Post change in cortisol reactivity were medium-sized, while the interaction was small. Similarly, an additional RM-ANOVA demonstrated a non-significant main effect of time (F(1, 21) = 0.77, p = ns, η2 = 0.036), a non-significant main effect of study group (F(1, 21) = 1.29, p = ns, η2 = ), and a non-significant interaction effect of study group by time (F(1, 21) = 0.87, p = ns, η2 = 0.040) for sTNFαRII reactivity (Figure 5, panel B). Effect sizes for Pre-Post changes in sTNFαRII reactivity were small to medium in nature.
Figure 5.
Panel A: Pre- to post-intervention change in AUCI cortisol reactivity. Note that positive numbers represent an increase from baseline.
Panel B: Pre- to post-intervention change in AUCI sTNFαRII reactivity. Note that negative numbers represent a decrease from baseline.
DISCUSSION
Several findings emerged from this randomized, controlled pilot study. First, the hypnosis intervention was associated with large, as evidenced by effect sizes, reductions in PI and PU self-reported as 0–100 numeric ratings of cold pain. The effect of hypnosis on pain ratings supports our first hypothesis and is consistent with previous reports [4,5,6,7,8,10]. Although reductions in PI and PU were both significant, Hypnosis demonstrated the most robust effect for reduction in PU. This result is congruent with the findings of Rainville et al. [12] and Price and Barber [14], which demonstrated that pain reduction depended on the nature of the hypnotic suggestion and that the sensory and affective components of pain are differentially impacted by hypnosis as a function of the specific post-hypnotic suggestion used rather than hypnosis in general. Given that all participants in the current study randomized to Hypnosis were exposed to a combination of post-hypnotic suggestions for reductions in pain intensity and/or pain affect prior to choosing the post-hypnotic suggestion they liked best, it is reasonable that significant reductions were demonstrated for both ratings of PI and PU, with PU demonstrating the greatest reduction.
The second finding that emerged from this pilot study was that hypnosis was not significantly associated with suppression of cortisol or sTNFαRII reactivity to the acute painful stimulus. Specifically, there were no significant differences in cortisol or sTNFαRII reactivity between Hypnosis and Control over time at Pre or Post. Further, Pre-Post change in the summary indicators (AUCI) of cortisol and sTNFαRII were not significantly different between Hypnosis and Control. Results revealed a marginally significant main effect of time for cortisol at Pre and significant main effects of time for cortisol and sTNFαRII at Post. This suggests that the cold water pain was successful in eliciting increased cortisol and decreased sTNFαRII from baseline, particularly at Post; however, the reactivity of cortisol and sTNFαRII to cold water pain was comparable between Hypnosis and Control. These findings are not consistent with previous preliminary reports [see 37 for review] and do not lend support to our hypothesis that hypnosis is associated with suppression of cortisol and sTNFαRII reactivity. There are several potential explanations (among others) that deserve to be mentioned addressing the lack of associations among hypnosis, cortisol, and sTNFαRII in this pilot study.
It may be simply that the effects of hypnosis do not engage cortisol and sTNFαRII reactivity to acute painful stimulation. However, this does not appear to purely be the case because a limited evidence base suggests that hypnosis has the potential to modulate cortisol and pro-inflammatory cytokine markers in certain subjects [64,65,66]. Thus, it is possible that the current study's lack of hypnosis effects on cortisol and sTNFαRII reactivity is due to technical reasons. For example, this study's intervention methods seem to have produced a sufficient “dose” of hypnosis for reducing pain ratings; however, this may not have been the case for cortisol and sTNFαRII reactivity. It has yet to be determined whether cortisol and sTNFαRII respond to hypnosis in a dose-dependent manner, but if this is indeed the case, perhaps a larger dose of hypnosis was needed in our study to demonstrate effects on these outcomes. Another possible explanation was our inclusion of healthy college student participants. Acute painful stressors are typically associated with promotion of transient alterations of cortisol and pro-inflammatory cytokines in young healthy adults [67,68], and such reactivity is generally considered adaptive for maintaining homeostasis. Conversely, these alterations tend to be excessive, fail to return to normalcy, or otherwise function inadequately in chronic pain conditions [69,70,71,72], which is considered maladaptive. Therefore, hypnosis may be better for helping restore the maladaptive physiological reactivity of those with chronically painful conditions to an adaptive state rather than influencing the already adaptive reactivity of healthy young adults. One final explanation is that hypnosis may indirectly affect cortisol and sTNFαRII reactivity via pain reduction. Due to the small sample size, the current study could not reliability examine whether Pre-Post changes in PI and PU were associated with Pre-Post changes in cortisol or sTNFαRII. However, the hypothesis that hypnosis-induced reductions of PI and PU might mediate alterations of cortisol and sTNFαRII reactivity is of interest and remains to be tested. Lastly, it seems important to point out that the effect sizes for the associations of hypnosis with change in cortisol and sTNFαRII over time were small at Pre (η2 = 0.019 to 0.041) but were medium in size at Post (η2 0.051 to 0.092). Thus, hypnosis may well have been associated with Pre-Post alterations in cortisol and sTNFαRII; however, this study's small sample size likely prevented sufficient power for detecting the effect of hypnosis on Pre-Post change in cortisol and sTNFαRII.
