Abstract
Increased placental release of soluble VEGF receptor-1 (sFlt-1) is believed to play an important role in the pathogenesis of preeclampsia (PE). Although the reason for increased placental sFlt-1 release in PE is unknown, proteolytic effect has been proposed as one of the mechanisms that mediate sFlt-1 release in the placenta. In this study, using various protease inhibitors, we tested the possible role of proteases in sFlt-1 release by human placenta. Villous explants from normal term placentas were incubated with various protease inhibitors including serine protease inhibitors (PMSF, aprotini, and specific chymotrypsin inhibitor (CI)), cysteine protease inhibitor E-64, metalloendopeptidase inhibitor PAD, and universal metalloprotease (ADAM) inhibitor PTM. Culture medium was collected and measured for sFlt-1 by ELISA. Our results showed that villous tissue treated with CI and PTM produced significantly less sFlt-1 than those of controls. PMSF, aprotini, E-64, and PAD had no effect on sFlt-1 release. We further examined chymotrypsin-like protease/chymase and ADAM10 expressions in tissue sections from normal and PE placentas by immunohistochymistry. We found that immunostaining for chymase and ADAM10 was significantly increased in the layer of syncytiotrophoblasts in PE placentas compared to normal placentas. These results suggest chymotrypsin-like serine protease and ADAM10, but not cysteine protease and metalloendopeptidase, may play a role in inducing sFlt-1 release in PE placentas.
Keywords: protease, sFlt-1, placenta, preeclampsia
Introduction
Soluble forms of vascular endothelial cell growth factor receptor-1 (sFlt-1) bind to free VEGF and PlGF in the circulation. It exerts an antagonistic effect on VEGF and PlGF biological functions such as angiogenesis. Placenta is a major source of sFlt-1 in the maternal circulation during pregnancy (1). Notably, placenta from preeclampsia produces significantly more sFlt-1 than normal placenta does (2). The placenta-derived sFlt-1 is believed to contribute to the maternal systemic manifestations in preeclampsia including hypertension, proteinuria, and glomerular endotheliosis. (1). Thus, increased placental production of sFlt-1 plays an important role in the pathophysiology and pathogenesis of preeclampsia (3).
Study has shown that both Flt-1 and sFlt-1 mRNA expressions are proportionally increased in preeclamptic placenta (1). In spite of well-accepted evidence about the pivotal role of sFlt-1 in preeclampsia, the mechanism of increased sFlt-1 production remains elusive. Several explanations have been put forward. For example, alternative splicing of Flt-1 mRNA has been identified as a key regulating step in sFlt-1 production (4,5). Transcriptional regulation or mRNA stability is also a suggestive (4). Many studies have demonstrated that a soluble form of proteins is often derived from their membrane forms by proteolytic process via specific proteases usually referred to as “secretases” or “sheddases” (6,7). Thus, another possibility of increased sFlt-1 release by the placenta (trophoblasts) could be due to proteolytic effect, i.e. shed from the cell membrane by proteases.
A number of membrane integral proteins can be shed as soluble forms by proteases and the shed molecules are biological active and play an important role in disease processes. For example, the deposition of amyloid precursor protein metabolite βA4 is currently believed to be the central pathological event in the development of Alzeimer’s disease and this peptide can be shed from type I integral membrane protein β-amyloid precursor protein (APP) by three secretases (α-, β-, and γ-secretase) (8). Another example is angiotensin II converting enzyme (ACE). ACE converts angiotensin I to angiotensin II, which is critical for controlling blood pressure, fluid, and electrolyte homeostasis. The level of soluble ACE in plasma is altered in several diseases, such as hypertension, diabetes mellitus, and sarcoidosis (9). It has been demonstrated that soluble ACE can be induced by metalloprotease, whereas serine-, thio- or asparatic-proteases had no effect (9).
Abundant proteases are present in the placental trophoblasts, including matrix metalloproteases (MMPs) (10), a disintegrin and metalloprotease (ADAMs) (11), cathespins (12), caspases (13). Recently, we found that a serine protease (chymotrypsin-like-protease/chymase) activity was increased in placentas from preeclampsia (14) and the maternal protease activity was also elevated in preeclampsia (15). At present, there is little information available regarding the possible role of placental proteases in regulating placental sFlt-1 production. Thus, we conducted this in vitro study to test our hypothesis that proteolytic effect of proteases might modulate sFlt-1 release from the placenta.
Materials and Method
Placenta and patient Information
Placentas from normal pregnancy and preeclampsia were used in this study. Normal pregnancy is defined as pregnancy with normal blood pressure (<140/90mmHg), negative proteinuria, and absence of obstetrical and medical complications. Preeclampsia is defined as follows: sustained systolic blood pressure of ≥ 140 mmHg or a sustained diastolic blood pressure of ≥ 90mmHg on two separate readings; proteinuria measurement of 1+ or more on dipstick, or 24 hour urine protein collection with ≥ 300mg in the specimen. To avoid clinical phenotypic differences, patients complicated with HELLP syndrome (hemolysis, elevated liver enzyme and low platelet count), diabetes and/or renal disease were excluded. No patient had a sign of infection and smokers were excluded. This study was approved by the Institutional Review Board (IRB) for Human Research at LSUHSC-Shreveport. A total of 41 placentas (31 from normal and 10 from preeclampsia) were used in this study. The patient demographic data are summarized in Table 1.
