Abstract
Inflammation may play a role in increased risk of heart failure (HF) that is associated with obesity, metabolic syndrome (MS), and diabetes. This study investigated associations between inflammatory markers, MS, and incident HF in a population with a high prevalence of diabetes, obesity, and MS. The cohort consisted of 3098 American Indians without prevalent cardiovascular disease who had C‐reactive protein (CRP) and fibrinogen measured at the Strong Heart Study phase II examination. Independent associations between inflammatory markers, MS, and HF were analyzed by Cox hazard models. During a mean follow‐up of 11 years, 218 participants developed HF. After the adjustment for cardiovascular risk factors, fibrinogen, (hazard ratio [HR], 1.36; 95% confidence interval [CI], 1.15–1.59) but not CRP (HR, 1.25; 95% CI, 0.97–1.32) remained a significant HF predictor. In individuals without diabetes, concomitant presence of MS and elevated CRP or fibrinogen increased HF risk (for MS and CRP: HR, 2.02; 95% CI, 0.95–4.31; for CRP and fibrinogen: HR, 1.75; 95% CI, 0.83–3.72). In a population with a high prevalence of obesity, MS, and diabetes, elevated CRP and fibrinogen increased HF risk. These associations are attenuated by the adjustments for conventional risk factors suggesting that inflammation acts in concert with metabolic and clinical risk factors in increasing HF risk.
Heart failure (HF) is one of the leading causes of cardiovascular morbidity and mortality and is related, in most patients, to underlying coronary artery disease, hypertension, and diabetes. In patients with HF, regardless of etiology, elevated plasma concentrations of inflammatory markers are associated with worsening functional class and adverse outcomes. 1 , 2 The biological processes involved in the pathogenesis of left ventricular (LV) dysfunction are complex and only partially understood, but the data suggest that chronic inflammation may play a role in promoting cardiac fibrosis, inflammation, and cardiac remodeling. 3 , 4 , 5
The proinflammatory state is a common characteristic of clinical syndromes, such as obesity, metabolic syndrome (MS), and diabetes, that have been associated with LV dysfunction and increased risk of HF. 6 , 7 , 8 , 9 Reports of increasing obesity, MS, and diabetes in the US population suggest the increased importance of these conditions in HF risk 10 and a need for better understanding of the mechanisms involved in the progression of these conditions to overt HF.
Our group has previously reported that fibrinogen, reflecting both inflammation and thrombosis, is a strong correlate of preclinical cardiovascular disease. 11 , 12 A community‐based investigation has shown an association between elevated myeloperoxidase (MPO) levels, a leukocyte‐derived enzyme, and risk of incident HF, particularly in individuals without significant cardiovascular risk factors, 13 suggesting an independent role of MPO in cardiac remodeling.
The Strong Heart Study (SHS) is a longitudinal observational study, spanning more than 2 decades, designed to characterize the metabolic and cardiovascular health profile of American Indians. The SHS cohort, with its high prevalence of obesity and diabetes and elevated inflammatory markers, is an ideal population in which to investigate the link between metabolic risk factors, markers of inflammation, and HF. 14 The aim of this study was to determine associations between inflammatory markers, MS, and risk of HF in a population with a high prevalence of diabetes, obesity, and MS.
Methods
Study Population
The SHS is a population‐based cohort study of cardiovascular risk factors and disease in American Indians from Arizona, North and South Dakota, and Oklahoma who were first examined in 1989 to 2002 and remain under surveillance for cardiovascular disease (CVD). 14 CRP, fibrinogen, and urinary albumin were measured during SHS phase 2 (SHS2), which was conducted between 1993 and 1995, and included 3638 men and women (aged 49–78 years) who were considered for the present analysis. All participants provided informed consent.
Individuals were excluded from the current analyses if they had prevalent CVD, including myocardial infarction (MI), coronary heart disease (diagnosed by electrocardiography, coronary angiography, a combination of typical symptoms and a positive stress test, or need for a revascularization procedure), stroke, or history of HF. Prevalent and incident cardiovascular events were confirmed by the SHS Mortality and Morbidity Committee using specified criteria for causes of fatal and nonfatal cardiovascular events. 14
To avoid the confounding link between severe chronic kidney disease and HF, we excluded all participants with serum creatinine >2.5 mg/dL or estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2. Thus, the cohort for the present analysis comprised 3098 members of the SHS2 population without prevalent cardiovascular or severe chronic kidney disease.
