Abstract
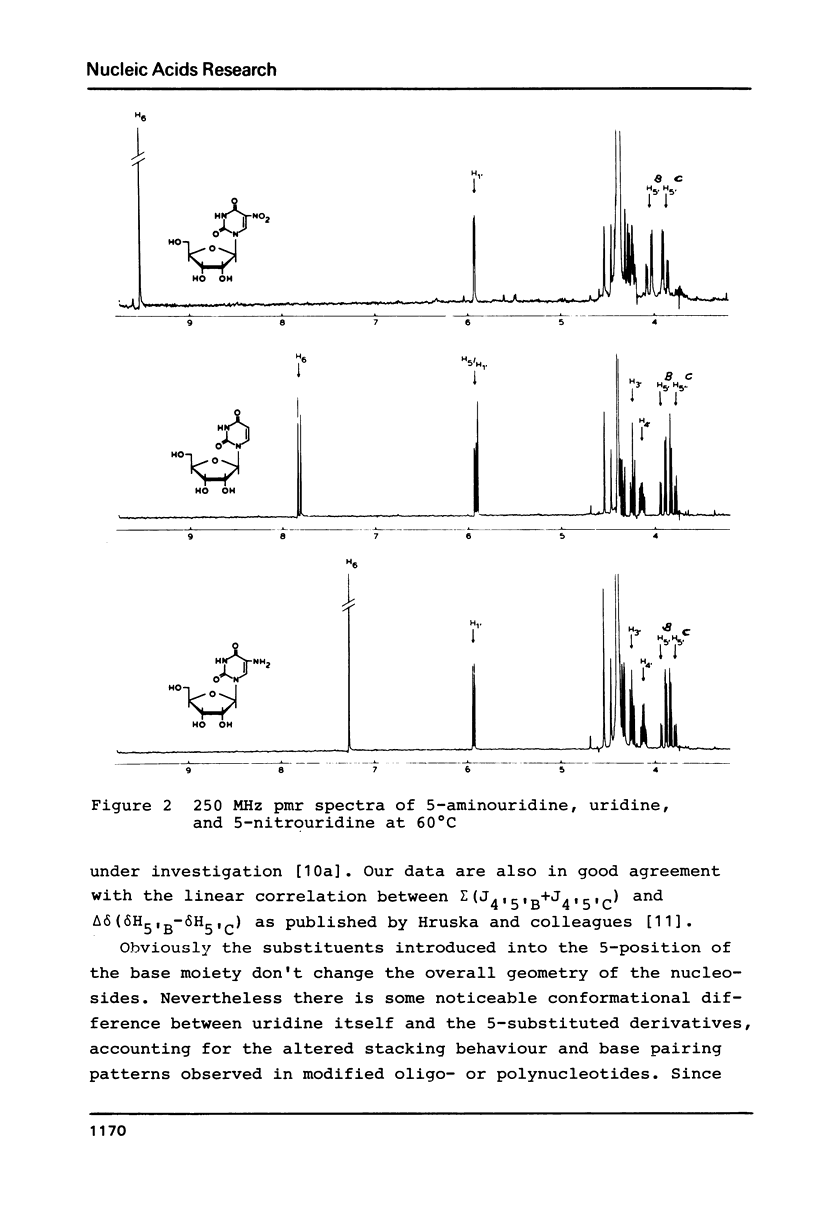
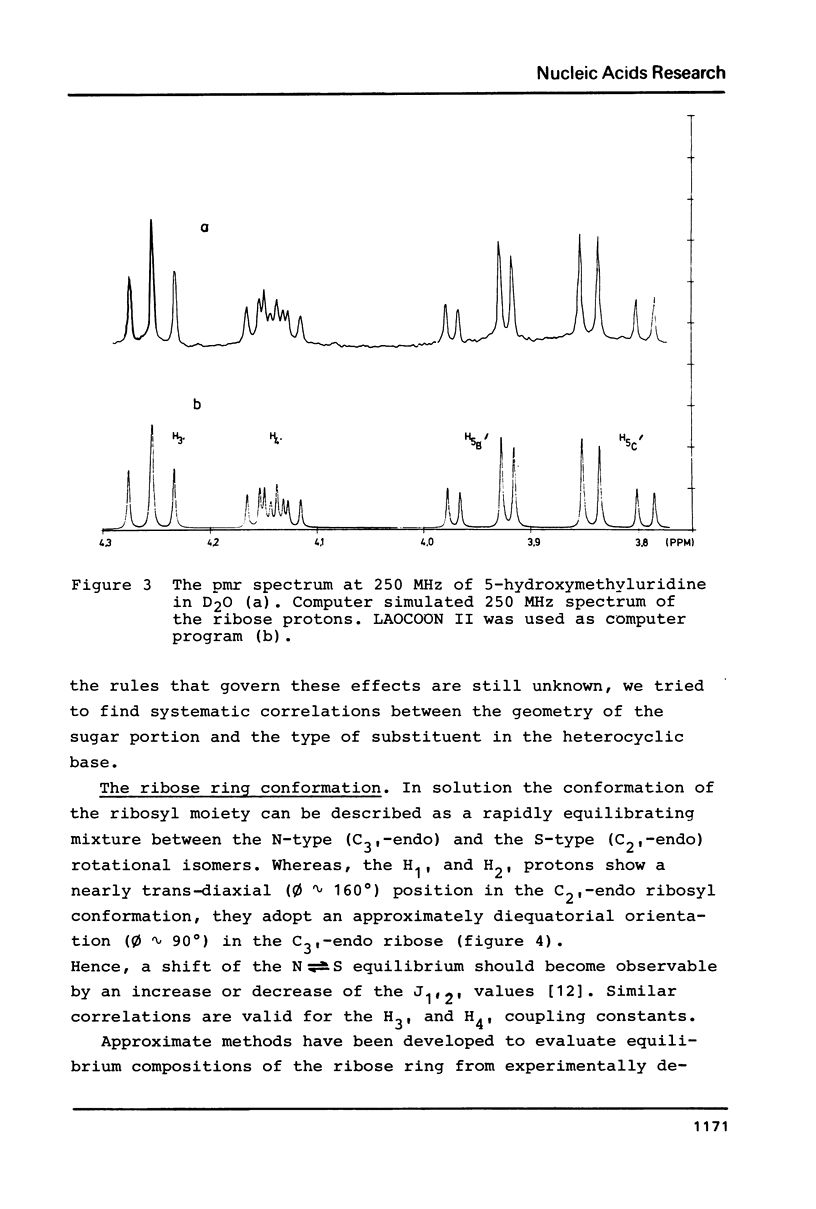
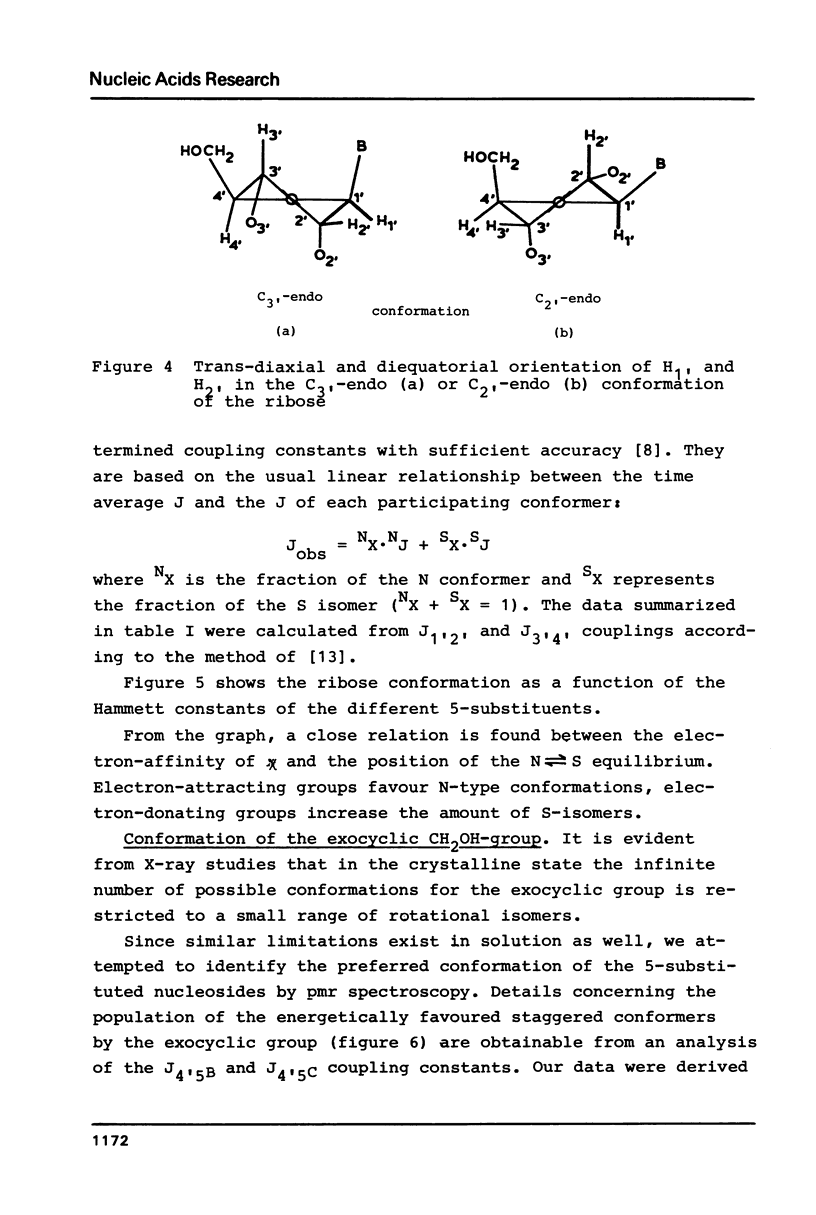
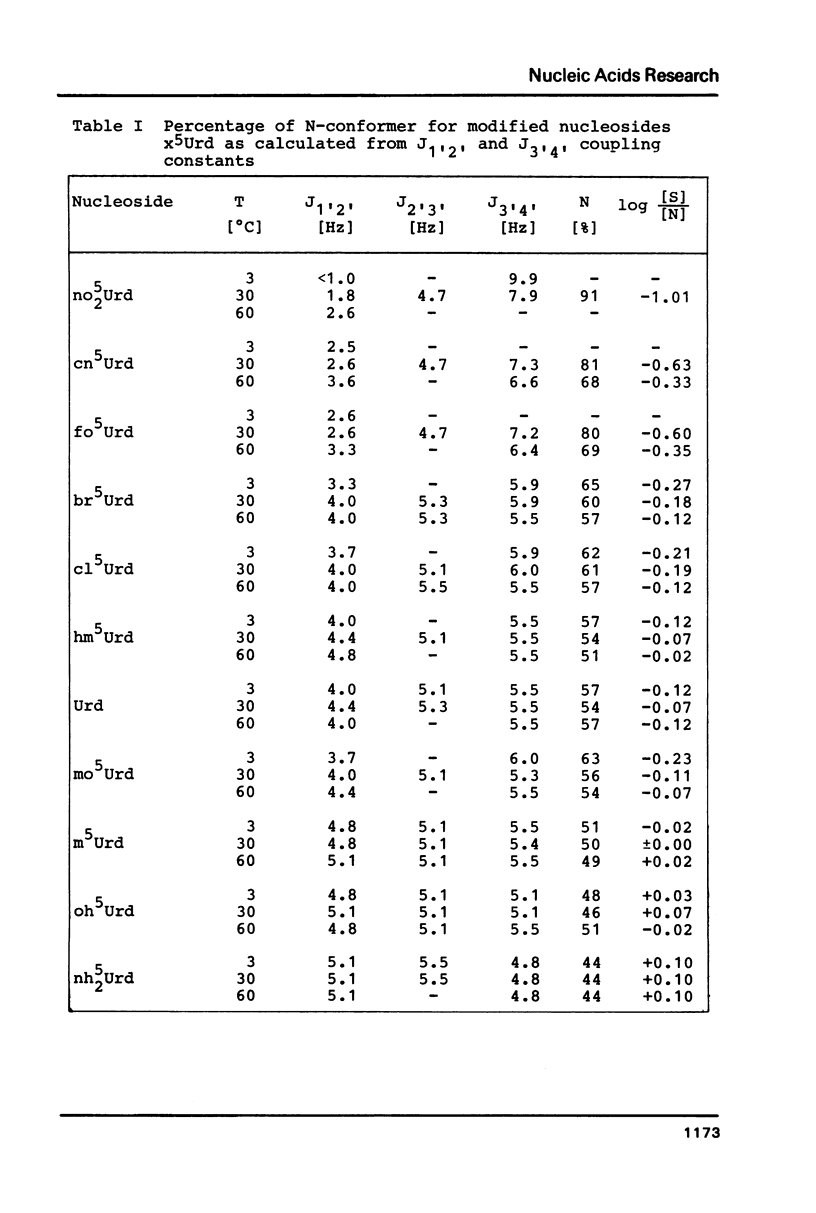
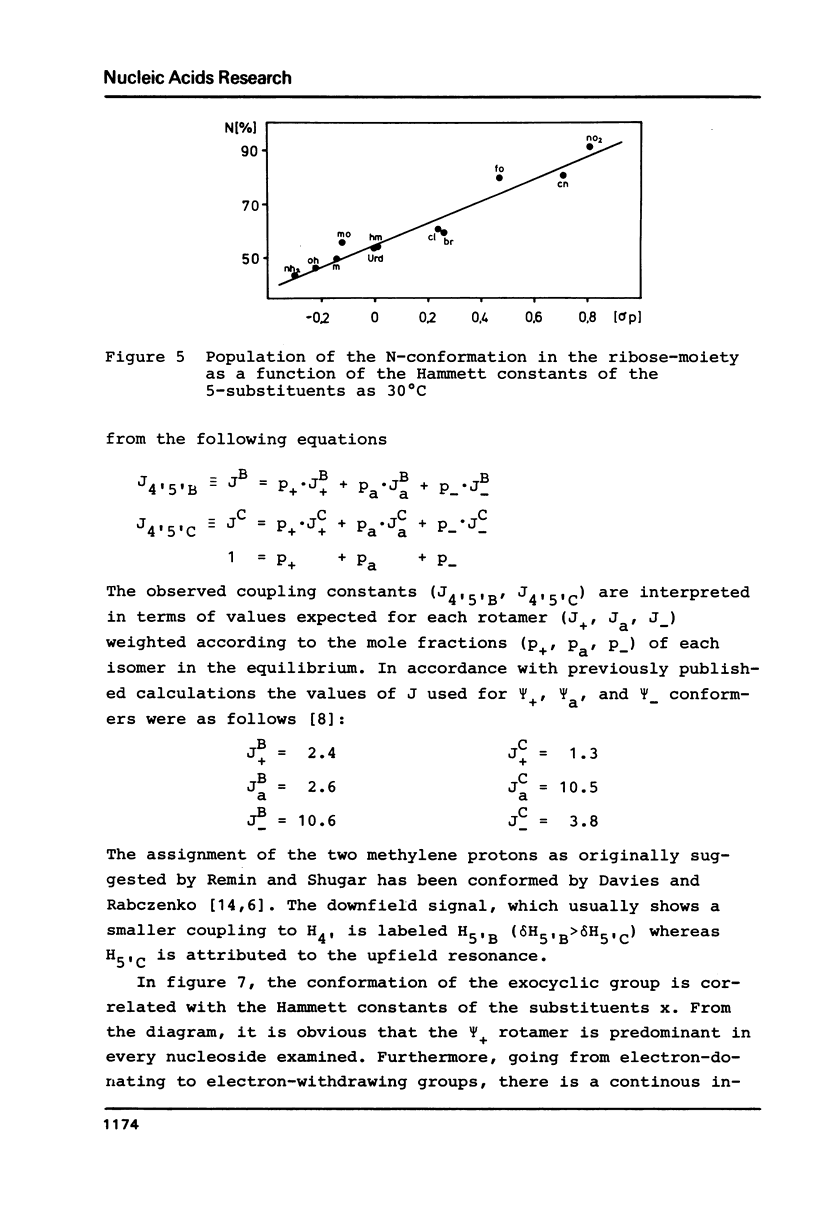
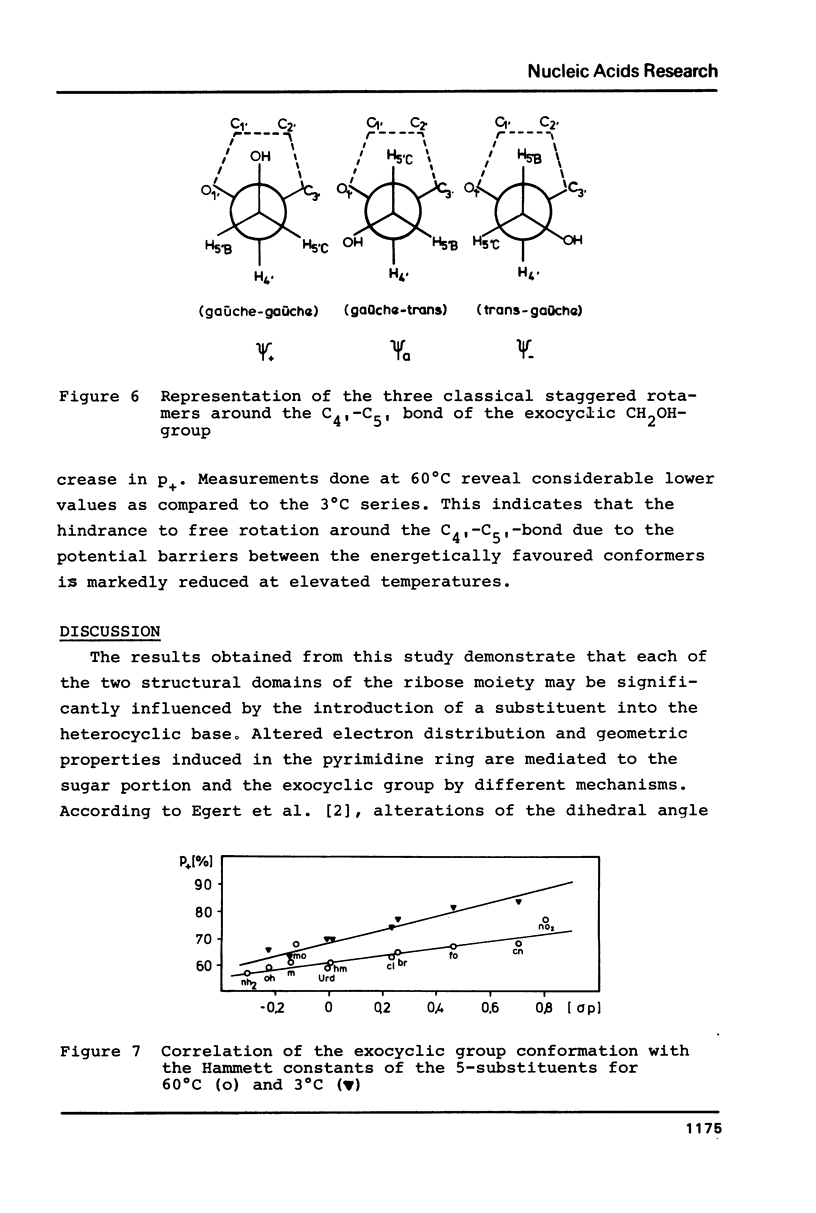
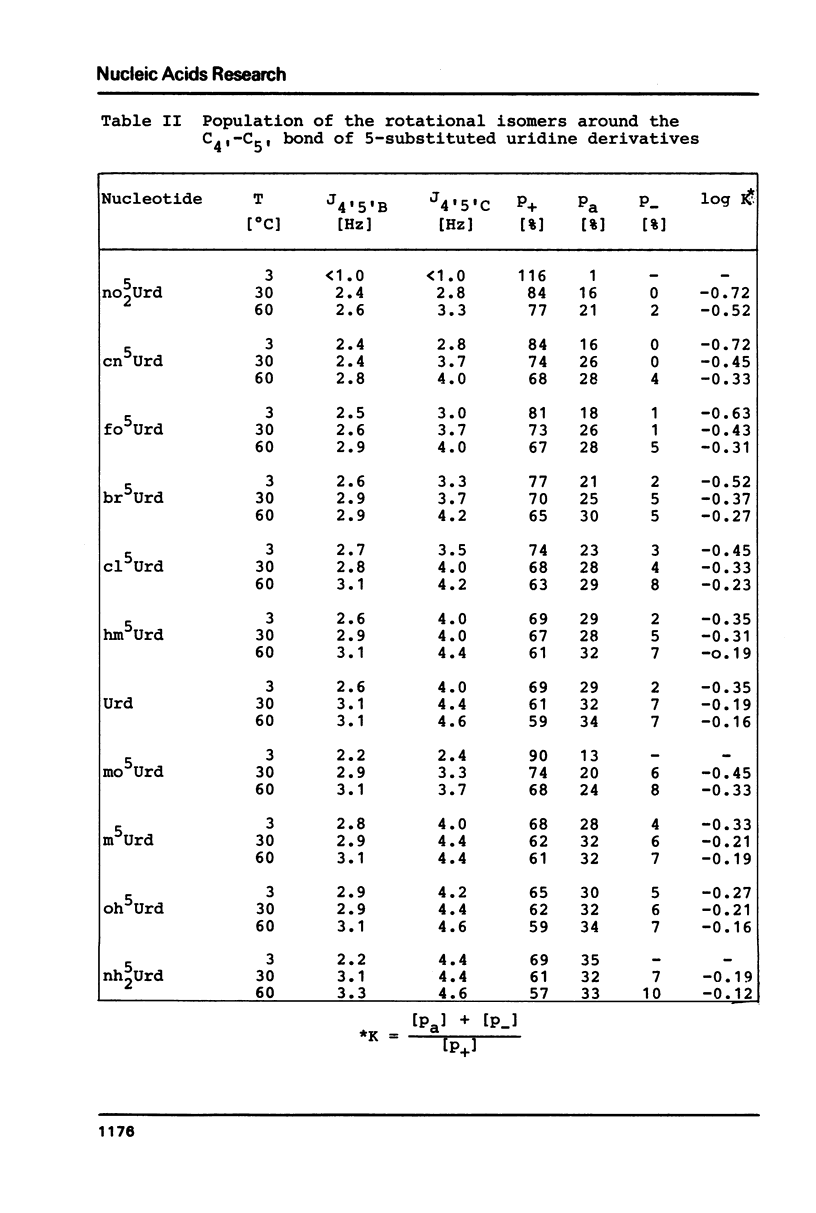
The proton magnetic resonance (pmr) spectra of 10 basemodified uridine derivatives x5Urd have been measured at 3 degrees, 30 degrees, and 60 degrees C