Table 1.
Demographic characteristics for normal and preeclamptic pregnancies from which placentas were used in the study
| Variables | Normal (n=31) | Preeclampsia (n=10) | p value |
|---|---|---|---|
| Maternal age (years) | 33 ± 6 | 33 ± 5 | --- |
| Racial Status | |||
| White | 5 | 1 | --- |
| Black | 26 | 9 | --- |
| Gestational Age (weeks) | 37 ± 5 | 34 ± 5 | 0.2025 |
| Blood Pressure (mmHg) | |||
| Systolic | 118 ± 11 | 169 ± 17 | <0.0001 |
| Diastolic | 74 ± 10 | 108 ± 15 | <0.0001 |
| Mode of Delivery | |||
| Vaginal | 10 | 1 | --- |
| Caesarean Section | 21 | 9 | --- |
Data presented as mean ± SD.
Placental explant culture
All placentas used in the culture experiments were from normal pregnant women. The tissue process was performed as previously described (13). Briefly, villous tissue pieces excluding chorionic and basal plates, and villous core vessels were removed from the placenta under sterile condition. Villous tissues were washed with phosphate buffered saline (PBS) repeatedly to remove red blood cells from the intervillous space. Then the tissue pieces (500mg/well) were incubated in 5 ml serum free Dulbecco’s Modified Eagles Medium (DMEM, Sigma Chemical Inc., St Louis, MO) with or without protease or protease inhibitors in culture for 6 hours (see below).
Since increased chymotrypsin-like protease/chymase activity was found in preeclamptic placentas (14), we first tested if protease chymotrypsin/chymase could promote sFlt-1 release/shedding by incubating villous explant with different concentration of chymotrypsin (Calbiochem, San Diego, CA). Villous tissue from normal placentas was incubated with concentrations of chymotrypsin at 1.0, 2.5, and 5.0 μg/ml for 6 hours in serum free DMEM. Because chymotrypsin belongs to serine protease family, we then determined the specificity of chymotrypsin induced sFlt-1 production. Specific chymotrypsin inhibitor (CI, Calbiochem) at concentrations of 0.5 and 5 μg/ml; non-specific serine protease inhibitors phenylmethylsulphonyl fluoride (PMSF) at concentrations of 50, 100, and 500μM; and aprotinin at concentrations of 1.0, 5.0 and 10.0 μg/ml were used in culture. To test if other proteases such as cysteine protease, metalloendopeptidase, and metalloprotease might also affect sFlt-1 shedding from placental tissues, cysteine protease inhibitor E-64 at concentrations of 10, 25, and 50μM; metalloendopeptidase inhibitor PAD at concentrations of 1, 5, and 10μM; and universal metalloprotease inhibitor 1,10 phenanthrolin monohydrate (PTM) at concentrations of 5 and 10mM were used in our culture system. The culture medium was collected at the end of incubation and stored at −80C until assay. PMSF, aprotini, PAD, and PTM were purchased from Sigma. The selected concentrations for aprotini and PTM were based on effective doses provided by the supplier (Sigma). The concentrations for PMSF, chymotrypsin inhibitor, PAD, and E-64 were based on published works (16–19).
Explant tissues were also collected after treatment with chymotrypsin inhibitor and PTM. Tissue pieces were either homogenized with protein lysis buffer to examine Flt-1 protein expression by immunoblot or fixed with formalin to examine Flt-1 immunostaining.
Measurement of soluble Flt-1
Soluble Flt-1 levels in the culture medium was measured by enzyme-linked immunosorbent assay (ELISA). The sFlt-1 ELISA kit was purchased from R&D systems, Inc, Minneapolis, MN. For assays, medium samples were diluted (10X) with dilution buffer provided by the ELISA kit, and then an aliquot of 100μl per sample was assayed. All samples were assayed in duplicate. The range of standard curve for sFlt-1 was 31.2 – 2000pg/ml. The assay procedures were followed by the manufacturer’s instruction. Within and between assays variations were less <8%. sFlt-1 production was calculated as pg/mg wet tissue/hour.
Immunohistochemistry
The tissue culture studies revealed that sFlt-1 release by placental tissue in culture could be attenuated by chymotrypsin inhibitor and metalloproteinase inhibitor, suggesting that serine protease chymotrypsin-like protease and metalloproteinase might contribute to sFlt-1 shedding in preeclamptic placentas. To test this possibility, immunoreactivities for chymase (a chymotrypsin-like protease) and metalloproteinase ADAM10 were examined in tissue sections from normal and preeclamptic placentas. Paraffin embedded tissue sections were immunostained with antibodies against chymase and ADAM10. The immunohistochemistry procedure was performed as previously described (20). Briefly, tissue sections were deparaffinized with xylene and serial ethanol solution. Antigen retrieval was achieved by boiling tissue slides with citrate buffer and endogenous peroxidase was quenched by 0.1% hydrogen peroxide. After blocking with goat serum, the slides were hybridized with mAb of chymase (Abcam, Cambridge, MA, AB2377) and rabbit anti-human ADAM10 antibody (Abcam, AB1997) at 4C over night. Slides stained with the same antibody were processed all together. Corresponding secondary antibodies, AB enzyme and DAB detection kit were purchased from Vector Laboratories, Inc. (Burlingame, CA). The nuclei were counterstained with haematoxylin. Stained slides were examined by an Olympus microscope (Olympus IX71, Tokyo, Japan). Images were captured by a digital camera with PictureFrame computer software (Uptronics, Inc., Sunnyvale, CA) and recorded to a microscope-linked PC.