Some important limitations of this study merit caution when interpreting the findings and will need to be addressed in future research. First, due to limited resources and the pilot nature of this study, we chose to incorporate a no-intervention control group rather than an active control group (e.g., relaxation training). As a result, it is not possible to conclude whether the obtained results are entirely due to the effects of hypnosis or to other potential analgesic effects such as placebo. Second, this study possessed a relatively small sample of healthy individuals known to be highly susceptible to hypnosis. As a consequence, the statistical power for detecting the Pre-Post intervention effects of hypnosis was low and the generalizability of these findings is not perfectly clear. Accordingly, replication of these effects in other larger samples appears necessary. Third, this study was only able to sample a single soluble receptor of a pro-inflammatory cytokine (sTNFαRII). Simultaneous assessment of many actual cytokines and/or their receptors in a biological sample would provide more comprehensive information rather than assessing the receptor of a single cytokine [73]. Hence, systemic measurement of multiple cytokines in oral fluids, plasma, and/or serum may provide a more detailed indication of the ability for hypnosis to alter T-helper responses (Th1 or Th2) following acute painful stimulation [74].
In conclusion, compared to Control, Hypnosis was associated with large reductions in ratings of PI and PU, but was not found to be associated with significant alterations of salivary cortisol or sTNFαRII reactivity over time. Dysfunctional HPA axis and pro-inflammatory cytokine activity have been suggested to be implicated in the development and perpetuation of a variety of painful conditions and phenomena. The ability of pharmacological agents to target HPA axis and pro-inflammatory cytokine activity as a means of pre-emptive analgesia and pain treatment has received considerable attention [75,76,77]. However, much less research has been dedicated to examining whether non-pharmacological treatments (e.g., hypnosis, cognitive-behavioral therapy, mindfulness-based relaxation) might also affect these physiological targets. Because of this, and because many of the effect sizes noted in this pilot study were in the moderate-large range, it appears that a larger replication study of the effects of hypnosis on pain-related HPA axis and pro-inflammatory cytokine activity is warranted. On balance, this study provides support for the viability of hypnosis as a meaningful contributor to non-pharmacological analgesia and underscores the potential for clinical hypnosis to alter physiological mechanisms believed to be implicated in the facilitation of pain.
Acknowledgment
Funding and support for this study and manuscript preparation was provided by NIH/NCCAM Grant R21AT003250-01A1 (L.McGuire) and by NIH Training Grant T32NS045551-06 provided to the University of Florida (B. Goodin). Special thanks to Dr. Russell Hibler for his expertise and training of research assistants for provision of hypnosis, and supervision of its implementation.
Footnotes
Conflict of Interest Statement There are no conflicts of interest, or any financial interests, to report with regard to this work for any of the authors.