in order to correlate the electronic effects of different substituents with the molecular conformation of the respective nucleosides. The results presented demonstrate the close relation between conformational parameters and the electron-affinity of the substituents as reflected by their Hammett constants. Going from electron-donating to electron-accepting groups, the portion of N-conformer in the ribose N in equilibrium S equilibrium increases from 44% to about 90%. In addition the percentage of gauche-gauche rotamer as measured for the exocyclic groups changes from about 30% in nh52Urd to more than 80% in no52Urd.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan S. I., Nelson J. H. Proton magnetic resonance studies of ribose dinucleoside monophoshates in aqueous solution. I. The nature of the base-stacking interaction in adenylyl 3'--5')adenosine. J Am Chem Soc. 1969 Jan 1;91(1):168–183. doi: 10.1021/ja01029a033. [DOI] [PubMed] [Google Scholar]
- Follmann H., Pfeil R., Witzel H. Pyrimidine nucleosides in solution. A study of intramolecular forces by proton magnetic resonance spectroscopy. Eur J Biochem. 1977 Aug 1;77(3):451–461. doi: 10.1111/j.1432-1033.1977.tb11686.x. [DOI] [PubMed] [Google Scholar]
- Hillen W., Gassen G. 5-Substituents in the uridine moiety and their effect on the conformation of ApU-type dinucleoside phosphates. Biochim Biophys Acta. 1978 Mar 29;518(1):7–16. doi: 10.1016/0005-2787(78)90111-9. [DOI] [PubMed] [Google Scholar]
- Hillen W., Gassen H. G. Physical and coding properties of poly(5-methoxyuridylic) acid. Biochim Biophys Acta. 1979 Apr 26;562(2):207–213. doi: 10.1016/0005-2787(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Hruska F. E., Danyluk S. S. Conformational changes of the ribose group in dinucleoside mono- and diphosphates. Temperature dependence. J Am Chem Soc. 1968 Jun 5;90(12):3266–3267. doi: 10.1021/ja01014a067. [DOI] [PubMed] [Google Scholar]
- Remin M., Shugar D. Conformation of the exocyclic 5'-CH 2 OH in nucleosides and nucleotides in aqueous solution from specific assignments of the H 5' and H 5'' signals in the NMR spectra. Biochem Biophys Res Commun. 1972 Aug 7;48(3):636–642. doi: 10.1016/0006-291x(72)90395-6. [DOI] [PubMed] [Google Scholar]