The intensity of chymase and ADAM10 immunostaining was evaluated semiquantitatively as previously described (21,22) using the following categories: 0 (no staining); 1+ (detectable but weak staining); 2+ (moderate or distinct staining), and 3+ (intensive staining). In each slide, five different areas with 15–20 villi per area were evaluated microscopically with an x20 objective magnification. For each specimen, a H-score value was derived by calculating the sum of the percentage of villi that stained in each intensity category and multiplying that value by the weighted intensity of the staining, using the formula H-score = Pi (i + l), where i represents the intensity score, and Pi is the corresponding percentage of villi.
Protein expression
Chymase and ADAM10 protein expressions were examined in normal and preeclamptic placental tissue homogenates by Western blot. Freshly delivered placental pieces were snap frozen and homogenized with lysis buffer. An aliquot of 50μg total cellular protein per sample was used to run SDS-PAGE and transferred to Hybond-protein transfer membrane (Amersham Corp, Arlington Heights, Ill). The membrane was probed with antibodies against chymase and ADAM10. Same antibodies were used as for immunostaining. Bands were visualized with an enhanced chemiluminescent detection kit (Amersham). β-actin expression was determined to verify the protein loading consistency for the samples.
Flt-1 expression
Flt-1 expression was examined by both immunohistochemistry and Western blot in explant tissues after treated with chymotrypsin inhibitor and PTM. In both assays, a polyclonal rabbit antibody against Flt-1 (sc-316, Santa Cruz) was used. This antibody recognizes the carboxyl-terminus of Flt-1 intracellular domain. For immunohistochemistry, explant tissues were fixed and tissue sections were stained with anti-Flt-1 antibody. The immunostaining procedure was the same as described above. For Western blot, explant tissues were collected after incubation and homogenized with lysis buffer. An aliquot of 50μg of total protein per sample was used to run SDS-PAGE and membranes were probed with anti-Flt-1 and β-actin antibodies.
Statistical Analysis
Data are expressed as means ± SE and analyzed by analysis of variance (ANOVA) with Student-Newman-Keuls test as a post hoc test. A computer software program Statview (Cary, NC) was used. A probability level of p<0.05 was considered statistically significant.