In contrast to other inflammatory markers, MPO was not measured at the phase 2 examination. To explore the potential association of MPO with incident HF in this cohort, we measured and compared MPO levels in 83 incident HF cases and 83 age‐, sex‐, and center‐matched (AZ, ND/SD, and OK) controls with available stored serum specimens.
Definitions
HF was defined as having 2 major or 1 major and 2 minor Framingham criteria concurrently in the absence of a condition, such as end‐stage renal failure leading to massive fluid overload. Major criteria were paroxysmal nocturnal dyspnea or orthopnea, neck vein distention, rales, cardiomegaly, acute pulmonary edema, S3 gallop, venous pressure >16 cm water, and hepatojugular reflux. Minor criteria were ankle edema, night cough, dyspnea upon exertion, hepatomegaly, pleural effusion, vital capacity <2 or 3 of predicted, or heart rate >120 beats per minute. Weight loss >4.5 kg within 5 days in response to treatment could serve as a major or minor criterion.
MS (diagnosed at baseline [phase II] examination) was defined via Adult Treatment Panel III (ATP III) criteria. 15 Individuals with ≥3 of the following criteria were considered as having MS: waist circumference >102 cm in men and >88 cm in women, triglycerides >150 mg/dL, high‐density lipoprotein cholesterol (HDL‐C) <40 mg/dL in men and <50 mg/dL in women, blood pressure >130/85 mm Hg or use of antihypertensive drugs, and fasting glucose >100 mg/dL to <126 mg/dL. A total of 930 patients met the criteria for MS. Individuals with fasting glucose >126 mg/dL or using antidiabetes medications were classified as diabetic (n=1414) and were excluded from the MS group. Albuminuria was defined as urinary albumin‐creatinine ratio >30 mg/g. Obesity was defined as body mass index (BMI) >30, which classified 1669 participants as obese.
Laboratory Assays
Urinary albumin, fasting glucose, and lipid profile were measured by standard methods. CRP and fibrinogen were measured by validated methods. 11 , 16 , 17 , 18 A commercially available, CardioMPO Test (PrognostiX Inc, Cleveland, OH) was used to determine MPO concentration.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables and as percentages for categoric variables. Median and interquartile ranges also are presented for continuous variables that were not normally distributed. Chi‐square and Student t tests were used to examine differences in proportions and means of categoric and continuous variables, respectively, between those with and without incident HF. Wilcoxon rank‐sum tests were used for continuous variables not normally distributed.
Person‐years were calculated from the date of the baseline (phase 2) examination to the date of incident HF, date of death if the participant died, or December 31, 2007. The distributions of fibrinogen and CRP were divided into quartiles. Participants whose values were in the fourth quartile were considered positive for that inflammatory marker.
Cox proportional hazard models were used to analyze independent associations of inflammatory markers, including CRP and fibrinogen, with incident HF. The initial multivariate model was adjusted for age and sex. Subsequent models were further adjusted for BMI, hypertension, low‐density lipoprotein cholesterol (LDL‐C), diabetes, current smoking, and eGFR. The results of these models are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Conditional logistic regression was performed to assess the association between incident HF and MPO concentration. Results of these models are reported as odds ratios (ORs) and 95% CIs.
Cox proportional hazard models were used to analyze the association of MS with incident HF, among those without diabetes. HRs and 95% CIs were estimated with the use of 4 models as follows: model 1 was univariate, model 2 was adjusted for CRP, model 3 was adjusted for fibrinogen, and model 4 was adjusted for conventional risk factors including age, sex, LDL‐C, current smoking, and eGFR. HRs and 95% CIs for incident HF were estimated by the combined categories of presence or absence of MS and elevated inflammatory markers, defined as CRP and fibrinogen >75th percentile.
Cox proportional hazard models were also used to analyze the associations of diabetes, inflammation and HF. In the analysis of diabetes without albuminuria, participants with albuminuria were excluded. HRs and 95% CIs for incident HF were estimated by the combined categories of presence or absence of diabetes (with or without albuminuria) and elevated inflammatory markers, defined as CRP and fibrinogen >75th percentile.
Cumulative hazard curves of incident HF were produced among participants categorized by elevated inflammatory markers. A log‐rank test was calculated to examine differences between the 4 groups defined by elevation vs normality of the 2 inflammatory markers. Two‐tailed P values <.05 were considered statistically significant. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC). All graphics were created using R software, version 2 (Free Software Foundation, Boston, MA).