References
- 1.Montgomery GH, David D, Winkel G, Silverstein JH, Bovbjerg DH. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth Analg. 2002;94:1639–45. doi: 10.1097/00000539-200206000-00052. PubMed: 12032044. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery GH, DuHamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: how effective is hypnosis? Int J Clin Exp Hypn. 2000;48:138–153. doi: 10.1080/00207140008410045. PubMed: 10769981. [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Stubner S, Krings T, Meister IG, Rex S, Thron A, Roissaint R. Clinical hypnosis modulates functional magnetic resonance imaging signal intensities and pain perception in a thermal stimulation paradigm. Reg Anesth Pain Med. 2004;29(6):549–556. doi: 10.1016/j.rapm.2004.09.002. PubMed: 15635514. [DOI] [PubMed] [Google Scholar]
- 4.Berger MM, Davadant M, Marin C, Wasserfallen CP, Maravic P, Koch N, Raffoul W, Chiolero RL. Impact of a pain protocol including hypnosis in major burns. Burns. 2010;36:639–646. doi: 10.1016/j.burns.2009.08.009. PubMed: 19880257. [DOI] [PubMed] [Google Scholar]
- 5.Wiechman Askay S, Patterson DR, Jensen MP, Sharar SR. A randomized controlled trial of hypnosis for burn wound care. Rehab Psych. 2007;52(3):247–253. [Google Scholar]
- 6.Wright BR, Drummond PD. Rapid induction analgesia for the alleviation of procedural pain during burn care. Burns. 2000;26:275–282. doi: 10.1016/s0305-4179(99)00134-5. PubMed: 10741595. [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsen R, Baad-Hansen L, Svensson P. Hypnosis in the management of persistent idiopathic orofacial pain: Clinical and psychosocial findings. Pain. 2008;136(1–2):44–52. doi: 10.1016/j.pain.2007.06.013. PubMed: 17689192. [DOI] [PubMed] [Google Scholar]
- 8.Castel A, Perez M, Sala J, Padrol A, Rull M. Effect of hypnotic suggestion on fibromyalgia pain: Comparison between hypnosis and relaxation. Eur J Pain. 2007;11:463–468. doi: 10.1016/j.ejpain.2006.06.006. PubMed: 16889999. [DOI] [PubMed] [Google Scholar]
- 9.Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull. 2003;129:495–521. doi: 10.1037/0033-2909.129.4.495. PubMed: 12848218. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MP, Hanley MA, Engel JM, Romano JM, Barber J, Cardenas DD, Kraft GH, Hoffman AJ, Patterson DR. Hypnotic analgesia for chronic pain in persons with disabilities: A case series. Int J Clin Exp Hypn. 2005;53(2):198–228. doi: 10.1080/00207140590927545. PubMed: 16025734. [DOI] [PubMed] [Google Scholar]
- 11.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. PubMed: 2952934. [DOI] [PubMed] [Google Scholar]
- 12.Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159–171. doi: 10.1016/S0304-3959(99)00048-2. PubMed: 10467921. [DOI] [PubMed] [Google Scholar]
- 13.Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cogn Neurosci. 1999;11:110–125. doi: 10.1162/089892999563175. PubMed: 9950718. [DOI] [PubMed] [Google Scholar]
- 14.Price DD, Barber J. An analysis of factors that contribute to the efficacy of hypnotic analgesia. J Abnorm Psychol. 1987;96:46–51. doi: 10.1037//0021-843x.96.1.46. PubMed: 3558949. [DOI] [PubMed] [Google Scholar]
- 15.Rainville P, Hofbauer RK, Bushnell MC, Duncan GH, Price DD, Paus T, Carrier B. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neruosci. 2002;14:887–901. doi: 10.1162/089892902760191117. PubMed: 12191456. [DOI] [PubMed] [Google Scholar]
- 16.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. PubMed: 9252330. [DOI] [PubMed] [Google Scholar]
- 17.Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92(5):1257–1267. doi: 10.1097/00000542-200005000-00013. PubMed: 10781270. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MP. The neurophysiology of pain perception and hypnotic analgesia: implications for clinical practice. Am J Clin Hyp. 2008;51:123–135. doi: 10.1080/00029157.2008.10401654. PubMed: 18998379. [DOI] [PubMed] [Google Scholar]
- 19.Lang EV, Benotsch EG, Fick LJ, Lutgendorf S, Berbaum ML, Berbaum KS, Logan H, Spiegel D. Adjunctive non-pharmacological analgesia for invasive medical procedures: A randomized trial. Lancet. 2000;355:1486–1490. doi: 10.1016/S0140-6736(00)02162-0. PubMed: 10801169. [DOI] [PubMed] [Google Scholar]