Results
Serine protease induced sFlt-1 release
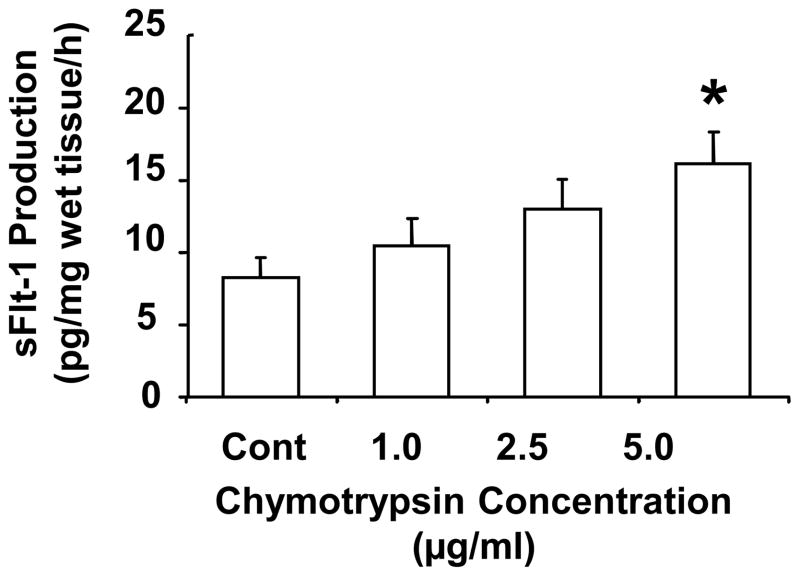
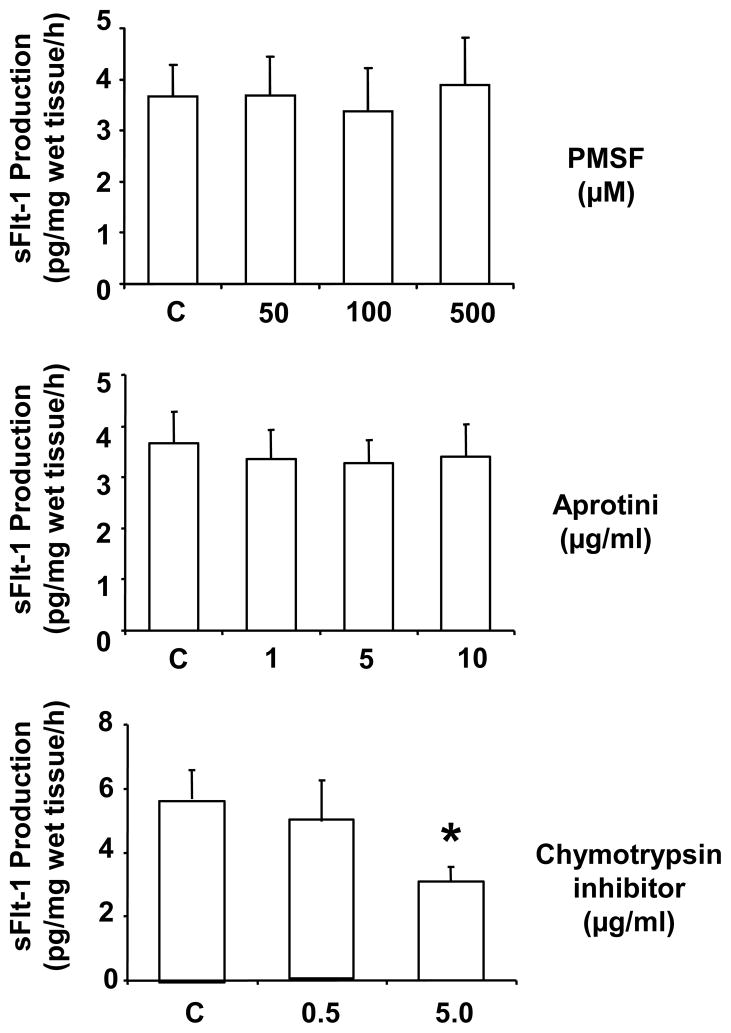
To determine if chymotrypsin could promote sFlt-1 release/shedding, villous tissues were treated with chymotrypsin at concentrations of 1.0, 2.5 and 5.0 μg/ml. We found that chymotrypsin could promote villous tissue to release sFlt-1 and this effect was in a dose-dependent manner (Figure 1). To further examine the specificity of serine protease effects, villous tissues were incubated with PMSF, aprotini, and specific chymotrypsin inhibitor (CI) at different concentrations for 6 hours, and then sFlt-1 production was determined. PMSF and aprotini are non-specific serine protease inhibitor. As shown in Figure 2, we found that sFlt-1 release was dose-dependently inhibited by chymotrypsin inhibitor, whereas neither PMSF nor aprotini had inhibitory effect (Figure 2). These data suggest that chymotrypsin-like protease/chymase may contribute to sFlt-1 shedding.
Figure 1.
Chymotrypsin promotes sFlt-1 release by placentas from normal pregnancies. Villous tissues from normal placentas (n=5) were incubated with chymotrypsin at concentrations of 1.0, 2.5 and 5.0μg/ml for 6 hours. Chymotrypsin induced sFlt-1 release in dose-dependent manner, * p<0.05: 5μg/ml vs. control.
Figure 2.
Effects of serine proteases on placental sFlt-1 release. Villous tissues from normal placentas (n=7) were incubated with serine protease inhibitors PMSF, aprotini, and chymotrypsin inhibitor (CI) at various concentrations for 6 hours. CI could reduce sFlt-1 release in a dose dependent manner, *p<0.05: CI 5μg/ml vs. control. In contrast, PMSF and aprotini had no effect on sFlt-1 release with any doses used in the study.
Metalloproteinase and sFlt-1 release
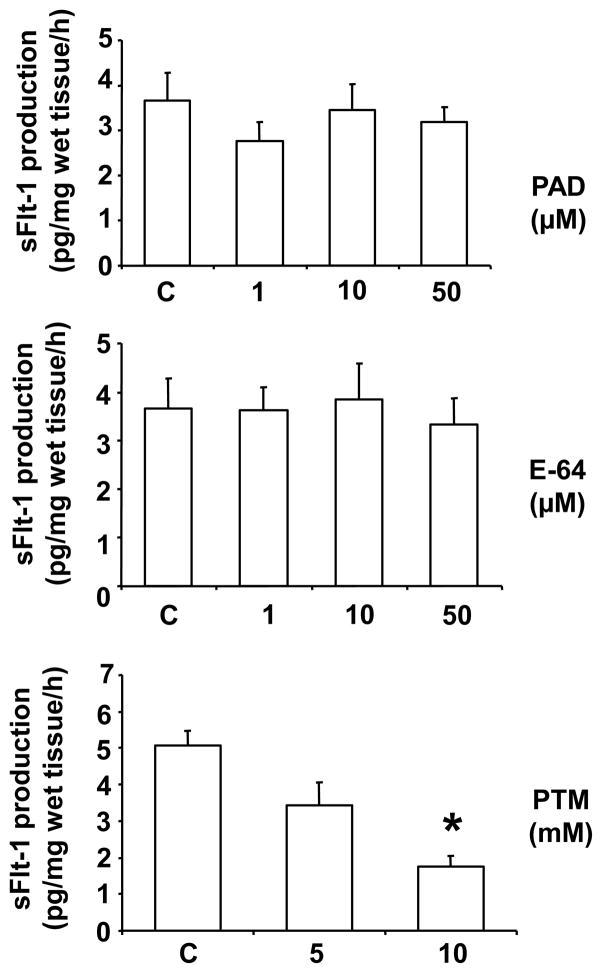
Next, we determined whether metalloendopeptidase, cysteine protease, and metalloproteinase might also affect placental tissue release of sFlt-1. Metalloendopeptidase inhibitor PAD, cysteine protease inhibitor E-64, and metalloproteinase inhibitor 1,10 phenanthrolin monohydrate (PTM) were used in our culture system. Interestingly, PTM, but not PAD and E-64, dose-dependently inhibited villous tissue release of sFlt-1 (Figure 3). These data indicate that metalloproteinase, but not metalloendopeptidase and cysteine protease, exerts biological effects on sFlt-1 shedding.