Results
Of 3098 SHS2 participants free of CVD at baseline, 218 developed HF during a mean 11‐year follow up (incidence, 6.4 per 1000 person‐years). Incident MI occurred in 255 patients (incidence rate, 7.6 per 1000 person‐years). At baseline, diabetes, obesity, hypertension, and albuminuria were more prevalent among participants with incident HF compared with those who remained HF‐free. Individuals who developed HF also had lower HDL‐C and higher CRP and fibrinogen levels, while total cholesterol and LDL‐C were similar between the groups (Table I).
Table I.
Baseline Characteristics of Study Participants With or Without Incident Heart Failure (N=3098)
| Participants With Incident HF (n=218) | Participants Without Incident HF (n=2880) | P Value | |
|---|---|---|---|
| Age, y | 61.3±7.7 | 59.4±7.8 | .001 |
| Women, % | 72.0 | 62.4 | .004 |
| Body mass index, kg/m2 | 32.4±7.2 | 31.2±6.4 | .017 |
| Waist circumference, cm | 109.7±15.4 | 106.4±14.8 | .001 |
| Obesity (BMI ≥30), % | 61.9 | 53.5 | .016 |
| Diabetes, % | 71.8 | 44.1 | <.001 |
| Metabolic syndrome, No. (%)a (excluding DM) | 37/63 (58.7) | 893/1621 (55.1) | .568 |
| Hypertension, % | 57.8 | 41.4 | <.001 |
| SBP, mm Hg | 134±21 | 128±19 | .001 |
| DBP, mm Hg | 75±11 | 75±10 | .697 |
| Total cholesterol, mg/dL | 192±41 | 190±39 | .503 |
| LDL, mg/dL | 120±36 | 118±33 | .583 |
| HDL, mg/dL | 39±11 | 42±14 | <.001 |
| Triglycerides, mg/dL | 145 (100–204) | 129 (91–183) | <.001b |
| Current cigarette smoking, % | 31.3 | 31.8 | .872 |
| eGFR, mL/min/1.73 m2 | 81±27 | 87±36 | .006 |
| UACr, mg/g | 46 (10–313) | 12 (6–45) | <.001b |
| Fibrinogen, mg/dL | 377 (337–436) | 347 (306–396) | <.001b |
| CRP, mg/dL | 4.7 (2.4–8.4) | 3.7 (2.0–6.8) | .004b |
| MPO, pmol/L (83 cases + 83 controls) | 699 (436–1023) | 678 (466–922) | .848c |
| Incident MI, % | 19.3 | 7.4 | <.001 |
Abbreviations: CRP, C‐reactive protein; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; LDL, low‐density lipoprotein; MI, myocardial infarction; MPO, myeloperoxidase; SBP, systolic blood pressure; UACr, urinary albumin to creatinine ratio. Data are presented as mean ± standard deviation, percentages, or median (interquartile range [IQR]). Chi‐square tests were used for differences in proportions and Student t tests for differences in means. aMetabolic syndrome criteria included presence 3 of 5 criteria (increased waist circumference, elevated triglycerides, low high‐density lipoprotein (HDL), hypertension, and fasting glucose >100 (see Methods for details). Diabetics were excluded from metabolic syndrome definition. bWilcoxon rank‐sum test was used if distributions were not normal and median and IQR were reported. cWilcoxon signed‐rank test was used for the matched paired samples.
Both markers of systemic inflammation, CRP and fibrinogen, were significant predictors of incident HF in analyses that were adjusted for age and sex (Table II). Fibrinogen, but not CRP, remained a statistically significant HF predictor after additional adjustment for conventional CV risk factors, including diabetes, BMI, hypertension, smoking, LDL‐C, and eGFR (Table II). In a limited sample of cases and controls, elevated serum MPO was not associated with incident HF in univariate or multivariate analyses (Table II).
Table II.