- 20.De Jongh RF, Vissers KC, Meert TF, Booji LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;96(4):1096–1103. doi: 10.1213/01.ANE.0000055362.56604.78. PubMed: 12651667. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson MR, La Vincente SF, Somogyi AA. In vitro opioid induced proliferation of peripheral blood immune cells correlates with in vivo cold pressor pain tolerance in humans: A biological marker of pain tolerance. Pain. 2004;110(3):751–755. doi: 10.1016/j.pain.2004.05.017. PubMed: 15288416. [DOI] [PubMed] [Google Scholar]
- 22.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: Integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67:783–790. doi: 10.1097/01.psy.0000181276.49204.bb. PubMed: 16204439. [DOI] [PubMed] [Google Scholar]
- 23.Sommer C, Kress M. Recent findings on how pro-inflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1–3):184–187. doi: 10.1016/j.neulet.2003.12.007. PubMed: 15135924. [DOI] [PubMed] [Google Scholar]
- 24.Watkins LR, Maier SF, Goehler LE. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. PubMed: 8719529. [DOI] [PubMed] [Google Scholar]
- 25.Angst MS, Clark JD, Carvalho B, Tingle M, Schelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139(1):15–27. doi: 10.1016/j.pain.2008.02.028. PubMed: 18396374. [DOI] [PubMed] [Google Scholar]
- 26.Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. PubMed: 15494200. [DOI] [PubMed] [Google Scholar]
- 27.Martinez V, Fletcher D, Bouhassira D, Sessler D, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: Quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105(3):815–821. doi: 10.1213/01.ane.0000278091.29062.63. PubMed: 17717244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon JG, Friedberg JS, Gelfand JA, Tompkins RG, Burke JF, Dinarello CA. Circulating interleukin-1 beta and tumor necrosis factor-alpha concentrations after burn injury in humans. Crit Care Med. 1992;20:1414–1419. doi: 10.1097/00003246-199210000-00009. PubMed: 1395662. [DOI] [PubMed] [Google Scholar]
- 29.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG. Charcterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. PubMed: 18391855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hobson KG, Havel PJ, McMurtry AL, Lawless MB, Palmieri TL, Greenhalgh DD. Circulating leptin and cortisol after burn injury: Loss of diurnal pattern. J Burn Care Rehabil. 2004;25(6):491–499. doi: 10.1097/01.bcr.0000144532.02792.6e. PubMed: 15534457. [DOI] [PubMed] [Google Scholar]
- 31.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: Evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–117. doi: 10.1016/j.pain.2004.12.007. PubMed: 15733636. [DOI] [PubMed] [Google Scholar]
- 32.Johansson AC, Gunnarsson LG, Linton SJ, Bergkvist L, Stridsberg M, Nilsson O, Cornefjord M. Pain, disability and coping reflected in the diurnal cortisol variability in patients scheduled for lumbar disc surgery. Eur J Pain. 2008;12(5):633–640. doi: 10.1016/j.ejpain.2007.10.009. PubMed: 18077197. [DOI] [PubMed] [Google Scholar]
- 33.Ohtori S, Inoue G, Ito T, Koshi T, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in verterbral endplates of patients with discogenic low back pain and modic type 1 or type 2 changes on MRI. Spine. 2006;31(9):1026–1031. doi: 10.1097/01.brs.0000215027.87102.7c. PubMed: 16641780. [DOI] [PubMed] [Google Scholar]
- 34.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Ann Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. PubMed: 19400724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBeth J, Silman AJ, Gupta A, Chiu YH, Ray D, Morriss R, Dickens C, King Y, Macfarlane GJ. Moderation of psychosocial risk factors through dysfunction of the hypothamic-pituitary-adrenal stress axis in the onset of chronic widespread musculoskeletal pain. Arthritis Rheum. 2007;56(1):360–371. doi: 10.1002/art.22336. PubMed: 17195240. [DOI] [PubMed] [Google Scholar]