Figure 3.
Effects of cysteine protease, metalloendopeptidase, and metalloproteinase on placental sFlt-1 release. Villous tissues from normal placentas (n=7) were incubated with cysteine protease inhibitor E-64, metalloendopeptidase inhibitor phosphoramidon (PAD) and metalloproteinase inhibitor 1,10-phenanthroline monohydrate (PTM) at various concentrations for 6 hours. We found PTM, but not E-64 or PAD, could reduce sFlt-1 release, *p<0.05: PTM 10mM vs. control.
Chymase and ADAM10 expressions
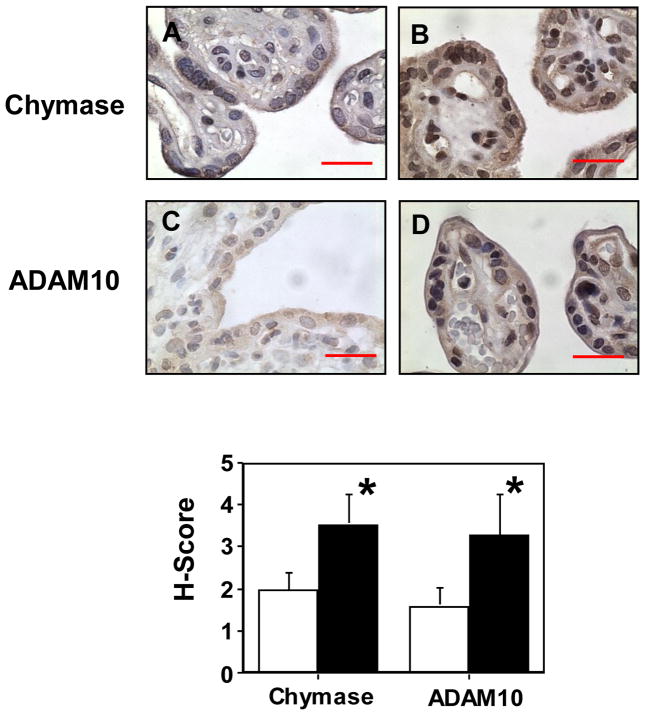
Chymase and ADAM10 expressions were examined by both immunohistochymsitry and Western blot. For immunohistochymsitry, tissue sections from 10 normal and 10 preeclamptic placentas were stained with antibodies against chymase and ADAM10, a member of a disintegrin and metalloprotease (ADAMs). As shown in Figure 4, both chymase and ADAM10 were intensively stained in the syncytiotrophoblasts of preeclamptic placentas compared to normal placentas. The H-score for chymase was 3.536±0.687 in preeclamptic placentas, which was significantly higher than 1.984±0.386 in normal placentas, p<0.05. Consistently, the H-score for ADAM10 was also significantly higher in preeclamptic than in normal placentas, 3.295±0.941 vs. 1.985±0.395, p<0.05, respectively.
Figure 4.
Immunostaining of chymase and ADAM10 in villous tissue sections from normal and preeclamptic placentas. Both chymase and ADAM10 immnostaining were increased in tissue sections from preeclamptic placentas compared to normal placentas. Normal: A and C; PE: B and D; A and B were stained for chymase; and C and D were stained for ADAM10, bar = 20 micron, respectively. Positive staining was mainly seen in the syncytiotrophoblast layer. For the H-score graph, open column: normal; and solid column: PE, p<0.05, respectively.
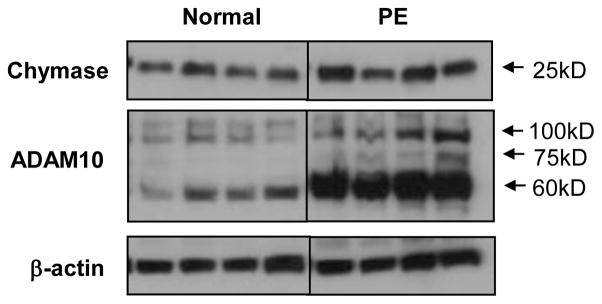
Placental tissue protein expressions for chymase and ADAM10 were examined in 4 normal and 4 preeclamptic placentas. The results are shown in Figure 5. Consistent with immunostaining results, both chymase and ADAM10 expressions were markedly increased in preeclamptic placentas compared to normal placentas. For ADAM10 expression, three molecular weight bands 100kDa, 75kDa, and 60kDa were detected in preeclamptic placental samples. The 100kDa size corresponds to unprocessed (pro-form) form of ADAM10; The 60kDa size is the active form of ADAM10; and the 75kDa size refers to partially processed form (23). Our results clearly showed that the expression of active form of ADAM10 is markedly increased in preeclamptic placental tissues compared to normal placental tissues (Figure 5).
Figure 5.