Adjusted HRs of Incident Heart Failure in Relation to Markers of Inflammation (N=3098)
| Incident Heart Failure HR (95% CI) per SD Log Increasea,b | P Valuea,b | |
|---|---|---|
| CRP | ||
| Age‐ and sex‐adjusted model | 1.25 (1.09–1.44) | .001 |
| Multivariate modelc | 1.13 (0.97–1.32) | .124 |
| Fibrinogen | ||
| Age‐ and sex‐adjustedmodel | 1.60 (1.39–1.86) | <.001 |
| Multivariate modelc | 1.36 (1.15–1.59) | <.001 |
| Odds Ratio (95% CI) per SD Log Increasea,d | P Valuea,d | |
| MPOd (N=166) | ||
| Age‐ and sex‐matched | 1.04 (0.78–1.37) | .800 |
| Multivariate modelc | 1.01 (0.68–1.51) | .961 |
Abbreviations: CI, confidence interval; HR, hazard ratio. aRespective values for standard deviations (SDs) were fibrinogen=0.226 mg/dL, C‐reactive protein (CRP)=0.987 mg/dL, and myeloperoxidase (MPO)=0.586 pmol/L. All the biomarkers were log‐transformed to achieve normality. bCox proportional models were used to analyze the associations between CRP/fibrinogen and HF. cAdjusted for age, sex, body mass index, hypertension, low‐density lipoprotein cholesterol, diabetes, smoking, and estimated glomerular filtration rate. dConditional logistic regression was used to assess the association between the MPO level and incident heart failure.
To investigate associations among inflammation, risk factors, and HF, we used Cox proportional models to examine HF risk in individuals with MS alone (Table III), as well as with elevated inflammatory markers (Table IV). Participants with diabetes were excluded from this analysis. MS was present in 930 of 1684 patients (55%). Individuals with MS had no significantly increased risk of developing HF compared with participants without MS (HR, 1.05; 95% CI, 0.64–1.74). Adjustments for CRP, fibrinogen, and conventional risk factors did not significantly affect this relationship (Table III). Among this group, individuals with elevated triglycerides (TGs >150 mg/dL) or with increased waist circumference (WC >102 cm in men or WC >88 cm in women) did not have an increased risk of incident HF (adjusted HR, 0.89; 95% CI, 0.50–1.59 for elevated TG, and HR, 1.10; 95% CI, 0.58–2.09 for increased WC; adjusted for age, sex, smoking, LDL cholesterol and eGFR).
Table III.
Unadjusted and Adjusted HRs for Incident HF in Relation to Metabolic Syndrome and Markers of Inflammation (Excluding DM, N=1684)
| HR | 95% CI | P Value | |
|---|---|---|---|
| Metabolic syndrome | 1.05 | 0.64–1.74 | .838 |
| Metabolic syndrome adjusted for CRP | 1.02 | 0.61–1.72 | .927 |
| Metabolic syndrome adjusted for fibrinogen | 0.98 | 0.58–1.66 | .951 |
| Metabolic syndrome adjusted for conventional risk factorsa | 1.14 | 0.67–1.95 | .624 |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; DM, diabetes mellitus; HR, hazard ratio. aAdjusted for age, sex, low‐density lipoprotein cholesterol, smoking, and estimated glomerular filtration rate.
Table IV.
HRs for Incident HF by Combined Categories of Metabolic Syndrome and Inflammatory Markers (Excluding DM, N=1684)
| MS | Inflammatory Markera | CRP | Fibrinogen | ||
|---|---|---|---|---|---|
| N | Adjusted HR (95% CI)b | No. | Adjusted HR (95% CI)b | ||
| No | No | 594 | 1 (referent) | 610 | 1 (referent) |
| No | Yes | 131 | 1.19 (0.44–3.23) | 111 | 0.96 (0.32–2.86) |
| Yes | No | 714 | 0.94 (0.50–1.75) | 740 | 0.86 (0.46–1.60) |
| Yes | Yes | 205 | 2.02 (0.95–4.31) | 169 | 1.75 (0.83–3.72) |
Abbreviations: CI, confidence interval; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; MS, metabolic syndrome. aInflammatory markers: C‐reactive protein (CRP) or fibrinogen >75% percentile (CRP ≥7 mg/dL; fibrinogen ≥398 mg/dL). bAdjusted for age, sex, low‐density lipoprotein cholesterol, smoking, and estimated glomerular filtration rate.
We compared risk of incident HF in individuals with MS and normal inflammatory markers with risk associated with MS and elevated inflammatory markers. Table IV shows borderline increased incident HF risk in individuals with MS and CRP or fibrinogen >75th percentile (HR, 2.0; 95% CI, 0.95–4.31 and HR, 1.8; 95% CI, 0.83–3.72, respectively).