- 36.Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 37.Gruzelier JH. A review of the impact of hypnosis, relaxation, guided imagery and individual differences on aspects of immunity and health. Stress. 2002;5:147–163. doi: 10.1080/10253890290027877. [DOI] [PubMed] [Google Scholar]
- 38.Weber C, Arck P, Mazurek B, Klapp BF. Impact of a relaxation training on psychometric and immunological parameters in tinnitus sufferers. J Psychosom Res. 2002;52:29–33. doi: 10.1016/s0022-3999(01)00281-1. PubMed: 11801262. [DOI] [PubMed] [Google Scholar]
- 39.Zautra AJ, Davis MC, Reich JW, Nicassio P, Tennen H, Finan P, Kratz A, Parrish B, Irwin MR. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psych. 2008;76:408–421. doi: 10.1037/0022-006X.76.3.408. PubMed: 18540734. [DOI] [PubMed] [Google Scholar]
- 40.Shor RE, Orne EC. Harvard Group Scale of Hypnotic Suceptibility: Form A. Consulting Psychologists Press; Palo Alto, CA: 1962. [Google Scholar]
- 41.Weitzenhoffer AM, Hilgard ER. Stanford Hypnotic Susceptibility Scale: Form C. Consulting Psychologists Press; Palo Alto, CA: 1962. [Google Scholar]
- 42.Spiegel D, Bierre P, Rootenberg J. Hypnotic alteration of somatosensory perception. Am J Psychiatry. 1989;146:749–754. doi: 10.1176/ajp.146.6.749. PubMed: 2729425. [DOI] [PubMed] [Google Scholar]
- 43.Kihlstrom JF, Register PA. Optimal scoring of amnesia on the Harvard Group Scale of Hypnotic Susceptibility, Form A. Int J Clin Exp Hypn. 1984;32:51–57. doi: 10.1080/00207148408416000. PubMed: 6693222. [DOI] [PubMed] [Google Scholar]
- 44.Shor RE, Orne MT, O'Connell DN. Validation and cross-validation of a scale of self-reported personal experiences which predicts hypnotizability. J Psychol. 1962;53:55–75. [Google Scholar]
- 45.Appel PR, Bleiberg J. Pain reduction is related to hynotizability but not to relaxation or to reduction in suffering: A preliminary investigation. Am J Clin Hyp. 2006;48(2–3):153–161. doi: 10.1080/00029157.2005.10401512. PubMed: 16482842. [DOI] [PubMed] [Google Scholar]
- 46.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10(6):556–572. doi: 10.1016/j.jpain.2009.02.002. PubMed: 19380256. [DOI] [PubMed] [Google Scholar]
- 47.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90:261–269. doi: 10.1016/S0304-3959(00)00406-1. PubMed: 11207398. [DOI] [PubMed] [Google Scholar]
- 48.Goodin BR, Quinn NB, King CD, Page GG, Haythornthwaite JA, Edwards RR, Stapleton L, McGuire L. Psychophysiology. 2011. Salivary cortisol and soluble tumor necrosis factor-α receptor II responses to multiple experimental modalities of acute pain. in press. PubMed: 21895688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. PubMed: 8047637. [DOI] [PubMed] [Google Scholar]
- 50.Lang EV, Lutgendorf S, Logan H, Benotsch EG, Laser E, Spiegel D. Nonpharmacologic analgesia and anxiolysis for interventional radiological procedures. Sem Intervention Rad. 1999;16:113–12. [Google Scholar]
- 51.Kiernan BD, Dane JR, Phillips LH, Price DD. Hypnotic analgesia reduces R-III nociceptive reflex: further evidence concerning the multifactorial nature of hypnotic analgesia. Pain. 1995;60:39–47. doi: 10.1016/0304-3959(94)00134-Z. PubMed: 7715940. [DOI] [PubMed] [Google Scholar]
- 52.Breivik H, Borchgrevnik PC, Allen SM, Rosselan LA, Romundstad L, Breivik Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103. PubMed: 18487245. [DOI] [PubMed] [Google Scholar]
- 53.Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. PubMed: 14747646. [DOI] [PubMed] [Google Scholar]
- 54.Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin Diagn Lab Immunol. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. PubMed: 9665958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumor necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. PubMed: 7859870. [DOI] [PubMed] [Google Scholar]
- 56.Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M, Shirtcliff EA. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Horm Behav. 2004;46:39–46. doi: 10.1016/j.yhbeh.2004.01.006. PubMed: 15215040. [DOI] [PubMed] [Google Scholar]
- 57.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. PubMed: 942051. [DOI] [PubMed] [Google Scholar]