Protein expressions for chymase and ADAM10 in normal and preeclamptic placentas by Western blot. Both chymase and ADAM10 expressions were markedly increased in preeclamptic (n=4) compared to normal (n=4) placentas. For ADAM10 expression, two bands were detected in normal tissues and three bands were detected in preeclamptic tissues. The 100kDa size corresponds to unprocessed (pro-form) form; the 60kDa size is the active form; and the 75kDa size refers to partially processed form. The active form of ADAM10 is markedly increased in preeclamptic placental tissues compared to normal placental tissues.
Flt-1 expression
To determine if proteolytic inhibition could affect Flt-1 expression in syncytiotrophoblasts, Flt-1 expression was examined by both immunostaining and immunoblotting in explant tissues after chymotripsin inhibitor and PTM treatment. Chymotrypsin inhibitor at concentration of 5μg/ml and PTM at 10mM were used. This was examined in three placentas. Consistent results were obtained. As shown in Figure 6, both inhibitors had no effect on Flt-1 expression compared to control samples.
Figure 6.
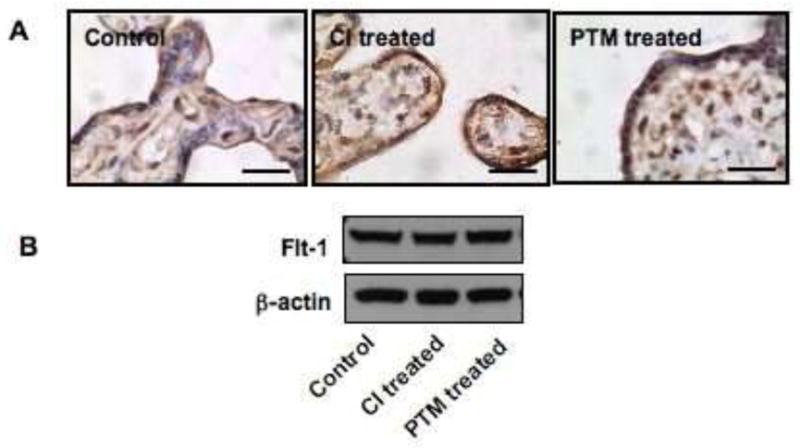
Flt-1 expression in chymotrypsin inhibitor and PTM treated villous tissue. A: immunostaining; and B: immunoblotting. No difference was observed for Flt-1 expression in explant tissues treated with chymotrypsin inhibitor and PTM compared to untreated controls.
Discussion
VEGF receptor-1 (Flt-1) is a transmembrane protein. Soluble Flt-1 exists in the circulation as a truncated form of full-length Flt-1. During pregnancy the placenta is a major source of sFlt-1 in the maternal circulation and increased placenta release of sFlt-1 is believed to play an important role in the pathophysiology and pathogenesis in preeclampsia (1,24). Using specific protease inhibitors, we examined if proteases could promote sFlt-1 release from placental trophoblasts. We found sFlt-1 release could be produced by chymotrypsin-like serine protease/chymase and metalloproteinase, but not by cysteine protease and metalloendopeptidase, in placental tissues from normal pregnancies. The specificity of chymotrypsin-like protease/chymase induced sFlt-1 release is supported by 1) sFlt-1 release was dose-dependently induced by chymotrypsin and specific chymotrypsin inhibitor dose-dependently reduced sFlt-1 release; and 2) non-specific serine protease inhibitors PMSF and aprotini had no effects. PMSF is a non-specific serine protease inhibitor and its proteolytic inhibition occurs when a concentration between 0.1 – 1mM PMSF is used. Aprotini is called bovine pancreatic trypsin inhibitor (BPTI). It inhibits a number of serine proteases including trypsin, plasmin, and kallikrein with the concentration in a range of 125 to 300 KIU/ml. The concentrations for PMSF and aprotini used in our study are in the dose effective range and compatible with the published work (25). However, no effect was noticed in sFlt-1 release in our culture condition by PMSF and aprotini.
We recently found that chymase expression and activity were increased in placental trophoblasts in preeclampsia (14). The localization of chymotrypsin/chymase expression in placental syncytiotrophoblasts makes it a candidate for cell membrane protein shedding. Several studies have shown the sheddase effect of chymotrypsin-like protease. For example, in corneal epithelial cells chymase degradates fibronectin and occludin, leading to disturbed barrier function of the eye (26). Chymotrypsin is able to cleave L-selectin on leukocytes (27). Chymotrypsin-like protease/chymase could also shed CD44 from myoepithelial cells (28) and the myoepithelial cell-specific CD44 shedding contributes to the anti-invasive and anti-angiogenic phenotype of myoepithelial cells (29). Taken together, these studies suggest that chymotrypsin-like protease/chymase might act like a cell membrane sheddase and play an active role in regulating sFlt-1 release from the placenta.