To investigate associations among inflammation, HF, diabetes, and albuminuria, we compared hazard ratios for incident HF in diabetic patients without albuminuria and diabetics with albuminuria, alone and in the presence of elevated inflammatory markers. In diabetics without albuminuria, concomitant presence of elevated inflammatory markers further increased the risk of HF (from HR 2.40 to 3.30 for diabetes and CRP, and from HR 2.27 to 3.48 for diabetes and fibrinogen) (Table V). Diabetics with proteinuria were at high risk for incident HF, that was further increased in the presence of elevated fibrinogen (from HR 3.11 to 4.63 for diabetes and albuminuria and fibrinogen) but not elevated CRP levels (from HR 3.76 to 3.40 for diabetes with albuminuria and CRP) (Table VI).
Table V.
HRs for Incident HF by Combined Categories of Diabetes Without Albuminuria and Inflammatory Markers (Excluding Diabetics With Albuminuria, N=2330)
| DM Without Albuminuriaa | Inflammatory Markerb | CRP | Fibrinogen | ||
|---|---|---|---|---|---|
| No. | Adjusted HR (95% CI)c | No. | Adjusted HR (95% CI)c | ||
| No | No | 1278 | 1 (referent) | 1253 | 1 (referent) |
| No | Yes | 362 | 1.50 (0.85–2.67) | 372 | 1.34 (0.76–2.38) |
| Yes | No | 429 | 2.40 (1.49–3.87) | 445 | 2.27 (1.38–3.72) |
| Yes | Yes | 208 | 3.30 (1.86–5.85) | 188 | 3.48 (2.00–6.05) |
Abbreviations: CI, confidence interval; HR, hazard ratio. aDiabetes melitus (DM) without albuminuria (defined as urinary albumin/creatinine ratio >30 mg/g). bInflammatory markers: C‐reactive protein (CRP) or fibrinogen >75% percentile (CRP ≥7 mg/dL; fibrinogen ≥398 mg/dL). cAdjusted for age, sex, LDL cholesterol, smoking, and estimated glomerular filtration rate.
Table VI.
HRs for Incident HF by Combined Categories of Diabetes With Albuminuria and Inflammatory Markers (N=3098)
| DM With Albuminuriaa | Inflammatory Markerb | CRP | Fibrinogen | ||
|---|---|---|---|---|---|
| No. | Adjusted HR (95% CI)c | No. | Adjusted HR (95% CI)c | ||
| No | No | 1764 | 1 (referent) | 1838 | 1 (referent) |
| No | Yes | 517 | 1.78 (1.18–2.69) | 425 | 1.86 (1.22–2.83) |
| Yes | No | 517 | 3.76 (2.66–5.32) | 421 | 3.11 (2.11–4.60) |
| Yes | Yes | 239 | 3.40 (2.13–5.43) | 332 | 3.48 (3.16–6.79) |
Abbreviations: CI, confidence interval; HR, hazard ratio;. aDefined as urinary albumin/creatinine ratio >30 mg/g). bInflammatory markers: C‐reactive protein (CRP) or fibrinogen >75% percentile (CRP ≥7 mg/dL; fibrinogen ≥398 mg/dL). cAdjusted for age, sex, low‐density lipoprotein cholesterol, smoking, and estimated glomerular filtration rate.
The cumulative hazard for HF by categories of inflammatory markers is shown in the Figure. Elevated CRP alone did not significantly increase incident HF risk; however, elevated fibrinogen was associated with a significant increase in HF risk (HR, 1.60; 95% CI, 1.39–1.86).
Figure FIGURE.
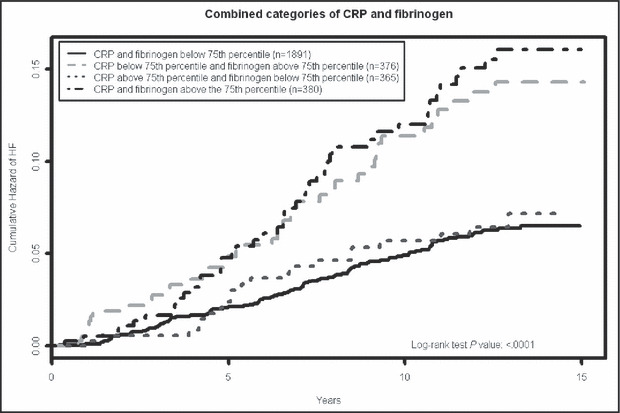
Kaplan–Meier plots of cumulative hazards for incident heart failure (HF) for all Strong Heart Study phase 2 (SHS2) participants by categories of C‐reactive protein (CRP) and fibrinogen (N=3098). The 75th percentile (CRP ≥7 mg/dL, fibrinogen ≥398 mg/dL) was used as a cutoff to consider an individual “positive” for a given inflammatory marker. Group 1 represents the individuals with CRP and fibrinogen levels <75th percentile; group 2 with CRP <75th percentile and fibrinogen >75th percentile; group 3 with CRP >75th percentile and fibrinogen <75th percentile; and group 4 had both inflammatory markers >75th percentile. Log‐rank test was used for comparison among the groups.