- 58.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. PubMed: 15122924. [DOI] [PubMed] [Google Scholar]
- 59.Matheson G, Shue KL, Bart CA. Validation study of a short-form hypnotic experience questionnaire and its relationship to hypnotizability. Am J Clin Hyp. 1989;32:17–26. doi: 10.1080/00029157.1989.10402792. PubMed: 2773816. [DOI] [PubMed] [Google Scholar]
- 60.Kelly PJ. Doctoral dissertation. University of Waterloo; Waterloo, Ontario, Canada: 1985. The relationship between hypnotic ability and hypnotic experience. [Google Scholar]
- 61.Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomized controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. PubMed: 10480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum, Hillsdale: 1988. [Google Scholar]
- 63.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. PubMed: 12892658. [DOI] [PubMed] [Google Scholar]
- 64.Domangue BB, Margolis CG, Lieberman D, Kaji H. Biochemical correlates of hypnoanalgesia in arthritic pain patients. J Clin Psychiatry. 1985;46:235–238. [PubMed] [Google Scholar]
- 65.Zachariae R, Bjerring P, Zachariae C, Arendt-Nielsen L, Nielsen T, Elderup E, Larsen CS, Gotliebsen K. Monocyte chemotactic activity in sera after hypnotically induced emotional states. Scand J Immunol. 1991;34:71–79. doi: 10.1111/j.1365-3083.1991.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 66.Wood GJ, Tanavoli S, Bughi S, Tanavoli S, Morrison J, Zadeh HH. Hypnosis, differential expression of cytokines by T-cell subsets, and the hypothalamic-pituitary-adrenal axis. Am J Clin Hypn. 2003;45:179–196. doi: 10.1080/00029157.2003.10403525. [DOI] [PubMed] [Google Scholar]
- 67.de Kloet ER. Hormones and the stressed brain. Ann NY Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. PubMed: 15240347. [DOI] [PubMed] [Google Scholar]
- 68.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. PubMed: 12490958. [DOI] [PubMed] [Google Scholar]
- 69.Clauw DJ, Chrousos G. Chronic pain and fatigue syndromes: Overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. 1997;4:143–153. doi: 10.1159/000097332. PubMed: 9500148. [DOI] [PubMed] [Google Scholar]
- 70.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237(2–3):243–250. doi: 10.1016/s0304-3940(97)00810-0. PubMed: 9453216. [DOI] [PubMed] [Google Scholar]
- 71.Parker AJ, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med. 2001;31(8):1331–1345. doi: 10.1017/s0033291701004664. PubMed: 11722149. [DOI] [PubMed] [Google Scholar]
- 72.Lisowska B, Maslinksi W, Maldyk P, Zabek J, Baranowska E. The role of cytokines in inflammatory response after total knee arthroplasty in patients with rheumatoid arthritis. Rheumatol Int. 2008;28(7):667–671. doi: 10.1007/s00296-007-0508-1. PubMed: 18071707. [DOI] [PubMed] [Google Scholar]
- 73.Prabhakar U, Eirikis E, Reddy M, Silvestro E, Spitz S, Pendley C, Davis HM, Miller BE. Validation and comparative analysis of a multiplexed assay for the simultaneous quantitative measurement of Th1/Th2 cytokines in human serum and human peripheral blood mononuclear cell culture supernatants. J Immunol Meth. 2004;291:27–38. doi: 10.1016/j.jim.2004.04.018. PubMed: 15345302. [DOI] [PubMed] [Google Scholar]
- 74.Sachdeva N, Yoon HS, Oshima K, Garcia D, Goodkin K, Asthana D. Biochip array-based analysis of plasma cytokines in HIV patients with immunological and virological discordance. Scand J Immunol. 2007;65:549–554. doi: 10.1111/j.1365-3083.2007.01906.x. PubMed: 17523947. [DOI] [PubMed] [Google Scholar]
- 75.Chou R, Hoyt Huffman L. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. PubMed: 17909211. [DOI] [PubMed] [Google Scholar]
- 76.Pinto-Ribeiro F, Moreira V, Pego JM, Leao P, Almeida A, Sousa N. Anti-nociception induced by chronic glucocorticoid treatment is correlated to local modulation of spinal neurotransmitter content. Mol Pain. 2009;5(41):1–11. doi: 10.1186/1744-8069-5-41. PubMed: 19630968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roelofs P, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: An updated Cochrane review. Spine. 2008;33(16):1766–1774. doi: 10.1097/BRS.0b013e31817e69d3. PubMed: 18580547. [DOI] [PubMed] [Google Scholar]