ADAM (a disintegrin and metalloprotease) composes of a large family of about 40 type-I transmembrane proteins. Several members of this family have been shown to function during gestation. For example, ADAM19 was reported to play a role in blastocyst implantation (30) and subsequent trophoblast invasion (31). Increased ADAM12 expression was found in placentas from preeclampsia (32), which may explain the reason for reduced membrane-bound heparin-binding–EGF (HB-EGF) expression in preeclampsia (33) since ADAM 12 is a sheddase of HB-EGF (34). In line with these findings, our results show that universal ADAM inhibitor PTM could reduce sFlt-1 release from normal placentas and the inhibitory effect was in a dose-dependent manner, indicating that ADAM members may also involve in sFlt-1 shedding. Among ADAM family, ADAM10 is a proved sheddase (35). In vitro studies have shown that ADAM10 is responsible for the CXC-chemokine ligand 16 shedding from keratinocytes (36). Again in keratinocyte cell line HaCat, ADAM10 could induce CD46 shedding and increased CD46 shedding attributes to the cell apoptotic process (37). ADAM10 also induces N-cadherin cleavage and mediates E-cadherin shedding (38,39).
In the present study, we examined chymase and ADAM10 expressions by immunostaining and by Western blot in placental tissues from normal and preeclamptic pregnancies. We found that both chymase and ADAM10 immunoreactivities were localized in syncytiotrophoblast layer within the placenta and both chymase and ADAM10 expressions were increased in placentas from preeclampsia compared to normal pregnancies. The increased ADAM10 expression in preeclamptic placentas is in line with up-regulation of ADAM12 expression found in preeclamptic placentas (32).
Considering the sheddase nature of ADAM10 in epithelial cells, it is plausible to speculate that enhanced ADAM10 expression on preeclamptic placental trophoblasts contributes to the increased production of sFlt-1 in preeclampsia. The PTM inhibitor experiment suggests this might well be the case. However, further work is needed to confirm the specificity of ADAMs in sFlt-1 shedding when specific ADAM inhibitors are available, since PTM is a universal inhibitor for ADAMs. The mechanism of the enhanced ADAM10 expression in preeclamptic placenta is not known, though recently, oxidative stress could induce ADAM9 upregulation in human prostate cancer cells (40). Based on the significant role of oxidative change in preeclamptic placentas, it might be of interest to investigate the interrelationship between oxidative stress and ADAM10 up-regulation in the pathophysiology of preeclampsia.
Effect of proteolytic inhibition on cellular Flt-1 expression was examined by both immunostaining and Western blot in villous explant tissues after chymotrypsin and PTM treatment. We did not notice any difference for Flt-1 immunoreactivity and expression in syncytiotrophoblasts or villous tissue with chymotrypsin and PTM treatment compared to untreated control tissues.
Human placenta is rich of proteases, such as serine proteases, caspases, MMPs, and ADAMs, etc. In this study, using various protease inhibitors in the explant culture studies we found that serine protease chymotrypsin-like protease/chymase and metalloprotease ADAM, but not cysteine protease and metalloendopeptidase, were involved in sFlt-1 release. It is highly likely that membrane proteases of chymotrypsin/chymotrypsin and ADAMs play an important role in orchestrating sFlt-1 production or sFlt-1 shedding. Our data support the notion that the proteolytic process of placental trophoblast membrane integral protein Flt-1 contributes to shedding of its soluble form into the extra-placental compartment during pregnancy. Further studies are needed to decipher which family member(s) of serine proteases and ADAMs are responsible for the increased sFlt-1 production from placentas during pregnancy disorders including preeclampsia.
Acknowledgments
This study was presented in part at the 55th Annual Meeting of Society for Gynecologic Investigation, San Diego, CA, March 26–29, 2008, and supported by grants from National Institute of Health, NICHD (HD36822) and NHLBI (HL65997).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93(1):260–266. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75(1):1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Smith SK, Day KA, Clark DE, Licence DR, Charnock-Jones DS. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol Endocrinol. 1999;13(4):537–545. doi: 10.1210/mend.13.4.0265. [DOI] [PubMed] [Google Scholar]
- 5.Thomas CP, Andrews JI, Liu KZ. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J. 2007;21(14):3885–3895. doi: 10.1096/fj.07-8809com. [DOI] [PubMed] [Google Scholar]
- 6.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;200(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tedder TF. Cell-surface receptor shedding: a means of regulating function. Am J Respir Cell Mol Biol. 1991;5(4):305–306. doi: 10.1165/ajrcmb/5.4.305. [DOI] [PubMed] [Google Scholar]