Discussion
This study showed that, in a population with a high prevalence of obesity, MS, and diabetes, elevated markers of inflammation predicted increased incident HF risk. Adjustment for conventional cardiovascular factors significantly attenuated these associations, suggesting that inflammatory markers act with other metabolic risk factors in increasing HF risk. In populations characterized by the presence of MS, but not diabetes, elevated CRP and fibrinogen increase incident HF risk, even after adjustment for conventional risk factors.
Systemic inflammation has been associated with HF in different populations, 6 , 19 , 20 and inflammatory markers may be involved directly, or as a marker of underlying conditions, in the progression from obesity and MS to LV dysfunction and HF. In the SHS cohort, elevated CRP concentrations showed a modest association with HF that was attenuated after adjustment for cardiovascular risk factors. These results are in agreement with previous SHS findings that identified high median CRP concentrations compared with other populations, as well as limited value of CRP in predicting cardiovascular events in diabetic patients. 21 The high prevalence of diabetes in this population has been proposed as a main factor for the observed differences. In the present study, we also analyzed the role of elevated CRP in those with MS alone, excluding individuals with diabetes, and showed a trend towards increased HF risk in patients with MS who also had elevated CRP levels. These results are in agreement with other reports 6 , 19 , 20 of increased HF risk with elevated CRP in populations without or with low prevalence of diabetes. When we extended the analysis to the diabetic population, we found that elevated CRP levels were associated with further increase in incident HF risk in diabetic patients without albuminuria, but not in diabetics with albuminuria. Our results emphasize the dynamic relationship between subclinical inflammation, MS, and overt diabetes, and suggest that the role of inflammation may be more important at intermediate levels of disease (such as MS and diabetes without albuminuria) rather than at either extreme.
In contrast to CRP, fibrinogen was more strongly associated with incident HF in this cohort and this association persisted after adjusting for conventional risk factors. In addition to its role in inflammation, fibrinogen is prothrombotic and has been correlated with cardiovascular events and HF independently from known cardiovascular factors and LV hypertrophy. 6 , 11 Fibrinogen also can affect microcirculation by increasing blood viscosity and erythrocyte aggregation, as well as by altering endothelial reactivity. 22 Although fibrinogen concentrations are significantly correlated with other inflammatory markers, a recent study from the SHS population showed novel genetic determinants of fibrinogen concentrations, 23 suggesting possible parallel and independent pathways regulating the synthesis and metabolism of acute phase reactants.
No association was observed between MPO concentrations and incident HF in the case‐control subset. This finding is in contrast to recent reports on the role of MPO in the development and progression of HF 13 , 24 ; however, our investigation was limited by the small number of frozen serum samples in which MPO was measured. Therefore, the current results may reflect a lack of statistical power to detect significant differences in HF events and should not be interpreted as evidence for the absence of the association between the MPO and HF.
Strengths and Limitations
This study’s strengths include its well‐characterized cohort with high cardiovascular risk, long follow‐up, and carefully adjudicated cardiovascular events including HF. We used the ATP III definition of MS and excluded those with diabetes from the MS analysis to investigate the role of inflammation and metabolic factors in the absence of diabetes. This study focused on a population with a high prevalence of MS and inflammation that has proved to be a model for understanding of diabetic CV disease. The exclusion of diabetic patients from the MS analysis allowed us to assess HF risk in individuals with multiple cardiovascular risk factors and without diabetes, which on its own is a powerful independent HF predictor. 8
This study’s limitations include the small number of events and the small number of serum samples with MPO measurements and/or measures of novel inflammatory markers, such as interleukin 6 and tumor necrosis factor α.