- 8.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321(Pt 2):265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oppong SY, Hooper NM. Characterization of a secretase activity which releases angiotensin-converting enzyme from the membrane. Biochem J. 1993;292 ( Pt 2):597–603. doi: 10.1042/bj2920597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27(8):783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Huppertz B, Bartz C, Kokozidou M. Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006;37(6):509–517. doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Mason RW. Emerging functions of placental cathepsins. Placenta. 2008;29(5):385–390. doi: 10.1016/j.placenta.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Levy R, Nelson DM. To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta. 2000;21(1):1–13. doi: 10.1053/plac.1999.0450. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Gu Y, Zhang Y, Lewis DF, Alexander JS, Granger DN. Increased chymotrypsin-like protease (chymase) expression and activity in placentas from women with preeclampsia. Placenta. 2007;28:263–269. doi: 10.1016/j.placenta.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Gu Y, Lewis DF, Alexander JS, Granger DN. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens Pregn. 2010 doi: 10.3109/10641950802001842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang Y, Lewis DF, Gu Y, Li H, Granger DN, Alexander JS. Protease chymotrypsin mediates the endothelial expression of P- and E-selectin, but not ICAM and VCAM, induced by placental trophoblasts from preeclamptic pregnancies. Placenta. 2003;24:851–861. doi: 10.1016/s0143-4004(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 17.Achmad TH, Winterscheidt A, Lindemann C, Rao GS. Oxidized low density lipoprotein acts on endothelial cells in culture to enhance endothelin secretion and monocyte migration. Methods Find Exp Clin Pharmacol. 1997;19(3):153–159. [PubMed] [Google Scholar]
- 18.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66(13):6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 19.Vlata Z, Porichis F, Tzanakakis G, Tsatsakis A, Krambovitis E. A study of zearalenone cytotoxicity on human peripheral blood mononuclear cells. Toxicol Lett. 2006;165(3):274–281. doi: 10.1016/j.toxlet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Gu Y, Lewis DF, Wang Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29 (1):81–88. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzeloglu-Kayisli O, Kayisli UA, Al-Rejjal R, Zheng W, Luleci G, Arici A. Regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) expression by estradiol and progesterone in human endometrium. J Clin Endocrinol Metab. 2003;88(10):5017–5026. doi: 10.1210/jc.2003-030414. [DOI] [PubMed] [Google Scholar]
- 22.Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172(6):1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCulloch DR, Akl P, Samaratunga H, Herington AC, Odorico DM. Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res. 2004;10(1):314–323. doi: 10.1158/1078-0432.ccr-0846-3. [DOI] [PubMed] [Google Scholar]
- 24.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. New England Journal of Medicine. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 25.Stonelake PS, Jones CE, Neoptolemos JP, Baker PR. Proteinase inhibitors reduce basement membrane degradation by human breast cancer cell lines. Br J Cancer. 1997;75(7):951–957. doi: 10.1038/bjc.1997.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebihara N, Funaki T, Murakami A, Takai S, Miyazaki M. Mast cell chymase decreases the barrier function and inhibits the migration of corneal epithelial cells. Curr Eye Res. 2005;30(12):1061–1069. doi: 10.1080/02713680500346625. [DOI] [PubMed] [Google Scholar]
- 27.Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9(2):255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MC, Alpaugh ML, Nguyen M, Deato M, Dishakjian L, Barsky SH. Myoepithelial-specific CD44 shedding is mediated by a putative chymotrypsin-like sheddase. Biochem Biophys Res Commun. 2000;279(1):116–123. doi: 10.1006/bbrc.2000.3918. [DOI] [PubMed] [Google Scholar]
- 29.Alpaugh ML, Lee MC, Nguyen M, Deato M, Dishakjian L, Barsky SH. Myoepithelial-specific CD44 shedding contributes to the anti-invasive and antiangiogenic phenotype of myoepithelial cells. Exp Cell Res. 2000;261(1):150–158. doi: 10.1006/excr.2000.5056. [DOI] [PubMed] [Google Scholar]
- 30.Wang HX, Zhao YG, Wang HM, Yang Q, Lin HY, Sang QX, Zhu C. Expression of adamalysin 19/ADAM19 in the endometrium and placenta of rhesus monkey (Macaca mulatta) during early pregnancy. Mol Hum Reprod. 2005;11(6):429–535. doi: 10.1093/molehr/gah183. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M, Qiu W, Li Y, Sang QA, Wang Y. Dynamic change of Adamalysin 19 (ADAM19) in human placentas and its effects on cell invasion and adhesion in human trophoblastic cells. Sci China C Life Sci. 2009;52(8):710–718. doi: 10.1007/s11427-009-0102-8. [DOI] [PubMed] [Google Scholar]
- 32.Gack S, Marmé A, Marmé F, Wrobel G, Vonderstrass B, Bastert G, Lichter P, Angel P, Schorpp-Kistner M. Preeclampsia: increased expression of soluble ADAM 12. J Mol Med. 2005;83(11):887–896. doi: 10.1007/s00109-005-0714-9. [DOI] [PubMed] [Google Scholar]
- 33.Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, et al. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360(9341):1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- 34.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8(1):35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 35.Anderegg U, Eichenberg T, Parthaune T, Haiduk C, Saalbach A, Milkova L, Ludwig A, Grosche J, Averbeck M, Gebhardt C, et al. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. J Invest Dermatol. 2009;129(6):1471–1482. doi: 10.1038/jid.2008.323. [DOI] [PubMed] [Google Scholar]
- 36.Scholz F, Schulte A, Adamski F, Hundhausen C, Mittag J, Schwarz A, Kruse ML, Proksch E, Ludwig A. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. J Invest Dermatol. 2007;127(6):1444–1455. doi: 10.1038/sj.jid.5700751. [DOI] [PubMed] [Google Scholar]
- 37.Hakulinen J, Keski-Oja J. ADAM10-mediated release of complement membrane cofactor protein during apoptosis of epithelial cells. J Biol Chem. 2006;281(30):21369–21376. doi: 10.1074/jbc.M602053200. [DOI] [PubMed] [Google Scholar]
- 38.Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24(4):742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102(26):9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung SY, Kubo H, Shigemura K, Arnold RS, Logani S, Wang R, Konaka H, Nakagawa M, Mousses S, Amin M, et al. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer Res. 2006;66(19):9519–9526. doi: 10.1158/0008-5472.CAN-05-4375. [DOI] [PubMed] [Google Scholar]