Conclusions
Elevated CRP and fibrinogen predict increased risk of incident HF in a population with a high prevalence of obesity, MS, and diabetes. The attenuation of these associations with adjustment for conventional cardiovascular factors suggests that inflammation acts in concert with other conventional risk factors in increasing HF risk.
Acknowledgments and disclosures: Dr B.V. Howard has served on the advisory boards of Merck/Schering Plough and has received research support in the form of drug donations from Merck/Schering Plough. The other authors have nothing to declare. Funding was provided by the National Heart, Lung, and Blood Institute, National Institutes of Health, cooperative agreements grants (HL41642, HL41652, HL41654, HL65521 and M10RR0047‐34). Biostatistical support for this project has been funded in whole or in part with federal funds (Grant # UL1RR031975) from the National Center for Research Resources, National Institutes of Health, through the Clinical and Translational Science Awards Program, a trademark of DHHS, part of the Roadmap Initiative, “Re‐Engineering the Clinical Research Enterprise.” ?The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service or the National Institutes of Health. We thank Rachel Schaperow, MedStar Health Research Institute, for editing the manuscript.
References
- 1. Anand IS, Latini R, Florea VG, et al. C‐reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–1434. [DOI] [PubMed] [Google Scholar]
- 2. Torre‐Amione G, Kapadia S, Benedict C, et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 1996;27:1201–1206. [DOI] [PubMed] [Google Scholar]
- 3. Nagai T, Anzai T, Kaneko H, et al. C‐reactive protein overexpression exacerbates pressure overload‐induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011;57:208–215. [DOI] [PubMed] [Google Scholar]
- 4. Schulz R, Heusch G. C‐reactive protein: just a biomarker of inflammation or a pathophysiological player in myocardial function and morphology? Hypertension. 2011;57:151–153. [DOI] [PubMed] [Google Scholar]
- 5. Verma S, Wang CH, Li SH, et al. A self‐fulfilling prophecy: C‐reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. [DOI] [PubMed] [Google Scholar]
- 6. Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. [DOI] [PubMed] [Google Scholar]
- 7. Chinali M, de Simone G, Roman MJ, et al. Cardiac markers of pre‐clinical disease in adolescents with the metabolic syndrome: the Strong Heart Study. J Am Coll Cardiol. 2008;52:932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Simone G, Devereux RB, Chinali M, et al. Diabetes and incident heart failure in hypertensive and normotensive participants of the strong heart study. J Hypertens. 2010;28:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi‐Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmieri V, Celentano A, Roman MJ, et al. Relation of fibrinogen to cardiovascular events is independent of preclinical cardiovascular disease: the Strong Heart Study. Am Heart J. 2003;145:467–474. [DOI] [PubMed] [Google Scholar]
- 12. Palmieri V, Celentano A, Roman MJ, et al. Fibrinogen and preclinical echocardiographic target organ damage: the Strong Heart Study. Hypertension. 2001;38:1068–1074. [DOI] [PubMed] [Google Scholar]
- 13. Tang WH, Katz R, Brennan ML, et al. Usefulness of myeloperoxidase levels in healthy elderly subjects to predict risk of developing heart failure. Am J Cardiol. 2009;103:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389–2395. [DOI] [PubMed] [Google Scholar]
- 15. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 16. Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C‐reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 17. Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high‐sensitivity C‐reactive protein assay. Clin Chem. 1999;45:2136–2141. [PubMed] [Google Scholar]
- 18. Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta‐analysis. JAMA. 2005;294:1799–1809. [DOI] [PubMed] [Google Scholar]
- 19. Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki T, Katz R, Jenny NS, et al. Metabolic syndrome, inflammation, and incident heart failure in the elderly: the Cardiovascular Health study. Circ Heart Fail. 2008;1:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Best LG, Zhang Y, Lee ET, et al. C‐reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation. 2005;112:1289–1295. [DOI] [PubMed] [Google Scholar]
- 22. Lominadze D, Dean WL, Tyagi SC, Roberts AM. Mechanisms of fibrinogen‐induced microvascular dysfunction during cardiovascular disease. Acta Physiol (Oxf). 2010;198:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Best LG, North KE, Li X, et al. Linkage study of fibrinogen levels: the Strong Heart Family study. BMC Med Genet. 2008;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang WH, Tong W, Troughton RW, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. [DOI] [PubMed] [Google Scholar]


